Abstract
Aims
Lansoprazole is affected by polymorphism of CYP2C19. The aim of this study was to examine the effects of fluvoxamine, a CYP2C19 inhibitor, on the pharmacokinetics of each lansoprazole enantiomer among three different CYP2C19 genotype groups.
Methods
Eighteen healthy subjects, of whom six each were homozygous extensive metabolizers (homEMs), heterozygous extensive metabolizers (hetEMs), or poor metabolizers (PMs) for CYP2C19, participated in the study. Each subject received either placebo or fluvoxamine, 25 mg twice daily for 6 days, then a single oral dose of 60 mg of racemic lansoprazole. The plasma concentrations of lansoprazole enantiomers and lansoprazole sulphone were subsequently measured for 24 h post lansoprazole administration using liquid chromatography.
Results
In the homEMs and hetEMs, fluvoxamine significantly increased the AUC(0, ∞) and Cmax and prolonged the elimination half-life of both (R)- and (S)-lansoprazole, whereas in the PMs, the only statistically significant effect of fluvoxamine was on the AUC(0, ∞) for (R)-lansoprazole. The mean fluvoxamine-mediated percent increase in the AUC(0, ∞) of (R)-lansoprazole in the homEMs compared with the PMs was significant (P = 0.0117); however, Cmax did not differ among the three CYP2C19 genotypes. On the other hand, fluvoxamine induced a significant percent increase in both the AUC(0, ∞) and Cmax for (S)-lansoprazole in the homEMs compared with the hetEMs (P = 0.0007 and P = 0.0125, respectively) as well as compared with the PMs (P < 0.0001 for each parameter). The mean R : S ratio for AUC(0, ∞) of lansoprazole in the homEMs was significantly different between the placebo and the fluvoxamine treatment groups (12.7 (9.1, 16.8) vs 6.4 (5.4, 7.4), respectively, P < 0.0001), though not in the PMs (5.5 (4.3, 6.7) vs 5.9 (5.3, 6.5), respectively).
Conclusions
The magnitude of the contribution of CYP2C19 to the metabolism of (S)-lansoprazole is much greater compared with that of the (R)-enantiomer. In extensive metabolizers, hepatic CYP2C19 plays an important role in the absorption and elimination of lansoprazole, particularly the (S)-enantiomer.
Keywords: CYP2C19, enantiomer, fluvoxamine, lansoprazole
Introduction
Lansoprazole is a proton pump inhibitor (PPI) that inhibits gastric acid secretion by interacting with (H+/K+)-ATPase in gastric parietal cells [1 This drug is extensively metabolized by CYP2C19 and CYP3A4 to two major plasma metabolites, 5-hydroxylansoprazole and lansoprazole sulphone, respectively [2–4]. The CYP2C19-catalyzed hydroxylation pathway is the main disposition route of lansoprazole and hence, the disposition of lansoprazole is strongly influenced by CYP2C19 genetic polymorphism [5, 6].
Lansoprazole is administered clinically as a racemic mixture of the (R)- and (S)-enantiomers. It has been reported that the plasma concentrations of (R)-lansoprazole are higher than those of the (S)-enantiomer following an oral dose of 30 mg of racemic lansoprazole in both extensive metabolizers (EMs) and poor metabolizers (PMs) [7]. The differences between the pharmacokinetics of lansoprazole enantiomers are assumed to be influenced by enantioselective metabolism [8, 9]. Kim et al. reported that the affinity and the intrinsic clearance for CYP2C19 catalyzed 5-hydroxylation of (S)-lansoprazole is higher than for the (R)-enantiomer [8]. Therefore, one can expect that the disposition of (S)-lansoprazole is more intensely affected by CYP2C19 polymorphism than that of the (R)-enantiomer. Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is a known potent CYP1A2 inhibitor and it also inhibits CYP2C19 [10, 11]. Thus when lansoprazole is administered in the presence of fluvoxamine, one can assume a resultant increase in the plasma concentration of (S)-lansoprazole compared with that of the (R)-enantiomer.
On the basis of the above-described background and hypothesis, we investigated the effect of fluvoxamine on the disposition of lansoprazole enantiomers in relation to the CYP2C19 genotype status.
Methods
Subjects
Eighteen healthy Japanese subjects (homozygous extensive metabolizer group (homEMs, n = 6), heterozygous extensive metabolizer group (hetEMs, n = 6) and poor metabolizer group (PMs, n = 6)) were selected to participate in this study. The subjects enrolled in the present study are the same as those who participated in our previous study [12]. The mean age was 25.1 ± 3.8 years (range 21–34 years) and the mean weight was 56.6 ± 13.3 kg (range 40–86 kg). There were no differences among the three CYP2C19 genotypes (homEMs, hetEMs and PMs) with respect to age (24.7 ± 3.8, 25.0 ± 4.5 and 25.7 ± 3.6 years, respectively), body weight (57.2 ± 15.6, 53.0 ± 10.5 and 59.5 ± 15.0 kg, respectively), body mass index (20.9 ± 3.8, 20.5 ± 2.5 and 21.3 ± 3.5 kg m−2, respectively) and male : female ratios (3 : 3 in each group) [12]. None of the subjects had a history of significant medical illness or hypersensitivity to any drug nor were any of the subjects smokers. The study protocol was approved by the ethics committee of Hirosaki University Hospital, and all subjects gave their written informed consent prior to study participation.
Study protocol
A randomized double-blind placebo-controlled crossover study design was conducted at intervals of 2 weeks. After fluvoxamine (25 mg) in capsule form containing a tablet (Luvox®, Fujisawa Pharmaceutical Co., Ltd, Osaka, Japan) or matched placebo (in capsule form with the same appearance and size as that of fluvoxamine) was given orally twice a day (09.00 h, 21.00 h) for 6 days, each subject received an oral dose of 60 mg of lansoprazole (Takepron®, Takeda Pharmaceutical Co. Ltd, Osaka, Japan) with 240 ml of tap water at 09.00 h. Venous blood samples were taken before lansoprazole administration and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h after the drug was taken to measure the plasma concentration of lansoprazole enantiomers and lansoprazole sulphone. The samples were centrifuged at 3000 g immediately after collection and stored at −80°C until analysis. All subjects fasted for 10 h before the administration of lansoprazole and had a standard meal 4 h later. Alcohol and caffeinated beverages were forbidden during the study period.
CYP2C19 genotyping
The genotyping procedure used to identify the CYP2C19 wild-type gene and its two mutant alleles, CYP2C19*2 in exon 5 and CYP2C19*3 in exon 4, was performed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method [13]. CYP2C19 genotype analysis revealed five different patterns as follows: *1/*1 in six; *1/*2 in three; *1/*3 in three; *2/*2 in five and *2/*3 in one patient. Subjects with these genotype patterns were divided into three groups: the homEMs (*1/*1, n = 6), the hetEMs (*1/*2 and *1/*3, n = 6) and the PMs (*2/*2 and *2/*3, n = 6).
Analysis of lansoprazole enantiomers and their metabolites in plasma
The plasma concentrations of lansoprazole enantiomers and lansoprazole sulphone were determined by HPLC with solid-phase extraction method of Miura et al.[14]. The HPLC column used was a Chiral CD-Ph (250 mm × 4.6 mm I.D., Shiseido Co. Ltd, Tokyo, Japan). The mobile phase consisted of 0.5 m NaClO4 : acetonitrile : methanol (60 : 30 : 10, v : v). A flow rate of 0.5 ml min−1 was used at ambient temperature, and the wavelength was set at 285 nm. The lower limit of quantification for this assay was 10 ng ml−1 for lansoprazole enantiomers and 5 ng ml−1 for lansoprazole sulphone. The coefficient of variation of inter- and intraday assays (n = 6) was less than 8.0% and the accuracy (n = 6) was within 8.4% for all analytes (concentration range of 10–4000 ng ml−1).
Pharmacokinetic analysis
Pharmacokinetic analysis of the lansoprazole enantiomers and lansoprazole sulphone was carried out by a standard noncompartmental method using WinNonlin (Pharsight Co., CA, version 4.0.1). The elimination half-life was obtained by log-linear regression of the terminal phase of the concentration-time data for at least three sampling points (elimination half-life = ln2/λz where λz = elimination rate constant). The total area under the observed plasma concentration-time curve (AUC) was calculated using the linear trapezoidal rule. Extrapolation of AUC from the last measurable concentration (Ct) to infinity (AUC(t, ∞)) was performed by adding the value Ct/λz (where Ct = plasma concentration at t h after lansoprazole administration). The maximum plasma concentration (Cmax) and time required to reach the peak (tmax) were directly obtained from the profile.
Statistical analysis
All results were expressed as mean values ± SD and 95% confidence intervals (CI). Statistical comparisons of the parameters were supplemented with the multiple comparison procedure of Fisher using the Stat View program (SAS Institute, Cary, NC, version 5.0). A P value of less than 0.05 was considered to be statistically significant.
Results
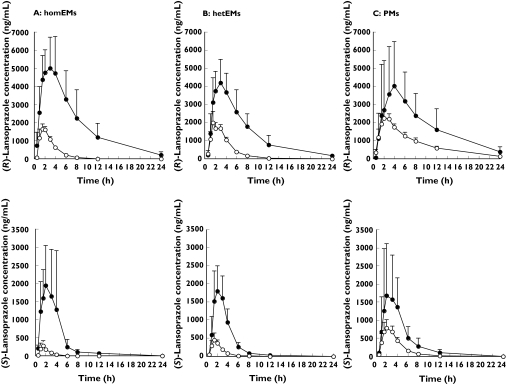
The mean plasma concentrations of the (R)- and (S)-enantiomers of lansoprazole were significantly increased by fluvoxamine in the three different CYP2C19 genotype groups (Figure 1). The plasma concentration-time profile of (R)- and (S)-lansoprazole in the presence of fluvoxamine was similar among the three CYP2C19 genotype groups. Fluvoxamine significantly increased the AUC(0, ∞) and Cmax and prolonged the elimination half-life of both (R)- and (S)-lansoprazole in the homEMs and hetEMs, whereas in the PMs there were no statistically significant differences in pharmacokinetic parameters for the subjects given placebo and those given fluvoxamine (Table 1), with the exception of the AUC(0, ∞) of (R)-lansoprazole where a significant difference was seen. The mean AUC(0, ∞) of (R)- and (S)-lansoprazole in the homEMs fluvoxamine group was 2.2- and 1.9-fold higher, respectively, than the those in the PMs who did not take fluvoxamine (45032 (28 786, 61 278) vs 20 132 (17 275, 22 989), 7322 (3354, 11 290) vs 3892 (3098, 4686) ng ml−1 h) (Table 1). The mean lansoprazole R/S ratio for the AUC(0, ∞) in the homEMs differed significantly between the placebo and the fluvoxamine treatment groups (12.7 (9.1, 16.8) vs 6.4 (5.4, 7.4), respectively, P < 0.0001), though not in the PMs (5.5 (4.3, 6.7) vs 5.9 (5.3, 6.5), respectively).
Figure 1.
The effect of fluvoxamine on the disposition of (R)-lansoprazole (upper panel) and (S)-lansoprazole (lower panel) in homozygous EMs (A), heterozygous EMs (B) and PMs (C). Subjects received a single oral dose of 60 mg of racemic lansoprazole following administration of placebo (○) or 25 mg of fluvoxamine (•) twice a day for 6 days. The results are shown as the mean ± SD
Table 1. Pharmacokinetic parameters of lansoprazole enantiomers with fluvoxamine in three CYP2C19 genotype groups.
| Homozygous EMs | Heterozygous EMs | PMs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study group | Placebo | Fluvoxamine | P value | Placebo | Fluvoxamine | P value | Placebo | Fluvoxamine | P value |
| (R)-Lansoprazole | |||||||||
| Cmax (ng ml−1) (95% CI) | 1957 ± 413 (1627, 2288) | 5698 ± 1839 (4227, 7170) | 0.0014 | 2196 ± 405 (1872, 2520) | 4526 ± 1318 (3471, 5580) | 0.0061 | 2516 ± 357 (2230, 2802) | 4460 ± 2500 (2460, 6460) | 0.1025 |
| tmax (h) (95% CI) | 1.9 ± 0.6 (1.4, 2.4) | 2.4 ± 1.1 (1.5, 3.3) | 0.3533 | 2.0±0.8 (1.7, 2.9) | 3.0±1.1 (2.2, 4.0) | 0.1711 | 2.4 ± 0.9 (1.7, 3.1) | 4.4 ± 2.3 (2.6, 6.2) | 0.0751 |
| Half-life (h) (95% CI) | 1.3 ± 0.3 (1.1, 1.5) | 4.3 ± 1.2 (3.3, 5.3) | 0.0001 | 1.5 ± 0.2 (1.3, 1.7) | 3.4 ± 1.2 (2.4, 4.4) | 0.0029 | 5.0 ± 1.0 (4.2, 5.8) | 6.0 ± 1.4 (4.9, 7.1) | 0.1805 |
| AUC(0, ∞) (ng ml−1 h) (95% CI) | 5009 ± 919 (4274, 5744) | 45 032 ± 20 303 (28 786, 61 278) | 0.0007 | 7300 ± 1008 (6493, 8107) | 31 210 ± 10 813 (22 558, 39 862) | 0.0004 | 20 132 ± 3570 (17 275, 22 989) | 45 735 ± 26 463 (24 560, 66 910) | 0.0396 |
| (S)-Lansoprazole | |||||||||
| Cmax (ng ml−1) (95% CI) | 337 ± 135 (229, 145) | 2356 ± 1219 (1381, 3331) | 0.0029 | 528 ± 166 (395, 661) | 1949 ± 773 (1331, 2568) | 0.0026 | 1156 ± 253 (954, 1358) | 2079 ± 1374 (980, 3178) | 0.1645 |
| tmax (h) (95% CI) | 1.7 ± 0.7 (1.1, 2.3) | 2.2 ± 1.1 (1.3, 3.1) | 0.3741 | 1.8 ± 0.7 (1.2, 2.4) | 2.4 ± 0.7 (1.8, 3.0) | 0.1189 | 1.9 ± 0.6 (1.4, 2.4) | 2.9 ± 1.0 (2.1, 3.7) | 0.0639 |
| Half-life (h) (95% CI) | 0.6 ±0.1 (0.5, 0.7) | 1.2 ± 0.4 (0.9, 1.5) | 0.0020 | 0.7 ± 0.2 (0.5, 0.9) | 1.1 ± 0.2 (0.9, 1.3) | 0.0078 | 1.6 ± 0.5 (1.2, 2.0) | 2.9 ± 1.0 (2.1, 3.7) | 0.1784 |
| AUC(0, ∞) (ng ml−1 h) (95% CI) | 524 ± 189 (373, 675) | 7322 ± 4959 (3354, 11290) | 0.0075 | 967 ± 224 (788, 1146) | 6009 ± 2101 (4328, 7690) | 0.0002 | 3892 ± 992 (3098, 4686) | 7837 ± 5210 (3668, 12006) | 0.1240 |
| R/S-ratio | |||||||||
| AUC(0, ∞) (95% CI) | 12.7 ± 4.5 (9.1, 16) | 6.4 ± 1.3 (5.4, 7.4) | <0.0001 | 8.5 ± 1.9 (7.0, 10) | 5.0 ± 0.6 (4.8, 5.8) | 0.0186 | 5.5 ± 1.5 (4.3, 6.7) | 5.9 ± 0.8 (5.3, 6.5) | 0.8007 |
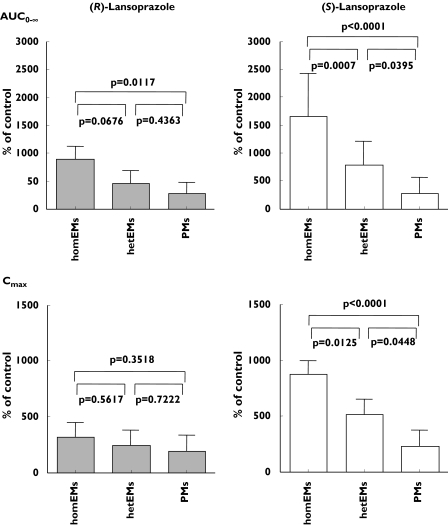
The mean fluvoxamine induced percent increase in the AUC(0, ∞) of (R)-lansoprazole in the homEMs compared with the PMs was significant (P = 0.0117), although no differences were observed in Cmax among the three CYP2C19 genotypes. In contrast, fluvoxamine cased a significant percent increase in both the AUC(0, ∞) and Cmax for (S)-lansoprazole in the homEMs compared with the hetEMs (P = 0.0007 and P = 0.0125, respectively) and compared with the PMs (P < 0.0001 for each parameter) (Figure 2). The influence of fluvoxamine on the CYP2C19-mediated metabolism of (S)-lansoprazole was much greater than that of the (R)-enantiomer (Figure 2).
Figure 2.
The change in AUC(0, ∞) and Cmax of (R)-lansoprazole (solid bars) and (S)-lansoprazole (open bars) by fluvoxamine among the three CYP2C19 genotype groups. Error bars indicate SD
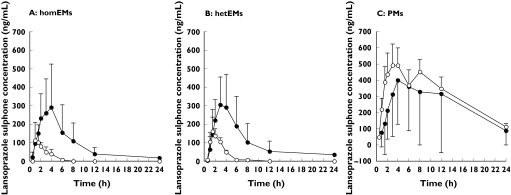
Meanwhile, fluvoxamine increased the mean plasma concentration of lansoprazole sulphone in the homEMs and hetEMs, whereas concentrations in the PMs were slightly decreased (Figure 3 and Table 2).
Figure 3.
The influence of fluvoxamine on the formation of lansoprazole sulphone in homozygous EMs (A), heterozygous EMs (B) and PMs (C). Subjects received a single oral dose of 60 mg of racemic lansoprazole following administration of placebo (○) or 25 mg of fluvoxamine (•) twice a day for 6 days. The results are shown as the mean ± SD
Table 2. Pharmacokinetic parameters of lansoprazole sulphone with fluvoxamine in three CYP2C19 genotype groups.
| Homozygous EMs | Heterozygous EMs | PMs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study group | Placebo | Fluvoxamine | P value | Placebo | Fluvoxamine | P value | Placebo | Fluvoxamine | P value |
| Lansoprazole sulphone | |||||||||
| Cmax (ng ml−1) (95% CI) | 112 ± 106 (27, 197) | 323 ± 203 (161, 486) | 0.0478 | 184 ± 142 (70, 298) | 334 ± 183 (187, 481) | 0.1448 | 568 ± 277 (346, 790) | 468 ± 326 (207, 729) | 0.5798 |
| (95% CI) | (27, 197) | (161, 486) | (70, 298) | (187, 481) | (346, 790) | (207, 729) | |||
| tmax (h) (95% CI) | 1.8 ± 0.7 (1.2, 2.4) | 2.6 ± 1.3 (1.6, 3.6) | 0.1909 | 2.0 ± 0.8 (1.4, 2.6) | 3.2 ± 0.8 (2.6, 3.8) | 0.0245 | 3.2 ± 1.0 (2.4 4.0) | 6.8 ± 4.1 (3.5, 10) | 0.0599 |
| (95% CI) | (1.2, 2.4) | (1.6, 3.6) | (1.4, 2.6) | (2.6, 3.8) | (2.4 4.0) | (3.5, 10) | |||
| Half-life (h) (95% CI) | 0.6 ± 0.2 (0.4, 0.8) | 2.1 ± 1.1 (1.2, 3.0) | 0.0078 | 0.6 ± 0.2 (0.4, 0.8) | 2.2 ± 0.8 (1.6, 2.8) | 0.0013 | 7.1 ± 3.4 (4.4, 9.8) | 6.7 ± 3.2 (4.1, 9.3) | 0.8218 |
| (95% CI) | (0.4, 0.8) | (1.2, 3.0) | (0.4, 0.8) | (1.6, 2.8) | (4.4, 9.8) | (4.1, 9.3) | |||
| AUC(0, ∞) (ng ml−1 h) (95% CI) | 197 ±204 (34, 360) | 1759 ± 1463 (588, 2930) | 0.0268 | 368 ± 239 (177, 559) | 1699 ± 1113 (808, 2590) | 0.0169 | 7756 ± 4855 (3871, 11641) | 6886 ± 6327 (1823, 11949) | 0.9481 |
| (95% CI) | (34, 360) | (588, 2930) | (177, 559) | (808, 2590) | (3871, 11641) | (1823, 11949) | |||
Discussion
This is the first report on the effect of fluvoxamine, a CYP2C19 inhibitor, on the pharmacokinetics of lansoprazole enantiomers in relation to CYP2C19 genotype status. The effect of fluvoxamine on lansoprazole metabolism is greatest in individuals who are homEMs, less in hetEMs and least in PMs of CYP2C19. In the present study, fluvoxamine significantly increased the AUC(0, ∞) for (R)- and (S)-lansoprazole in the homEMs by 903% and 1664%, respectively, and in the hetEMs by 462% and 781%, respectively (Figure 2). Thus, the drug interaction is more marked between (S)-lansoprazole and fluvoxamine than between (R)-lansoprazole and fluvoxamine. Furthermore, mean R : S ratios of AUC(0, ∞) for lansoprazole in the presence of fluvoxamine were not significantly different between homEMs and PMs (6.4 vs 5.9), although in the placebo groups the ratio was significantly greater in the homEMs than in the PMs (12.7 vs 5.5, P < 0.05). These data show that the magnitude of the contribution of CYP2C19 to the metabolism of (S)-lansoprazole is much greater compared with that of the (R)-enantiomer. This finding is supported by previous in vitro data that describe a higher affinity and intrinsic clearance for CYP2C19-catalyzed hydroxylation of (S)-lansoprazole than of the (R)-enantiomer [8]. In contrast (S)-omeprazole is less influenced by CYP2C19 genetic polymorphism compared with (R)-omeprazole and racemic omeprazole [15–17]. This finding has led to the development of esomeprazole, an (S)-enantiomer of omeprazole, as the first single enantiomer PPI. Thus, the racemate is not simply a mixture of the two enantiomers but rather a distinct molecular compound or entity with properties quite distinct from those of the two optical isomers [18–22]. Although the clinical relevance on the effect of each enantiomer is not yet fully established, the pharmacological activities of (R)- and (S)-lansoprazole according to data obtained from in vitro studies are considered to be similar [23]. The disposition of (R)-lansoprazole appears to be less influenced by CYP2C19 than that of the (S)-enantiomer. Therefore, the use of only (R)-lansoprazole would be highly desirable for clinical application.
In this study, we were unable to evaluate the pharmacokinetic parameters of 5-hydroxylansoprazole enantiomers in the plasma samples obtained from each subject co-administered fluvoxamine, because the plasma concentrations of the enantiomers were below the limit of detection by the HPLC method we used (lower limit of quantification, 10 ng ml–l each) [14]. However, fluvoxamine inhibits 5-hydroxylation of lansoprazole and consequently significantly slows its elimination in the EMs. Fluvoxamine markedly increased Cmax and significantly prolonged the elimination half-life of lansoprazole, particularly the (S)-enantiomer; it seems that fluvoxamine inhibits the biotransformation of lansoprazole during both the absorption and elimination phases in the liver. Due to the inhibition of CYP2C19 activity by fluvoxamine, lansoprazole is preferentially metabolized to lansoprazole sulphone by CYP3A4 in EMs, whereas in the PMs of CYP2C19, fluvoxamine slightly inhibits the formation of lansoprazole sulphone, though not significantly. This result suggests that fluvoxamine inhibits CYP3A4 activity in PM subjects to some extent [24, 25], because lansoprazole sulphone is formed solely by CYP3A4 [2, 6, 7].
In conclusion, the plasma concentration of (S)-lansoprazole is considerably influenced by fluvoxamine compared with that of the (R)-enantiomer. In EMs subjects, hepatic CYP2C19 plays a more important role in the absorption and elimination of (S)-lansoprazole compared with those of the (R)-enantiomer.
Acknowledgments
Competing interests: None declared.
References
- 1.Nagaya H, Satoh H, Maki Y. Possible mechanism for the inhibition of acid formation by the proton pump inhibitor AG-1749 in isolated canine parietal cells. J Pharmacol Exp Ther. 1990;252:1289–92. [PubMed] [Google Scholar]
- 2.Pearce RE, Rodrigues AD, Goldstein JA, Parkinson A. Identification of the human P450 enzymes involved in lansoprazole metabolism. J Pharmacol Exp Ther. 1996;277:805–16. [PubMed] [Google Scholar]
- 3.Katsuki H, Nakamura C, Arimori K, Fujiyama S, Nakano M. Genetic polymorphism of CYP2C19 and lansoprazole pharmacokinetics in Japanese subjects. Eur J Clin Pharmacol. 1997;52:391–6. doi: 10.1007/s002280050307. [DOI] [PubMed] [Google Scholar]
- 4.Pichard L, Curi-Pedrosa R, Bonfils C, Jacqz-Aigrain E, Domerque J, Joyeux H, Cosme J, Guengerich FP. Oxidative metabolism of lansoprazole by human liver cytochromes P450. Mol Pharmacol. 1995;47:410–8. [PubMed] [Google Scholar]
- 5.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors – emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13:27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 6.Sohn DR, Kwon JT, Kim HK, Ishizaki T. Metabolic disposition of lansoprazole in relation to the S-mephenytoin 4′-hydroxylation phenotype status. Clin Pharmacol Ther. 1997;61:574–82. doi: 10.1016/S0009-9236(97)90137-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim KA, Shon JH, Park JY, Yoon YR, Kim MJ, Yun DH, Kim MK, Cha IJ, Hyun MH, Shin JG. Enantioselective disposition of lansoprazole in extensive and poor metabolizers of CYP2C19. Clin Pharmacol Ther. 2002;72:90–9. doi: 10.1067/mcp.2002.126176. [DOI] [PubMed] [Google Scholar]
- 8.Kim KA, Kim MJ, Park JY, Shon JH, Yoon YR, Lee SS, Liu KH, Chun JH, Hyun MH, Shin JG. Stereoselective metabolism of lansoprazole by human liver cytochrome P450 enzymes. Drug Metab Dispos. 2003;31:1227–34. doi: 10.1124/dmd.31.10.1227. [DOI] [PubMed] [Google Scholar]
- 9.Katsuki H, Hamada A, Nakamura C, Arimori K, Nakano M. Role of CYP3A4 and CYP2C19 in the stereoselective metabolism of lansoprazole by human liver microsomes. Eur J Clin Pharmacol. 2001;57:709–15. doi: 10.1007/s002280100374. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brosen K. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–8. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- 11.Yao C, Kunze KL, Trager WF, Kharasch ED, Levy RH. Comparison of in vitro and in vivo inhibition potencies of fluvoxamine toward CYP2C19. Drug Metab Dispos. 2003;31:565–71. doi: 10.1124/dmd.31.5.565. [DOI] [PubMed] [Google Scholar]
- 12.Miura M, Tada H, Yasui-Furukori N, Uno T, Sugawara K, Tateishi T, Suzuki T. Pharmacokinetic differences between the enantiomers of lansoprazole and its metabolite, 5-hydroxylansoprazole, in relation to CYP2C19 genotypes. Eur J Clin Pharmacol. 2004;60:623–8. doi: 10.1007/s00228-004-0809-1. [DOI] [PubMed] [Google Scholar]
- 13.De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–5. [PubMed] [Google Scholar]
- 14.Miura M, Tada H, Suzuki T. Simultaneous determination of lansoprazole enantiomers and their metabolites in plasma by liquid chromatography with solid–phase extraction. J Chromatogr B. 2004;804:389–95. doi: 10.1016/j.jchromb.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H, Okada A, Higaki M, Yokota H, Mashige F, Nakahara K. Stereospecific analysis of omeprazole in human plasma as a probe for CYP2C19 phenotype. J Pharm Biomed Anal. 2003;30:1817–24. doi: 10.1016/s0731-7085(02)00524-1. [DOI] [PubMed] [Google Scholar]
- 16.Tybring G, Bottiger Y, Widen J, Bertilsson L. Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish white subjects. Clin Pharmacol Ther. 1997;62:129–37. doi: 10.1016/S0009-9236(97)90060-6. [DOI] [PubMed] [Google Scholar]
- 17.Andersson T, Hassan-Alin M, Hasselgren G, Rohss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)–isomer of omeprazole. Clin Pharmacokinet. 2001;40:411–6. doi: 10.2165/00003088-200140060-00003. [DOI] [PubMed] [Google Scholar]
- 18.Howe R, Shanks RG. Optical isomers of propranolol. Nature. 1966;210:1336–8. doi: 10.1038/2101336a0. [DOI] [PubMed] [Google Scholar]
- 19.Blaschke G, Kraft HP, Fickentscher K, Kohler F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers. Arzneimittelforschung. 1979;29:1640–2. [PubMed] [Google Scholar]
- 20.Hutt AJ, Tan SC. Drug chirality and its clinical significance. Drugs. 1996;52:1–12. doi: 10.2165/00003495-199600525-00003. [DOI] [PubMed] [Google Scholar]
- 21.Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: therapeutic pitfalls. J Pharm Sci. 1989;78:695–715. doi: 10.1002/jps.2600780902. [DOI] [PubMed] [Google Scholar]
- 22.Walle T. Stereochemistry of the in vivo disposition and metabolism of propranolol in dog and man using deuterium–labeled pseudoracemates. Drug Metab Dispos. 1985;13:279–82. [PubMed] [Google Scholar]
- 23.Nagaya H, Inatomi N, Nohara A, Satoh H. Effects of the enantiomers of lansoprazole (AG−1749 on (H++K+)–ATPase activity in canine gastric microsomes and acid formation in isolated canine parietal cells. Biochem Pharmacol. 1991;42:1875–8. doi: 10.1016/0006-2952(91)90584-r. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Shioiri T, Muratake T, Kawashima Y, Sato S, Hagiwara M, Inoue Y, Shimoda K, Someya T. Effects of concomitant fluvoxamine on the metabolism of alprazolam in Japanese psychiatric patients: interaction with CYP2C19 mutated alleles. Eur J Clin Pharmacol. 2003;58:829–33. doi: 10.1007/s00228-003-0563-9. [DOI] [PubMed] [Google Scholar]
- 25.Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P−450 mediated drug–drug interactions: an update. Curr Drug Metab. 2002;3:13–37. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]