Abstract
Background and aims
Gemfibrozil, and particularly its combination with itraconazole, greatly increases the area under the plasma concentration-time curve [AUC(0, ∞)] and response to the cytochrome P450 (CYP) 2C8 and 3A4 substrate repaglinide. In vitro, gemfibrozil is a more potent inhibitor of CYP2C9 than of CYP2C8. Our aim was to investigate the effects of the gemfibrozil-itraconazole combination on the pharmacokinetics and pharmacodynamics of another meglitinide analogue, nateglinide, which is metabolized by CYP2C9 and CYP3A4.
Methods
In a randomized crossover study with two phases, nine healthy subjects took 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily or placebo for 3 days. On day 3, they ingested a single 30-mg dose of nateglinide. Plasma nateglinide and blood glucose concentrations were measured for up to 12 h.
Results
During the gemfibrozil-itraconazole phase, the AUC(0, ∞) and Cmax of nateglinide were 47% (range 23–74%; P < 0.0001) and 30% (range −8% to 104%; P = 0.0146) higher than during the placebo phase, respectively, but the tmax and t1/2 of nateglinide remained unchanged. The combination of gemfibrozil and itraconazole had no effect on the formation of the M7 metabolite of nateglinide but impaired its elimination. The blood glucose response to nateglinide was not significantly changed by coadministration of gemfibrozil and itraconazole.
Conclusions
The combination of gemfibrozil and itraconazole has only a limited influence on the pharmacokinetics of nateglinide. This is in marked contrast to the substantial effect of this combination on the pharmacokinetics of repaglinide. The findings suggest that in vivo gemfibrozil, probably due to its metabolites, is a much more potent inhibitor of CYP2C8 than of CYP2C9.
Keywords: CYP2C9, CYP3A4, drug interaction, gemfibrozil, itraconazole, nateglinide
Introduction
Concomitant treatment with gemfibrozil, a lipid-lowering drug, and the antimycotic itraconazole was recently found to decrease substantially the systemic elimination of repaglinide, a meglitinide analogue antidiabetic drug. This resulted in a nearly 20-fold increase in the area under the plasma repaglinide concentration-time curve [AUC(0, ∞)] and a marked increase in its blood glucose-lowering effect [1]. Repaglinide is eliminated by hepatic metabolism via the cytochromes P450 (CYP) 2C8 and 3A4 [2, 3]. Gemfibrozil inhibits CYP2C8 activity [4, 5], but not that of CYP3A4 [6]. Itraconazole is a potent inhibitor of CYP3A4, with an inhibition constant, Ki, of about 1 µm[7, 8], but does not inhibit CYP2C8 (IC50 >100 µm) [9]. Thus, inhibition of CYP2C8 by gemfibrozil and CYP3A4 by itraconazole probably explains the considerable effect of their coadministration on the pharmacokinetics of repaglinide. In vitro, gemfibrozil is a more potent inhibitor of CYP2C9 (Ki = 5.8 µm) [10] than of CYP2C8 (Ki = 75 µm) [4].
Nateglinide is another antidiabetic drug in the meglitinide class [11]. It differs from repaglinide in being metabolized by CYP2C9 (70%) and CYP3A4 (30%) [11, 12]. The oral bioavailability of nateglinide is about 70% [11] and that of repaglinide about 60% [13]. Both nateglinide and repaglinide are highly bound to plasma proteins (98–99%), primarily to albumin [11, 13]. Nateglinide is oxidatively biotransformed to several metabolites [12], with a quantitatively minor dehydro derivative (M7) being as pharmacologically active as the parent compound (Figure 1) [14]. Fluconazole, an inhibitor of CYP2C9 (Ki = 10 µm) [15, 16], CYP2C19 (Ki = 2 µm) [17] and CYP3A4 (Ki = 2 µm) [18–20], was shown to increase the AUC(0, ∞) of nateglinide by 48% [21]. In order to study the effect of gemfibrozil on CYP2C9 activity in vivo and because the combination of gemfibrozil and itraconazole causes a large increase in the plasma concentrations of repaglinide, we have studied the effect of coadministration of gemfibrozil and itraconazole on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects.
Figure 1.
Chemical structures of nateglinide, its M7 metabolite and repaglinide
Methods
Subjects
Nine healthy subjects (five men, four women; age range 19–25 years; weight range 50–89 kg) participated in the study after giving written informed consent. They were ascertained to be healthy by medical history, physical examination and routine laboratory tests prior to the study. None was a tobacco smoker or used any continuous medication. Ten subjects were originally recruited, but one dropped out because of a respiratory tract infection during the first study phase.
Study design
The study protocol was approved by the Ethics Committee for Studies in Healthy Subjects and Primary Care of the Helsinki and Uusimaa Hospital District and by the National Agency for Medicines. A randomized crossover study with two phases and a washout period of 4 weeks was carried out. The subjects took 100 mg itraconazole (first dose 200 mg; Sporanox, Janssen-Cilag, Borgo San Michele, Italy) and 600 mg gemfibrozil (Lopid, Parke-Davis, Freiburg, Germany), or placebo orally twice daily at 08.00 h and 20.00 h for 3 days. On day 3, after an overnight fast at 09.00 h and 1 h after the last doses of itraconazole and gemfibrozil, or placebo they ingested a single dose of 30 mg nateglinide (one half of a Starlix 60-mg tablet, Novartis Europharm Limited, Horsham, UK) with 150 ml water. The mean weight of the original tablets was 325 mg [n = 20, coefficient of variation (CV) 1.0%] and that of the halved tablets was 161 mg (n = 20, CV 1.2%). The subjects remained seated for the next 3 h. A standardized light breakfast was served at precisely 15 min after the administration of nateglinide, a standardized snack at 1 and 2 h after nateglinide, a standardized warm meal after 3 h and a standardized snack after 7 and 10 h. Food intake was identical on both days of nateglinide administration, during which the subjects were under direct medical supervision. Blood glucose concentrations were monitored on both these days, and intravenous glucose and intramuscular glucagon were available in case of severe hypoglycaemia. However, neither was needed.
Blood sampling and determination of blood glucose concentrations
On the days of administration of nateglinide, a forearm vein was cannulated with a plastic cannula and kept patent with a stylet. Blood samples (10 ml) were drawn into tubes that contained ethylenediaminetetraacetic acid, before the administration of nateglinide and 15, 30, 45, 60, 75 and 90 min and 2, 2.5, 3, 4, 5, 7, 9 and 12 h later. Blood glucose concentrations were measured immediately after each sample was taken by the glucose oxidase method using the Precision G Blood Glucose Testing System (Medisense, Bedford, MA, USA). Plasma was separated within 30 min after blood sampling and stored at −70 °C until analysis. The between-day CV for blood glucose analysis was 6.4% at 3.0 mmol l−1, 3.1% at 5.5 mmol l−1 and 3.5% at 17.3 mmol l−1 (n = 4).
Determination of plasma drug and metabolite concentrations
Plasma nateglinide and its M7 metabolite (a dehydro derivative) concentrations were measured by liquid chromatography-tandem mass spectrometry using a PE SCIEX API 3000 LC/MS/MS System (Sciex Division of MDS Inc., Toronto, Ontario, Canada) in the atmospheric pressure chemical ionization mode [12]. Repaglinide served as the internal standard. The ion transitions monitored were m/z 318–166 for nateglinide, m/z 316–166 for M7, and m/z 453–230 for repaglinide. These transitions represent the product ions of the [M+H]+ ions. The quantification limit was 2 ng ml−1 and the between-day CVs were 12% at 2 ng ml−1 (n = 5), 8.9% at 20 ng ml−1 (n = 5), 12% at 200 ng ml−1 (n = 6) and 12% at 1600 ng ml−1 (n = 6). M7 concentrations are given in arbitrary units (U ml−1) relative to the ratio of the area of the peak of M7 to that of the internal standard in the chromatogram of the [M+H]+ ions. Plasma gemfibrozil, itraconazole and hydroxyitraconazole concentrations were determined by high-performance liquid chromatography [22–24]. The limit of quantification was 100 ng ml−1 for gemfibrozil and 10 ng ml−1 for itraconazole and hydroxyitraconazole. The CVs for the determination of gemfibrozil were 2.7% at 400 ng ml−1, 3.0% at 2300 ng ml−1, 1.6% at 15 100 ng ml−1 and 4.3% at 30 300 ng ml−1 (n = 7). Those for itraconazole were 10.0% at 20 ng ml−1, 2.5% at 190 ng ml−1 and 3.3% at 1010 ng ml−1, and those for hydroxyitraconazole were 8.5% at 20 ng ml−1, 8.6% at 200 ng ml−1 and 3.8% at 1020 ng ml−1 (n = 9–14).
Pharmacokinetic analysis
The pharmacokinetics of nateglinide and M7 were characterized by peak concentration in plasma (Cmax), time to Cmax (tmax), areas under the concentration-time curve [AUC(0, 12 h) and AUC(0, ∞)], elimination half-life (t1/2) and apparent formation rate constant (kf) of M7, and those of gemfibrozil, itraconazole and hydroxyitraconazole by Cmax and AUC(0, 13 h). The terminal log-linear part of the concentration-time curve for nateglinide and M7 was identified visually. The elimination rate constant (ke) was determined by linear regression analysis of the log-linear part of the plasma drug concentration-time curve. The t1/2 was calculated from the equation t1/2 = ln2/ke. The AUC values were determined by a combination of the linear and log-linear trapezoidal rules with extrapolation to infinity, when appropriate, by dividing the last measured concentration by ke. The kf of M7 was calculated, in an analogous manner to that for the absorption rate constant (ka), by linear regression analysis of the log-linear part of M7 residual curve [25]. To compare the mechanism of the interaction between the gemfibrozil-itraconazole combination and nateglinide, with that between fluconazole and nateglinide, the kf of M7 was also determined for our previous interaction study between fluconazole and nateglinide. Both studies used the same nateglinide dosage and blood sampling protocol up to 7 h [21].
Pharmacodynamic analysis
The blood glucose response was characterized by the mean change in blood glucose concentration calculated by dividing the net (above baseline) AUC for blood glucose by the corresponding time interval. In addition, the maximum increase and the maximum decrease from the baseline blood glucose concentration were calculated.
Statistical analysis
Results are expressed as mean values ± SD in the text and tables and, for clarity, as mean values ± SEM in the figures. The pharmacokinetic and pharmacodynamic variables for nateglinide after the two pretreatments were compared with a paired t-test or, in the case of tmax, by the Wilcoxon signed rank test. The Pearson correlation coefficient was used to investigate relationships between the pharmacokinetic variables for gemfibrozil, itraconazole and hydroxyitraconazole and the degree of interaction with nateglinide, and between the pharmacokinetic and pharmacodynamic variables for nateglinide. All the data were analysed using the statistical program Systat for Windows, version 6.0.1 (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when P-value was <0.05.
Results
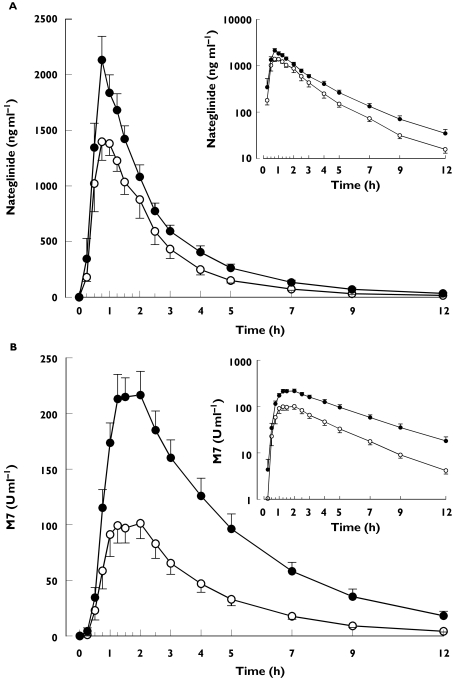
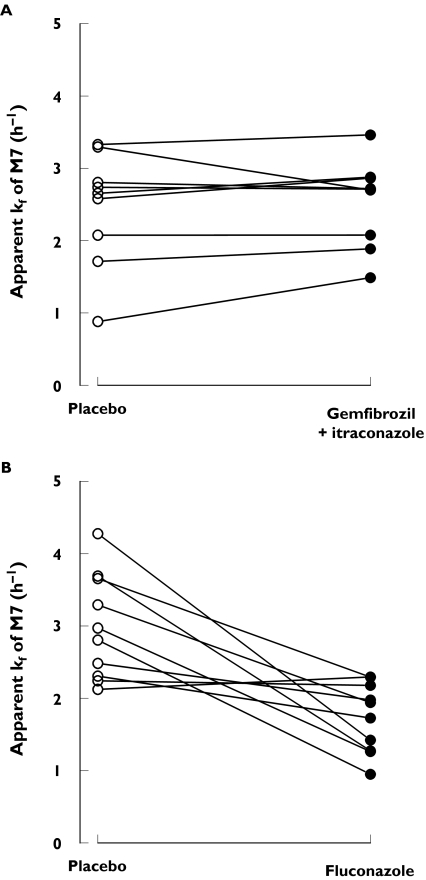
Coadministration of gemfibrozil and itraconazole raised the plasma concentrations of nateglinide only moderately (Table 1 and Figure 2). During the gemfibrozil-itraconazole phase, the AUC(0, ∞) and Cmax of nateglinide were 47% (range 23–74%]P < 0.0001) and 30% (range −8% to 104%; P = 0.0146) higher than during the placebo phase, respectively, but tmax and t1/2 remained unchanged. The gemfibrozil-itraconazole combination raised the AUC(0, ∞) and Cmax of the M7 metabolite of nateglinide by 166% (range 115–254%) and 92% (range 53–200%), respectively (P < 0.0001). The t1/2 of M7 was prolonged from 2.3 to 2.7 h (P = 0.0259) and the M7 to nateglinide AUC(0, ∞) ratio was increased by 83% (range 60–124%; P < 0.0001). The apparent formation rate constant (kf) of M7 remained unchanged following coadministration of gemfibrozil and itraconazole. In contrast, earlier work [21] showed that fluconazole reduces the kf of M7 by 42% (from 3.0 to 1.7 h−1, P = 0.0033) (Figure 3).
Table 1.
Pharmacokinetic data for nateglinide (given as a single 30-mg dose) and its M7 metabolite in nine healthy subjects after 3 days' treatment with placebo or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily
| Variable | Placebo phase (control) | Gemfibrozil + itraconazole phase | Gemfibrozil + itraconazole phase percentage of control (range) | Mean difference between phases (95% CI) | P |
|---|---|---|---|---|---|
| Nateglinide | |||||
| Cmax (ng ml−1) | 1697 ± 506 | 2196 ± 545 | 130 (92–204) | 498 (128, 869) | 0.0146 |
| tmax (min) | 60 (30–120) | 45 (45–75) | – | – | 0.3805 |
| t1/2 (h) | 1.8 ± 0.4 | 2.1 ± 0.4 | 113 (67–150) | 0.2 (−0.1, 0.6) | 0.1625 |
| AUC(0, 12 h) (ng h ml−1) | 3425 ± 1050 | 4984 ± 1385 | 146 (122–171) | 1559 (1101, 2016) | <0.0001 |
| AUC(0, ∞) (ng h ml−1) | 3469 ± 1049 | 5092 ± 1451 | 147 (123–174) | 1624 (1111, 2136) | <0.0001 |
| M7 | |||||
| kf (h−1) | 2.5 ± 0.8 | 2.4 ± 0.6 | 103 (82–169) | 0.1 (−0.2, 0.3) | 0.4838 |
| Cmax (U ml−1) | 120 ± 17 | 231 ± 22 | 192 (153–300) | 111 (89, 132) | <0.0001 |
| tmax (min) | 90 (60–120) | 120 (60–120) | – | – | 0.2568 |
| t1/2 (h) | 2.3 ± 0.3 | 2.7 ± 0.4 | 121 (91–162) | 0.5 (0.1, 0.9) | 0.0259 |
| AUC(0, 12 h) (U h ml−1) | 401 ± 166 | 1029 ± 317 | 256 (216–332) | 627 (494, 761) | <0.0001 |
| AUC(0, ∞) (U h ml−1) | 415 ± 171 | 1106 ± 372 | 266 (215–254) | 691 (516, 866) | <0.0001 |
| AUC(0, ∞) ratio (U ng−1) | 0.12 ± 0.04 | 0.22 ± 0.06 | 183 (160–224) | 0.09 (0.07, 0.11) | <0.0001 |
| (M7/nateglinide) | |||||
Values shown as mean ± SD unless otherwise indicated. tmax data as median (range). AUC(0, 12 h), area under the plasma concentration-time curve from time 0–12 h; AUC(0, ∞), area under the plasma concentration-time curve from time 0 to infinity; Cmax, peak plasma concentration; kf, apparent formation rate constant; t1/2, elimination half-life; tmax, time to peak plasma concentration.
Figure 2.
Mean ± SE mean plasma concentrations of nateglinide (A) and its M7 metabolite (B) in nine healthy subjects. A single oral dose of 30 mg nateglinide was given after 3 days' treatment with placebo or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily. Insets depict the same data on a semilogarithmic scale. ○, during placebo; •, during gemfibrozil-itraconazole
Figure 3.
Individual apparent formation rate constants (kf) for the M7 metabolite of nateglinide. A single oral dose of 30 mg nateglinide was given to nine healthy subjects after 3 days' treatment with placebo or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily (A) or to 10 healthy subjects after 4 days’ treatment with placebo or 200 mg fluconazole (first dose 400 mg) once daily (B] data from [18])
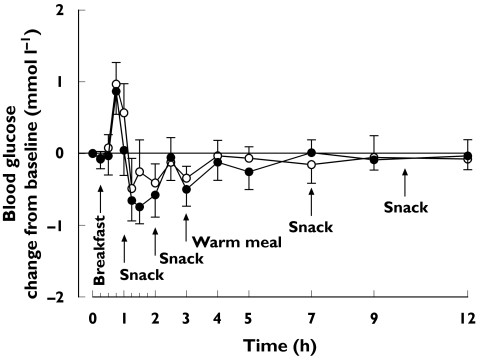
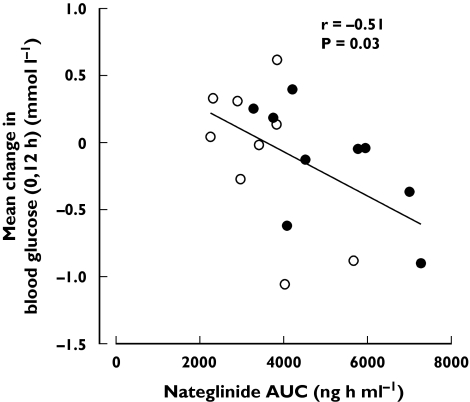
No significant differences were seen in the blood glucose response to nateglinide between the phases (Table 2 and Figure 4). None of the subjects experienced symptomatic hypoglycaemia. The mean change in blood glucose (0, 12 h) correlated inversely with the Cmax, AUC(0, 12 h) and AUC(0, ∞) of nateglinide (r = −0.55, P = 0.02; r = −0.52, P = 0.03; r = −0.51, P = 0.03, respectively) (Figure 5).
Table 2.
Blood glucose response to nateglinide (given as a single 30-mg oral dose) in nine healthy subjects after 3 days’ treatment with placebo or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily
| Variable | Placebo phase | Gemfibrozil + itraconazole phase | Mean difference between phases (95% CI) | P |
|---|---|---|---|---|
| Mean change 0,3 h (mmol l−1) | −0.1 ± 0.7 | −0.2 ± 0.7 | −0.2 (− 0.6, 0.3) | 0.4186 |
| Mean change 0,7 h (mmol l−1) | −0.1 ± 0.5 | −0.2 ± 0.5 | −0.1 (− 0.5, 0.3) | 0.5146 |
| Mean change 0,12 h (mmol l−1) | −0.1 ± 0.6 | −0.1 ± 0.4 | −0.1 (− 0.4, 0.3) | 0.7198 |
| Maximum increase (mmol l−1) | 1.3 ± 0.8 | 1.1 ± 0.7 | −0.2 (− 0.8, 0.5) | 0.5539 |
| Maximum decrease (mmol l−1) | 1.1 ± 0.7 | 1.2 ± 0.7 | 0.1 (− 0.5, 0.7) | 0.7195 |
Values shown as mean ± SD unless otherwise indicated.
Figure 4.
Mean ± SEM mean change in blood glucose concentrations in nine healthy subjects after a single oral dose of 30 mg nateglinide after 3 days’ treatment with placebo or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg) twice daily. ○, Blood glucose during placebo; •, blood glucose during gemfibrozil-itraconazole
Figure 5.
Relationship between the AUC(0, ∞) of a single 30-mg oral dose of nateglinide and mean change in blood glucose (0, 12 h) after 3 days' treatment with placebo (○) or 600 mg gemfibrozil and 100 mg itraconazole (first dose 200 mg; •) twice daily
The mean Cmax and AUC(0, 13 h) of gemfibrozil were 24.9 ± 8.8 µg ml−1 (range 14.6–24.9 µg ml−1) and 96.8 ± 27.0 µg h ml−1 (range 67.0–141.3 µg h ml−1). The mean Cmax and AUC(0, 13 h) of itraconazole and hydroxyitraconazole were 172 ± 70 ng ml−1 (range 53-267 ng ml−1) and 349 ± 138 ng ml−1 (range 129–558 ng ml−1), and 1525 ± 615 ng h ml−1 (range 445–2205 ng h ml−1) and 3744 ± 1423 ng h ml−1 (range 1248–5859 ng h ml−1), respectively. No correlations were evident between the pharmacokinetic variables for gemfibrozil, itraconazole, or hydroxyitraconazole and the degree of interaction with nateglinide.
Discussion
This work shows that concomitant treatment with gemfibrozil and itraconazole raises the plasma concentrations of nateglinide only moderately. The blood glucose response to nateglinide was not significantly changed by the gemfibrozil-itraconazole combination. This small interaction with nateglinide is in marked contrast to the substantial interaction of gemfibrozil and itraconazole with the other meglitinide analogue repaglinide.
The oral bioavailability of nateglinide is about 70% [11]. It is eliminated by oxidative biotransformation via CYP2C9 (70% of metabolism) and CYP3A4 (30% of metabolism) and by urinary excretion of unchanged nateglinide (15% of an oral dose) [11, 12]. In a previous investigation, fluconazole, an inhibitor of CYP2C9 [15, 16], CYP2C19 [17] and CYP3A4 [18, 19], raised the plasma concentrations of nateglinide and decreased the formation of its M7 metabolite, indicating that either CYP2C9 or CYP3A4 or both contribute to the biotransformation of nateglinide to M7 in vivo [21], although no direct in vitro evidence is available to support this view. In a genetic association study, the CYP2C9*3 allele was associated with a decreased clearance and increased plasma concentrations of nateglinide [26], supporting a significant role for CYP2C9 in its biotransformation in vivo.
Itraconazole is a potent inhibitor of CYP3A4 [7, 8], but does not markedly inhibit CYP2C9 or CYP2C8 activities [9, 27]. Gemfibrozil, on the other hand, inhibits both CYP2C8 and CYP2C9 in vitro [4, 10], but does not inhibit CYP3A4 [6]. Although gemfibrozil is a more potent inhibitor of CYP2C9 than of CYP2C8 in vitro [4, 10], in vivo it has the greatest effect on the pharmacokinetics of drugs metabolized primarily by CYP2C8. Thus, a 3-day treatment with 600 mg gemfibrozil twice daily raised the AUC(0, ∞) of repaglinide by about eight-fold [1], that of cerivastatin by about six-fold [5] and that of rosiglitazone by about 2.3-fold [28]. The same dose of gemfibrozil raised the AUC(0, ∞) of glimepiride, a sulphonylurea antidiabetic drug metabolized primarily by CYP2C9 [29], by only 20% [30]. In addition, gemfibrozil slightly, but significantly, decreased the plasma concentrations of the CYP2C9 probe substrate S-warfarin [31]. A major metabolite of gemfibrozil, its O-glucuronide, is a more potent inhibitor of CYP2C8-catalysed cerivastatin biotransformation than the parent drug (IC50 4 µmvs. 28 µm) [32]. Studies have indicated that gemfibrozil-O-glucuronide accumulates in rat liver [33–35]. If this metabolite behaves similarly in humans, potent inhibition of CYP2C8 activity in vivo would be expected.
The finding that fluconazole but not the gemfibrozil-itraconazole combination decreases the formation of M7 indicates a fundamental difference in the mechanism of these interactions, despite almost identical effects on the AUC(0, ∞) of parent nateglinide. This suggests that fluconazole inhibits the metabolism of nateglinide via both CYP2C9 and CYP3A4, and that the combination of gemfibrozil and itraconazole inhibits CYP3A4 only. The finding that gemfibrozil and itraconazole had no significant effect on the t1/2 of nateglinide, whereas they increased its AUC and Cmax, suggests that the interaction occurred mainly during the first-pass, probably through inhibition of presystemic metabolism of nateglinide by CYP3A4 in the gut wall and liver.
The active site of CYP2C8 is much larger (1438 Å3) [36] than that of CYP2C9 (470 Å3) [37], which is compatible with the preference of CYP2C8 for relatively large substrates such as repaglinide [3], cerivastatin [4], amiodarone [38], amodiaquine [39], paclitaxel [40], simvastatin acid [41] and verapamil [42]. It is evident that the large active site of CYP2C8 allows interaction even with glucuronic acid conjugates, such as gemfibrozil-O-glucuronide and diclofenac-glucuronide [43]. Formation and hepatic accumulation of gemfibrozil-O-glucuronide, and its interaction with the large active site of CYP2C8, could explain the much greater effect of gemfibrozil on CYP2C8 than on CYP2C9 activity in vivo.
In addition to inhibiting CYP enzymes, gemfibrozil and its glucuronide conjugate inhibit the organic anion transporting polypeptide 1B1 (OATP1B1, also known as OATP-C, OATP2 and LST-1) with IC50 values of 72 µm and 24 µm for cerivastatin uptake, respectively [32]. Furthermore, itraconazole inhibits P-glycoprotein with an IC50 value of 2 µm[44, 45]. OATP1B1 is a liver-specific transporter that mediates uptake of its substrates from blood into hepatocytes [46] and P-glycoprotein mediates efflux of its respective substrates into the gut lumen, biliary tract and the tubule lumen in the kidney [47]. It is not yet known whether nateglinide is a substrate of OATP1B1 or P-glycoprotein or other drug transporter, although it is not a substrate of PEPT1, PEPT2, or MCT1 [48, 49]. Thus, the possibility exists that gemfibrozil or itraconazole influence the pharmacokinetics of nateglinide through an effect on a drug transporter. Like nateglinide, gemfibrozil and itraconazole are highly bound to plasma proteins [50, 51] and it cannot be ruled out that either gemfibrozil, itraconazole or both displace nateglinide from plasma proteins. Such an effect could attenuate a decrease in the clearance of nateglinide.
The findings that coadministration of gemfibrozil and itraconazole increased the Cmax, AUC and t1/2 of M7 without any effect on its formation suggest that these impair the further metabolism of M7 [12]. Although this metabolite is as potent a blood glucose-lowering agent as the parent nateglinide (all other metabolites are much less active) [14], increased M7 concentrations during coadministration of gemfibrozil and itraconazole are probably of minor clinical significance, because they are much lower than those of nateglinide. Under normal circumstances, total exposure to M7 is only about 5% of that of nateglinide [12].
Cases of serious hypoglycaemia have been associated with concomitant use of gemfibrozil and repaglinide, and this combination is contraindicated in many countries [52, 53]. Gemfibrozil alone raises the AUC(0, ∞) of repaglinide about eight-fold, itraconazole alone by about 1.4-fold and their combination causes a nearly 20-fold increase [1]. Although the interaction of nateglinide with gemfibrozil-itraconazole was substantially smaller than that of repaglinide, the dosage of nateglinide may required lowering if it is used with this combination.
It has been shown that the magnitude of other pharmacokinetic interactions involving nateglinide are generally smaller than those affecting other antidiabetic drugs. For example, rifampicin at 600 mg daily for 5 days decreased the AUC(0, ∞) of nateglinide by only 22% [54], whereas the same treatment caused a 57% decrease in the AUC(0, ∞) of repaglinide [55]. Similarly, fluconazole given at 200 mg (first dose 400 mg) daily for 4 days increased the AUC(0, ∞) of nateglinide by 48% [21] and that of glimepiride by 138% [16]. These findings together with the present results suggest that there may be less risk of drug interactions involving nateglinide compared with repaglinide or the sulphonylureas. However, this hypothesis needs to be confirmed in the clinical setting.
In conclusion, coadministration of gemfibrozil and itraconazole has only a limited effect on the pharmacokinetics of nateglinide. This is in marked contrast to the substantial influence of the gemfibrozil-itraconazole combination on the pharmacokinetics and pharmacodynamics of repaglinide. The findings suggest that in vivo gemfibrozil, probably due to its metabolites, is a more potent inhibitor of CYP2C8 than of CYP2C9.
Acknowledgments
We thank Mr Jouko Laitila, Mrs Kerttu Mårtensson, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance. This study was supported by grants from the Helsinki University Central Hospital Research Fund and Sigrid Juselius Foundation.
References
- 1.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 2.van Heiningen PN, Hatorp V, Kramer Nielsen K, Hansen KT, van Lier JJ, De Merbel NC, Oosterhuis B, Jonkman JH. Absorption, metabolism and excretion of a single oral dose of (14) C-repaglinide during repaglinide multiple dosing. Eur J Clin Pharmacol. 1999;55:521–5. doi: 10.1007/s002280050667. [DOI] [PubMed] [Google Scholar]
- 3.Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305–14. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JS, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 5.Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–91. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- 6.Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–9. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 7.Jurima-Romet M, Crawford K, Cyr T, Inaba T. Terfenadine metabolism in human liver. In vitro inhibition by macrolide antibiotics and azole antifungals. Drug Metab Dispos. 1994;22:849–57. [PubMed] [Google Scholar]
- 8.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:481–5. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 9.Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol. 2000;50:573–80. doi: 10.1046/j.1365-2125.2000.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen X, Wang JS, Backman JT, Kivistö KT, Neuvonen PJ. Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos. 2001;29:1359–61. [PubMed] [Google Scholar]
- 11.McLeod JF. Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Clin Pharmacokinet. 2004;43:97–120. doi: 10.2165/00003088-200443020-00003. [DOI] [PubMed] [Google Scholar]
- 12.Weaver ML, Orwig BA, Rodriguez LC, Graham ED, Chin JA, Shapino MJ, McLeod JF, Mangold JB. Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos. 2001;29:415–21. [PubMed] [Google Scholar]
- 13.Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet. 2002;41:471–83. doi: 10.2165/00003088-200241070-00002. [DOI] [PubMed] [Google Scholar]
- 14.Takesada H, Matsuda K, Ohtake R, Mihara R, Ono I, Tanaka K, Naito M, Yatagai M, Suzuki E. Structure determination of metabolites isolated from urine and bile after administration of AY4166, a novel d-phenylalanine-derivative hypoglycemic agent. Bioorg Med Chem. 1996;4:1771–81. doi: 10.1016/0968-0896(96)00187-3. [DOI] [PubMed] [Google Scholar]
- 15.Back DJ, Tjia JF, Karbwang J, Colbert J. In vitro inhibition studies of tolbutamide hydroxylase activity of human liver microsomes by azoles, sulphonamides and quinolines. Br J Clin Pharmacol. 1988;26:23–9. doi: 10.1111/j.1365-2125.1988.tb03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemi M, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ, Kivistö KT. Effects of fluconazole and fluvoxamine on the pharmacokinetics and pharmacodynamics of glimepiride. Clin Pharmacol Ther. 2001;69:194–200. doi: 10.1067/mcp.2001.114229. [DOI] [PubMed] [Google Scholar]
- 17.Wienkers LC, Wurden CJ, Storch E, Kunze KL, Rettie AE, Trager WF. Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab Dispos. 1996;24:610–4. [PubMed] [Google Scholar]
- 18.Back DJ, Tjia JF. Comparative effects of the antimycotic drugs ketoconazole, fluconazole, itraconazole and terbinafine on the metabolism of cyclosporin by human liver microsomes. Br J Clin Pharmacol. 1991;32:624–6. doi: 10.1111/j.1365-2125.1991.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olkkola KT, Ahonen J, Neuvonen PJ. The effects of the systemic antimycotics, itraconazole and fluconazole, on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Anesth Analg. 1996;82:511–6. doi: 10.1097/00000539-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111–80. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Niemi M, Neuvonen M, Juntti-Patinen L, Backman JT, Neuvonen PJ. Effect of fluconazole on the pharmacokinetics and pharmacodynamics of nateglinide. Clin Pharmacol Ther. 2003;74:25–31. doi: 10.1016/S0009-9236(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 22.Hengy H, Kölle EU. Determination of gemfibrozil in plasma by high performance liquid chromatography. Arzneimittelforschung. 1985;35:1637–9. [PubMed] [Google Scholar]
- 23.Remmel RP, Dombrovskis D, Canafax DM. Assay of itraconazole in leukemic patient plasma by reversed-phase small-bore liquid chromatography. J Chromatogr. 1988;432:388–94. doi: 10.1016/s0378-4347(00)80671-4. [DOI] [PubMed] [Google Scholar]
- 24.Allenmark S, Edebo A, Lindgren K. Determination of itraconazole in serum with high-performance liquid chromatography and fluorescence detection. J Chromatogr. 1990;532:203–6. doi: 10.1016/s0378-4347(00)83770-6. [DOI] [PubMed] [Google Scholar]
- 25.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 3. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 26.Kirchheiner J, Meineke I, Muller G, Bauer S, Rohde W, Meisel C, Roots I, Brockmöller J. Influence of CYP2C9 and CYP2D6 polymorphisms on the pharmacokinetics of nateglinide in genotyped healthy volunteers. Clin Pharmacokinet. 2004;43:267–78. doi: 10.2165/00003088-200443040-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kivistö KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol. 1998;46:49–53. doi: 10.1046/j.1365-2125.1998.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemi M, Backman JT, Granfors M, Laitila J, Neuvonen M, Neuvonen PJ. Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia. 2003;46:1319–23. doi: 10.1007/s00125-003-1181-x. [DOI] [PubMed] [Google Scholar]
- 29.Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivistö KT. Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther. 2002;72:326–32. doi: 10.1067/mcp.2002.127495. [DOI] [PubMed] [Google Scholar]
- 30.Niemi M, Neuvonen PJ, Kivistö KT. Effect of gemfibrozil on the pharmacokinetics and pharmacodynamics of glimepiride. Clin Pharmacol Ther. 2001;70:439–45. doi: 10.1067/mcp.2001.119723. [DOI] [PubMed] [Google Scholar]
- 31.Lilja JJ, Backman JT, Neuvonen PJ. Effect of gemfibrozil on the pharmacokinetics and pharmacodynamics of racemic warfarin in healthy subjects. Br J Clin Pharmacol. 2004 doi: 10.1111/j.1365-2125.2004.02323.x. doi: 10.1111/j.1365-2125.2004.02323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shitara Y, Hirano M, Sato H, Sugiyama Y. Gemfibrozil and its glucuronide inhibit the OATP2 (OATP1B1: SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin. J Pharmacol Exp Ther. 2004;311:228–36. doi: 10.1124/jpet.104.068536. [DOI] [PubMed] [Google Scholar]
- 33.Sabordo L, Sallustio BC, Evans AM, Nation RL. Hepatic disposition of the acyl glucuronide 1-O-gemfibrozil-beta-D-glucuronide: effects of clofibric acid, acetaminophen, and acetaminophen glucuronide. J Pharmacol Exp Ther. 2000;295:44–50. [PubMed] [Google Scholar]
- 34.Sabordo L, Sallustio BC, Evans AM, Nation RL. Hepatic disposition of the acyl glucuronide1-O-gemfibrozil-beta-D-glucuronide: effects of dibromosulfophthalein on membrane transport and aglycone formation. J Pharmacol Exp Ther. 1999;288:414–20. [PubMed] [Google Scholar]
- 35.Sallustio BC, Fairchild BA, Shanahan K, Evans AM, Nation RL. Disposition of gemfibrozil and gemfibrozil acyl glucuronide in the rat isolated perfused liver. Drug Metab Dispos. 1996;24:984–9. [PubMed] [Google Scholar]
- 36.Schoch GA, Yano JK, Wester MR, Griffin KJ, Stout CD, Johnson EF. Structure of human microsomal cytochrome P450 2C8. Evidence for a peripheral fatty acid binding site. J Biol Chem. 2004;279:9497–503. doi: 10.1074/jbc.M312516200. [DOI] [PubMed] [Google Scholar]
- 37.Williams PA, Cosme J, Ward A, Angove HC, Matak Vinkovic D, Jhoti H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003;424:464–8. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 38.Ohyama K, Nakajima M, Nakamura S, Shimada N, Yamazaki H, Yokoi T. A significant role of human cytochrome P450 2C8 in amiodarone N-deethylation: an approach to predict the contribution with relative activity factor. Drug Metab Dispos. 2000;28:1303–10. [PubMed] [Google Scholar]
- 39.Li XQ, Björkman A, Andersson TB, Ridderström M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300:399–407. doi: 10.1124/jpet.300.2.399. [DOI] [PubMed] [Google Scholar]
- 40.Sonnichsen DS, Liu Q, Schuetz EG, Schuetz JD, Pappo A, Relling MV. Variability in human cytochrome P450 paclitaxel metabolism. J Pharmacol Exp Ther. 1995;275:566–75. [PubMed] [Google Scholar]
- 41.Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56:120–4. doi: 10.1046/j.1365-2125.2003.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse D, Cosme J, Beaune P, Kroemer HK, Eichelbaum M. Cytochromes of the P450 2C subfamily are the major enzymes involved in the O-demethylation of verapamil in humans. Naunyn Schmiedebergs Arch Pharmacol. 1995;353:116–21. doi: 10.1007/BF00168924. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Samuel K, Subramanian R, Braun MP, Steams RA, Chiu SH, Evans DC, Baillie TA. Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther. 2002;303:969–78. doi: 10.1124/jpet.102.038992. [DOI] [PubMed] [Google Scholar]
- 44.Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother. 2002;46:160–5. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalava KM, Partanen J, Neuvonen PJ. Itraconazole decreases renal clearance of digoxin. Ther Drug Monit. 1997;19:609–13. doi: 10.1097/00007691-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 47.Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25:423–9. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Terada T, Sawada K, Saito H, Hashimoto Y, Inui K. Inhibitory effect of novel oral hypoglycemic agent nateglinide (AY4166) on peptide transporters PEPT1 and PEPT2. Eur J Pharmacol. 2000;392:11–7. doi: 10.1016/s0014-2999(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 49.Okamura A, Emoto A, Koyabu N, Ohtani H, Sawada Y. Transport and uptake of nateglinide in Caco-2 cells and its inhibitory effect on human monocarboxylate transporter MCT1. Br J Pharmacol. 2002;137:391–9. doi: 10.1038/sj.bjp.0704875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller DB, Spence JD. Clinical pharmacokinetics of fibric acid derivatives (fibrates) Clin Pharmacokinet. 1998;34:155–62. doi: 10.2165/00003088-199834020-00003. [DOI] [PubMed] [Google Scholar]
- 51.Schäfer-Korting M, Korting HC, Amann F, Peuser R, Lukacs A. Influence of albumin on itraconazole and ketoconazole antifungal activity: results of a dynamic in vitro study. Antimicrob Agents Chemother. 1991;35:2053–6. doi: 10.1128/aac.35.10.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wooltorton E. Repaglinide and gemfibrozil interaction: serious hypoglycemia. CMAJ. 2003;169:813. [Google Scholar]
- 53. EMEA public statement on repaglinide (Novonorm/Prandin): Contraindication of Concomitant Use of Repaglinide and Gemfibrozil [Online;. London 21 May 2003. Available from URL http://www.emea.eu.int/htms/human/drugalert/drugalert.htm.
- 54.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. Br J Clin Pharmacol. 2003;56:427–32. doi: 10.1046/j.1365-2125.2003.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]