Abstract
Aims
Our objective was to study in vivo the role of CYP2C and CYP3A4 in the disposition of 3-keto-desogestrel after administration of desogestrel, by using the selective inhibitors fluconazole (CYP2C) and itraconazole (CYP3A4).
Methods
This study had a three-way crossover design and included 12 healthy females, the data from 11 of whom were analyzed. In the first (control) phase all subjects received a single 150 µg oral dose of desogestrel alone. In the second and third phases subjects received a 4 day pretreatment with either 200 mg fluconazole or 200 mg itraconazole once daily in a randomized balanced order. Desogestrel was given 1 h after the last dose of the CYP inhibitor. Plasma 3-keto-desogestrel concentrations were determined for up to 72 h post dose.
Results
Pretreatment with itraconazole for 4 days significantly increased the area under the plasma concentration-time curve (AUC) of 3-keto-desogestrel by 72.4% (95% confidence interval on the difference 12%, 133%; P = 0.024) compared with the control phase, whereas fluconazole pretreatment had no significant effect (95% CI on the difference −42%, 34%). Neither enzyme inhibitor affected significantly the maximum concentration (95% CI on the difference 14%, 124% for itraconazole and −23%, 40% for fluconazole) or elimination half-life (95% CI on the difference −42%, 120% for itraconazole and −24%, 61% for fluconazole) of 3-keto-desogestrel.
Conclusions
According to the present study, the biotransformation of desogestrel to 3-keto-desogestrel did not appear to be mediated by CYP2C9 and CYP2C19 as suggested earlier. However, the further metabolism of 3-keto-desogestrel seems to be catalyzed by CYP3A4.
Keywords: cytochrome P450 (CYP) enzyme, desogestrel, drug interaction, 3-keto-desogestrel, metabolism
Introduction
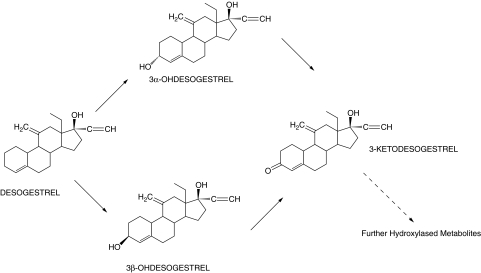
Desogestrel is a progestogenic 19-nortestoterone derivative [1], which is widely used for oral contraception both combined with ethinyl estradiol and alone in progestin-only pills. The parent drug does not possess progestogenic activity and bioactivation to 3-keto-desogestrel is needed for the contraceptive activity [2]. Previous in vitro studies have confirmed that 3-keto-desogestrel (etonogestrel) is the main identifiable metabolite of desogestrel being formed from two unstable intermediate metabolites, 3α-hydroxy- and 3β-hydroxydesogestrel [3–5] (Figure 1). Cytochrome P450 (CYP) enzymes catalyze the oxidative bioactivation of desogestrel and substantial first pass metabolism by the gut mucosa and the liver leading to formation of 3-keto-desogestrel has been reported [6]. Gentile et al. concluded that CYP2C9 and possibly CYP2C19 are the important enzymes in the conversion of the parent drug to 3-keto-desogestrel [5], whose further metabolism was suggested to be catalyzed by CYP3A4 [5]. Pharmacokinetic studies have provided evidence for bioactivation of desogestrel to 3-keto-desogestrel in vivo, with significant interindividual variation in the plasma concentration of the latter [7–10].
Figure 1.
The proposed metabolic pathway of desogestrel bioactivation in vitro [4, 5]. Figure reproduced from Gentile et al. [5]
Both CYP2C9 and CYP2C19 exhibit genetic polymorphism, with large interethnic differences in the frequency of mutant alleles [11–14]. In the western Caucasian population the frequencies of the poor metabolizer phenotypes are approximately 1% and 3% of the population for CYP2C9 and CYP2C19, respectively [15–18]. It has been previously shown that individuals who are hetero- or homozygous for CYP2C9*3, or homozygous for CYP2C9*2, have significantly impaired CYP2C9 activity compared with individuals with the wild-type genotype [19, 20]. Thus if desogestrel bioactivation is catalyzed by CYP2C9, the contraceptive efficacy of the drug may be affected by the polymorphism of this enzyme. Most poor metabolizers of CYP2C19 catalyzed reactions have been found to be homozygous for known mutant alleles of the CYP2C19 gene [21]. Consequently, subjects homozygous for CYP2C19*2 may be expected to have slower metabolism of desogestrel than subjects with the wildtype genotype, if desogestrel bioactivation is catalyzed by CYP2C19. Fluconazole has been shown to be a potent inhibitor of CYP2C9 catalyzed reactions both in vitro[22, 23] and in vivo[24–26]. Furthermore, fluconazole has been found to inhibit CYP2C19 catalyzed (S)-mephenytoin hydroxylation in vitro[27]. Another antifungal drug, itraconazole, is a well established specific inhibitor of CYP3A4 [28, 29], and is without effect on CYP2C enzyme catalyzed reactions in vitro and in vivo[30]. In the present study the role of CYP2C and CYP3A4 enzyme activities in the metabolic activation of desogestrel to 3-keto-desogestrel and on the further metabolism of 3-keto-desogestrel was investigated in vivo using the specific CYP enzyme inhibitors fluconazole and itraconazole.
Methods
Subjects and ethics
Twelve females (aged 20–24 years, body mass index 19–32 kg m−2, mean 22.6) participated in this study. The subjects were non smokers and the use of any concomitant medication was not allowed during the study. None of the subjects had used oral contraceptives for at least 2 months before the study. The subjects were ascertained to be in good health by medical history, physical examination and standard haematological and clinical chemistry tests. Pregnancy was excluded by a pregnancy test and the subjects were advised to use barrier methods for contraception during the study. Written informed consent was obtained from all the subjects and the study protocol was approved by the Ethics Committee of Hospital District of South-west Finland.
Protocol
This was an open, balanced, crossover study with three phases. In the first (control) phase all subjects received a single 150 µg dose of desogestrel (i.e. two tablets of Cerazette® 75 µg; Organon, Oss, the Netherlands) under the supervision of the study personnel. To determine the plasma concentration of 3-keto-desogestrel, venous blood samples were drawn immediately before and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 48 and 72 h after the ingestion of desogestrel. Plasma was separated and stored at −70 °C until analysis. In the second and third phases the subjects were pretreated, in a randomized order, with either 200 mg of fluconazole (i.e. two capsules of Diflucan® 100 mg; Pfizer, Amboise, France) or 200 mg of itraconazole (i.e. two capsules of Sporanox® 100 mg; Janssen-Cilag, Borgo San Michele, Latina, Italy) once daily for 4 days. On day 4 of the second and third phase, a single 150 µg dose of desogestrel was administered 1 h after taking the last fluconazole/itraconazole dose. Venous blood samples were collected as in the control phase for 72 h after the ingestion of desogestrel. The last dose of fluconazole, itraconazole and the hormone preparation were administered under the supervision of the study personnel. On the days 1–3 of the second and third phase, fluconazole and itraconazole were self-administered between 08.00 and 10.00 h. Compliance was monitored by tablet counting and a patient diary. The subjects were under medical supervision for 12 h after administration of the study drugs in all phases, fasted overnight before administration of desogestrel and continued fasting until a standardized lunch was served 4 h after ingestion. Alcohol, grapefruit and caffeine-containing beverages were forbidden during the study. Between the phases there was a washout period of 4 weeks to ascertain that the drugs were administered at the same phase of the menstrual cycle.
Analysis of 3-keto-desogestrel
The plasma samples were thawed and 500 µl were mixed with 30 µl 6% methanol containing 65 ng ml−1 gestodene as an internal standard. The samples were loaded into Oasis HLB (10 mg) solid phase extraction 96-well plates (Waters Corporation, Milford, MA, USA), which were preconditioned with 800 µl methanol and equilibrated with 800 µl water. After sample loading, the plates were washed consecutively with 300 µl water and 60% aqueous methanol, and the analytes were eluted with 200 µl acetonitrile. The eluted samples were dried and reconstituted with 130 µl 60% aqueous methanol and an aliquot (20 µl) was injected into the LC/MS/MS system. The final concentration of gestodene in the samples was 15 ng ml−1. LC/MS/MS multiple reaction monitoring (MRM) analyses were carried out using positive electrospray ionization on a Quattro II (Micromass, Manchester, UK) triple quadrupole mass spectrometer equipped with Waters Alliance 2695 LC. Chromatography was performed using a Waters Xterra RP8 (2.1 × 50 mm, 3.5 µm particle size) column and Phenomenex LunaC8 (2.0 × 4.0 mm) precolumn (Torrance, CA, USA). Mobile phases were 10 m m NH4OH (A, pH 9.8) and methanol (B), and linear gradient from 60% B to 90% B in 3 min was used. The column was then washed for 1 min with 90% B and equilibrated for 7 min at 60% B. Total analysis time was 11 min. The flow rate was 0.25 ml min−1. Retention times were 1.9 and 2.6 min for gestodene and 3-keto-desogestrel, respectively. The fragmentation reactions monitored by MRM were from m/z 311 to m/z 109 for gestodene and from m/z 325 to m/z 109 for 3-keto-desogestrel. The lower limit of quantification (LLQ) estimated from injections of standards was 0.5 ng ml−1, which corresponded roughly to a signal-to-noise ratio of 10. Interday and intraday precision determined at each of the seven standard concentrations was ≤15%.
Genotyping for CYP2C9*2 and *3 and CYP2C19*2 was performed using a 5′-nuclease assay [31]. First an UNG (Uracyl-N-Glycosylate) digestion for 1 min was performed at 50 °C followed by an UNG-enzyme inactivation and Taq polymerase activation for 10 min at 95°C and then a 40 cycle for 15 s at 95°C and for 1 min at 60°C. Each reaction utilized 15 ng of genomic DNA as a template in a 25 µl reaction using the 2x Taqman master mix (Applied Biosystems, New Jersey, USA). For CYP2C9*2, primers caa tgg aaa gaa atg gaa gga gat, aag ata gta gtc cag taa ggt cag tga tat g were used with the VIC-TTG AAC ACA GTC CTC A-MGB, 6-FAM-TTG AAC ACG GTC CTC-MGB probes, and for CYP2C9*3 the primers cag gaa gag att gaa cgt gtg att, cta tga att tgg gga ctt cga aa and the VIC-AGA GAT ACA TTG ACC TTC-MGB, 6-FAM-AGA TAC CTT GAC CTT CT-MGB probes were used. The primers were synthesized by Invitrogen and the MGB probes by Applied Biosystems (Cheshire, UK). TaqMan® Pre-Developed Assay Reagent for Allelic Discrimination (PDAR) #4312570 from Applied Biosystems was used for the analysis of CYP2C19*2.
Data analysis
The pharmacokinetic parameters for 3-keto-desogestrel were calculated using standard noncompartmental methods (WinNonlin™ version 3.11998–99; Pharsight Co., Mountain view, California, USA). The maximum concentration in plasma (Cmax) and time to maximum concentration (tmax) for each subject were derived directly from the plasma concentration data. The area under the plasma concentration-time curve (AUC) was calculated from zero to infinity using the linear trapezoidal rule. The half-life (t1/2) of 3-keto-desogestrel was estimated by least-squares regression analysis of the terminal part of log concentration-time curve. The absolute changes in the pharmacokinetic parameters were tested using the anova model for repeated measurements (SPSS® 11.0 for windows 2001; SPSS Inc., Chicago, USA). A paired t-test was used for posthoc analysis. The Friedman test and Wilcoxon signed rank test for pairwise comparison were used to compare tmax. The Bonferroni adjustment for repeated significance testing was used. The chosen statistical significance level was P < 0.05. Results are presented as mean ± SD. For tmax median with range is shown. The percentage differences between treatments were calculated within subjects, and 95% CIs are given.
Results
All subjects completed the study. The data from one subject was excluded from analysis because of interference from an unknown compound in the chromatographic determination of 3-keto-desogestrel. This subject denied use of other drugs during the study. One subject had the *2/*3 genotype for CYP2C9 and was homozygous wild-type for CYP2C19. Four of the subjects were heterozygous for the CYP2C19*2 mutation. One subject who was heterozygous for CYP2C19*2 had a genotype of CYP2C9*1*3, whereas the other subject who was heterozygous for CYP2C19*2 was homozygous wild-type for CYP2C9.
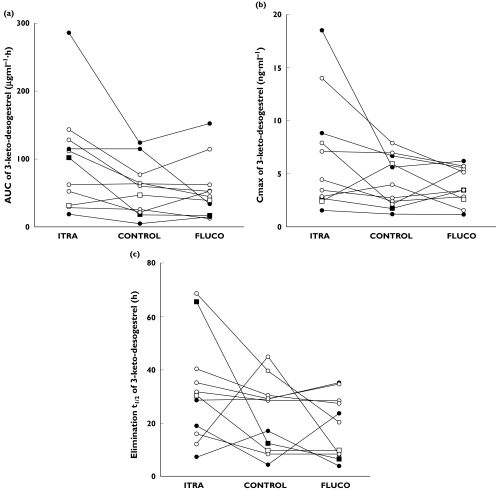
Compared with the control phase, there was a statistically significant increase of 72% in the AUC of 3-keto-desogestrel (95% CI 12%, 133%; P = 0.024) after the pretreatment with itraconazole (Table 1 and Figure 2a). However, no statistically significant difference was observed in the maximum concentration (Cmax) (55.5%, 95% CI −14%, 124%; P = 0.31) and in the elimination half-life (t1/2) (39%, 95% CI −42%, 120%; P = 0.58) (Table 1 and Figure 2b). During the itraconazole phase an increase in Cmax was observed in six out of 11 subjects, but no effect was seen in three and a decrease in Cmax was observed in two of the subjects. Prolongation of the t1/2 by itraconazole was apparent in seven out of 11 subjects, but no change was evident in two subjects and t1/2 was shortened in two subjects. In addition, the time to maximum concentration (tmax) was unaffected by itraconazole pretreatment compared with the control phase (P = 0.72). Pre-treatment with fluconazole produced no significant effects on the AUC (P = 0.08), Cmax (P = 1.0), t1/2 (P = 1.0), and tmax (P = 0.5) of 3-keto-desogestrel (Table 1).
Table 1. Pharmacokinetics parameters for 3-keto-desogestrel after administration of 150 µg desogestrel either alone (control) or after 4 days pretreatment with 200 mg fluconazole or 200 mg itraconazole (n = 11). The results are mean ± SD (median with range for tmax).
| Parameter | Control phase | Itraconazole phase | %-difference (95% CI) vs control | Fluconazole phase | %-difference (95% CI) vs control |
|---|---|---|---|---|---|
| AUC (µg ml−1 h) | 57.2 ± 38.5 | 98.6 ± 75.6 | 72% (12%, 133%) | 54.7 ± 43.1 | −4% (−42%, 34%) |
| Cmax (ng ml−1) | 4.3 ± 2.4 | 6.7 ± 5.4 | 55% (−14%, 124%) | 3.9 ± 1.8 | −8% (−23%, 40%) |
| t1/2 (h) | 23.2 ± 13.3 | 32.3 ± 19.6 | 39% (−42%, 120%) | 18.9 ± 11.7 | −18% (−24%, 61%) |
| tmax (h) | 2.5 (1.0–6.0) | 2.5 (1.5–4.0) | –(P = 0.72) | 2 (1.0–3.0) | –(0.47) |
Figure 2.
3-ketodesogestrel AUC (a), Cmax(b), and t1/2 (c) values after the administration of 150 µg desogestrel either alone (control) or after pretreatment with 200 mg fluconazole or 200 mg itraconazole daily for 4 days to 11 healthy females. Symbols for the CYP2C9/CYP2C19 genotype combinations: CYP2C9*1*1/CYP2C19*1*1 (○); CYP2C9*1*1/CYP2C19*1*2 (•); CYP2C9*2*3/CYP2C19*1*1 (□); CYP2C9*1*3/CYP2C10*1*2 (▪)
The AUC of 3-keto-desogestrel in the subject with the CYP2C9*2*3 genotype in the control phase was 47.8 µg ml−1 h, which was comparable with the mean AUC of 3-keto-desogestrel in that phase (Table 1). The mean AUC of 3-keto-desogestrel in the subjects heterozygous for the CYP2C19*2 mutation and in the subjects with the *1/*1 genotype for CYP2C19 were 53.1 and 52.0 µg ml−1 h, respectively. The pharmacokinetic parameters of 3-keto-desogestrel in the different CYP2C9/CYP2C19 genotypes are illustrated in Figure 2.
Discussion
In the present in vivo study, pretreatment with the CYP2C9 and CYP2C19 inhibitor fluconazole did not significantly affect the formation of 3-keto-desogestrel, the active metabolite of desogestrel. This observation does not support the results of previously published in vitro data [5], but suggests that the bioactivation of desogestrel is not dependent on CYP2C9 or CYP2C19. This conclusion is further supported by the observation that bioactivation of desogestrel in the subject with the CYP2C9*2*3 genotype did not differ from that of the rest of the subjects. In addition, no difference was observed in the pharmacokinetics of desogestrel in the subjects heterozygous for the CYP2C19*2 mutation.
In contrast to fluconazole, pretreatment with the CYP3A4 inhibitor itraconazole caused a significant 72% increase in the AUC of 3-keto-desogestrel. This increase was observed in all but two subjects. Accordingly, our study supports the conclusion by Gentile et al. that the further degradation of 3-keto-desogestrel is catalyzed by the CYP3A4 enzyme [5]. Furthermore, the observation that the conversion of desogestrel to 3-keto-desogestrel was not diminished after the pretreatment with itraconazole suggests that desogestrel bioactivation is not catalyzed by the CYP3A4 enzyme. Despite the increase in AUC by itraconazole, the remaining pharmacokinetic parameters were not statistically significantly changed by either of these CYP inhibitors. However, the prolongation of elimination half-life (t1/2) after itraconazole pretreatment was evident in seven subjects, but the study did not have the power to make this difference statistically significant.
Desogestrel is a prodrug, and the formation of 3-keto-desogestrel is crucial for the biological effects of the compound [2]. In the present study we demonstrated that drugs inhibiting CYP2C9 and CYP2C19 are not likely to affect the contraceptive efficacy of desogestrel. Desogestrel and fluconazole are likely to be used concomitantly, and based on the results of the present study, it seems unnecessary to avoid this combination. However, the adverse effects of desogestrel may be enhanced if it is co-administered with CYP3A4 inhibitors, since the plasma concentrations of the biologically active metabolite 3-keto-desogestrel may be increased. In contrast, drugs that induce CYP3A4 may impair the therapeutic effects of 3-keto-desogestrel by decreasing the plasma concentrations of 3-keto-desogestrel as described previously [32] and enhancing the risk for unwanted pregnancies.
Madden et al. [4] indicated that primaquine, a potent CYP2D6 inhibitor, strongly inhibits the conversion of desogestrel to 3-keto-desogestrel in vitro. However, a more recent in vitro study found no correlation between the activities of CYP2D6 and CYP1A2 and the bioactivation of desogestrel [5, 33].
In conclusion and contradictory to previous in vitro data, the bioactivation of desogestrel to 3-keto-desogestrel appears to be independent of CYP2C and CYP3A4 activities. Further in vivo studies are needed to investigate the role of the other CYP isoforms in desogestrel metabolism.
Acknowledgments
Mrs Elina Kahra is thanked for her skilful assistance in organizing the study.
None of the authors holds stocks or has a share in any drug company, and none has received any financial support or is working for drug company.
This work was supported by Turku University Hospital research fund EVO13390 (Laine).
References
- 1.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Viinikka L, Ylikorkala O, Nummi S, Virkkunen P, Ranta T, Alapiessa U, Yihko R. Biological effects of a new and potent progestagen. A clinical study. Acta Endocrinol (Copenh) 1976;83:429–38. doi: 10.1530/acta.0.0830429. [DOI] [PubMed] [Google Scholar]
- 3.Viinikka L. Metabolism of a new synthetic progestagen, Org 2969, by human liver in vitro. J Steroid Biochem. 1979;10:353–7. doi: 10.1016/0022-4731(79)90319-4. [DOI] [PubMed] [Google Scholar]
- 4.Madden S, Back DJ, Orme ML. Metabolism of the contraceptive steroid desogestrel by human liver in vitro. J Steroid Biochem. 1990;35:281–8. doi: 10.1016/0022-4731(90)90285-z. [DOI] [PubMed] [Google Scholar]
- 5.Gentile DM, Verhoeven CH, Shimada T, Back DJ. The role of CYP2C in the in vitro bioactivation of the contraceptive steroid desogestrel. J Pharmacol Exp Ther. 1998;287:975–82. [PubMed] [Google Scholar]
- 6.Madden S, Back DJ, Martin CA, Orme ML. Metabolism of the contraceptive steroid desogestrel by the intestinal mucosa. Br J Clin Pharmacol. 1989;27:295–9. doi: 10.1111/j.1365-2125.1989.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viinikka L, Ylikorkala O, Vihko R, Wijnand HP, Booij M, van der Veen F. Metabolism of a new synthetic progestagen, Org 2969, in female volunteers. Pharmacokinetics after an oral dose. Eur J Clin Pharmacol. 1979;15:349–55. doi: 10.1007/BF00558439. [DOI] [PubMed] [Google Scholar]
- 8.Hasenack HG, Bosch AM, Kaar K. Serum levels of 3-keto-desogestrel after oral administration of desogestrel and 3-keto-desogestrel. Contraception. 1986;33:591–6. doi: 10.1016/0010-7824(86)90047-8. [DOI] [PubMed] [Google Scholar]
- 9.Back DJ, Grimmer SF, Shenoy N, Orme ML. Plasma concentrations of 3-keto-desogestrel after oral administration of desogestrel and intravenous administration of 3-keto-desogestrel. Contraception. 1987;35:619–26. doi: 10.1016/s0010-7824(87)80021-5. [DOI] [PubMed] [Google Scholar]
- 10.Bergink W, Assendorp R, Kloosterboer L, van Lier W, Voortman G, Qvist I. Serum pharmacokinetics of orally administered desogestrel and binding of contraceptive progestogens to sex hormone-binding globulin. Am J Obstet Gynecol. 1990;163:2132–7. doi: 10.1016/0002-9378(90)90553-j. [DOI] [PubMed] [Google Scholar]
- 11.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–22. [PubMed] [Google Scholar]
- 12.de Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–8. [PubMed] [Google Scholar]
- 13.Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–9. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Crespi CL, Miller VP. The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH. cytochrome P450 oxidoreductase. Pharmacogenetics. 1997;7:203–10. doi: 10.1097/00008571-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Goto F, Ray WA, McAllister CB, Jacqz E, Wilkinson GR, Branch RA. Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol Ther. 1985;38:402–8. doi: 10.1038/clpt.1985.194. [DOI] [PubMed] [Google Scholar]
- 16.Alvan G, Bechtel P, Iselius L, Gundert-Remy U. Hydroxylation polymorphisms of debrisoquine and mephenytoin in European populations. Eur J Clin Pharmacol. 1990;39:533–7. doi: 10.1007/BF00316090. [DOI] [PubMed] [Google Scholar]
- 17.Bertilsson L, Lou YQ, Du YL, Liu Y, Kuang TY, Liao XM, Wang KY, Reviriego J, Iselius L, Sjogvist F. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoin. Clin Pharmacol Ther. 1992;51:388–97. doi: 10.1038/clpt.1992.38. [DOI] [PubMed] [Google Scholar]
- 18.Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjoqvist F. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun. 1999;254:628–31. doi: 10.1006/bbrc.1998.9992. [DOI] [PubMed] [Google Scholar]
- 19.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–9. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 20.Yasar U, Tybring G, Hidestrand M, Oscarson M, Ingelman-Sundberg M, Dahl ML, Eliasson E. Role of CYP2C9 polymorphism in losartan oxidation. Drug Metab Dispos. 2001;29:1051–6. [PubMed] [Google Scholar]
- 21.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–55. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back DJ, Tjia JF, Karbwang J, Colbert J. In vitro inhibition studies of tolbutamide hydroxylase activity of human liver microsomes by azoles, sulphonamides and quinolines. Br J Clin Pharmacol. 1988;26:23–9. doi: 10.1111/j.1365-2125.1988.tb03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunze KL, Wienkers LC, Thummel KE, Trager WF, Warfarin-fluconazole I. Inhibition of the human cytochrome P450-dependent metabolism of warfarin by fluconazole: in vitro studies. Drug Metab Dispos. 1996;24:414–21. [PubMed] [Google Scholar]
- 24.Blum RA, Wilton JH, Hilligoss DM, Gardner MJ, Henry EB, Harrison NJ, Schentag JJ. Effect of fluconazole on the disposition of phenytoin. Clin Pharmacol Ther. 1991;49:420–5. doi: 10.1038/clpt.1991.49. [DOI] [PubMed] [Google Scholar]
- 25.Black DJ, Kunze KL, Wienkers LC, Gidal BE, Seaton TL, McDonnell ND, Evans JS, Bauwens JE, Trager WF. Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos. 1996;24:422–8. [PubMed] [Google Scholar]
- 26.Kaukonen KM, Olkkola KT, Neuvonen PJ. Fluconazole but not itraconazole decreases the metabolism of losartan to E-3174. Eur J Clin Pharmacol. 1998;53:445–9. doi: 10.1007/s002280050405. [DOI] [PubMed] [Google Scholar]
- 27.Wienkers LC, Wurden CJ, Storch E, Kunze KL, Rettie AE, Trager WF. Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab Dispos. 1996;24:610–4. [PubMed] [Google Scholar]
- 28.von Moltke LL, Greenblatt DJ, Schmider J, Duan SX, Wright CE, Harmatz JS, Shader RI. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 29.Olkkola KT, Ahonen J, Neuvonen PJ. The effects of the systemic antimycotics, itraconazole and fluconazole, on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Anesth Analg. 1996;82:511–6. doi: 10.1097/00000539-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–80. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 32.Pfrunder A, Schiesser M, Gerber S, Haschke M, Bitzer J, Drewe J. Interaction of St John's wort with low-dose oral contraceptive therapy: a randomized controlled trial. Br J Clin Pharmacol. 2003;56:683–90. doi: 10.1046/j.1365-2125.2003.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masimirembwa CM, Hasler JA, Johansson I. Inhibitory effects of antiparasitic drugs on cytochrome P450 2D6. Eur J Clin Pharmacol. 1995;48:35–8. doi: 10.1007/BF00202169. [DOI] [PubMed] [Google Scholar]