Abstract
Aim
To identify experience with and attitudes towards paediatric off-label prescribing in primary care.
Method
A prospective questionnaire survey was sent to a sample of Scottish primary care practices (346 doctors in 80 general practices located throughout Scotland).
Results
Two hundred and two (58%) completed questionnaires were returned. Over 70% of GPs admitted to being familiar with the concept, and 40% to knowingly prescribing off-label. The most important sources of paediatric prescribing information were the British National Formulary (81%), personal experience (71%) and previous prescription notes (45%). The most common reason given by GPs for off-label prescribing was prescribing for a younger age than recommended, although prescribing data confirm that age is the least important and dose the most important reason for such prescribing. When asked to comment upon different causes for off-label prescribing, 80% of respondents expressed appropriate awareness of and concern for the described scenarios. Over 97% of GPs ranked development of paediatric formulations and clearer dosage information more highly than clinical trials as a means to reducing off-label prescribing.
Conclusions
Despite high levels of off-label prescribing in primary care in the UK, the majority of GPs claimed to be familiar with the concept, although less than half were aware of this common practice. A clear disparity between perceived and actual reasons for off-label prescribing was noted, possibly due to a reliance on personal experience, colleague experience or previous patient prescription notes as a guide to prescribing.
Keywords: off-label prescribing, child, primary health care, attitude
Introduction
The prescribing of off-label medicines to children in primary care is common and widespread throughout the UK affecting up to 26% of children aged 0–16 years of age [1, 2]. Previous studies have demonstrated that the majority of off-label prescribing in primary care is due to the prescription of licensed medicines out of their dosage recommendations [1, 2] unlike secondary care where high rates of unlicensed prescribing occurs [3–5].
The problem of unlicensed and off-label prescribing to children has been recognized by individual researchers [4, 6, 7], organizations such as The Royal College of Paediatrics and Child Health [8, 9], national and European governments [10, 11] and the pharmaceutical industry [8]. However little is known about the level of knowledge and attitudes of primary care physicians regarding off-label prescribing to children, and the potential problems and risks of such practice. In order to reduce off-label prescribing primary care, physicians need to be aware of the situation and acknowledge their role in providing optimal treatment for children.
Methods
Following piloting in the general practice of one of the authors (MWT), a questionnaire investigating prior knowledge of off-label prescribing and licensing recommendations, acknowledgement of and perceived problems with off-label prescribing, prescribing information sources used in practice, and the importance of proposed methods for reducing off-label prescribing was sent to 346 doctors within 80 randomly selected primary care practices throughout Scotland.
In an attempt to increase response rate each practice was contacted by telephone and informed of the forthcoming questionnaire. If a response had not been returned within 4 weeks a further questionnaire was sent out. A total of 13 questions were included with a combination of tick-box responses, ranked five-point scale questions ranging from ‘very frequently’ to ‘never’ and from ‘very important’ to ‘very unimportant’ together with questions requiring free text responses. Responses were analysed using SPSS software with differences in responses between GPs who claimed a specific interest in paediatrics and those who did not tested using chi-square test and Jonckheere-Terpstra test with significance set at the 5% level.
Results
Within the 80 selected practices 346 questionnaires were sent to individual GPs of which 202 were returned completed, a response rate of 58.4%. At least one reply was obtained from 79 of the practices surveyed. A specific interest in paediatrics was claimed by 31% (62) of respondents who reported a larger proportion of their workload made up by children (38.7%vs. 19.0%; chi-square test, P = 0.005).
The presence or lack of a specific interest in paediatrics however, made no significant difference to the responses to subsequent questions.
The extent to which GPs used various information sources when prescribing for children is shown in Table 1. Of all sources the British National Formulary (BNF) was most commonly used, followed by previous experience and colleague experience; no respondent used any of the available paediatric formularies.
Table 1.
Information sources used by GPs when prescribing to children (number of GPs (%))
| ++ | + | / | – | –– | Median | |
|---|---|---|---|---|---|---|
| British National Formulary | 123 (60.9) | 41 (20.3) | 28 (13.9) | 9 (4.5) | 1 (0.5) | + |
| Monthly Index of Medical Specialities | 45 (22.4) | 42 (20.9) | 48 (23.9) | 50 (24.9) | 16 (8.0) | / |
| Summary of Product Characteristics | 1 (0.5) | 3 (1.5) | 21 (10.8) | 99 (51.0) | 70 (36.1) | – |
| Local formulary | 15 (7.8) | 28 (14.5) | 48 (24.9) | 58 (30.1) | 44 (22.8) | – |
| National guidelines | 5 (2.6) | 30 (15.5) | 70 (36.3) | 59 (30.6) | 29 (15.0) | / |
| Previous patient prescription notes | 17 (8.7) | 72 (36.7) | 74 (37.8) | 26 (13.3) | 7 (3.6) | / |
| Previous experience | 37 (18.6) | 104 (52.3) | 52 (26.1) | 6 (3.0) | 0 (0.0) | + |
| Colleague experience | 2 (1.0) | 45 (23.3) | 73 (37.8) | 54 (28.0) | 19 (9.8) | / |
++ very frequently, + frequently,/moderately, – rarely, –– never.
Although the concept of off-label prescribing was familiar to 73.7% (148) of respondents, over half (53.3%) were unaware that off-label prescribing is commonplace in general practice. A sizeable proportion (40.6%n = 82) also admitted that they knowingly prescribed off-label medicines to children, most commonly for a younger age group than recommended (79.3%, n = 65), at higher (25.6%, n = 21) or lower (23.3%, n = 19) dose than recommended or for a nonrecommended indication (22%, n = 18).
The reasons given for prescribing off-label medicines were hospital consultant advice (26.8%, n = 22), and claims that no licensed alternative was available (23.2%, n = 19). However more than 50% of respondents were specifically concerned by the lack of paediatric dosage information and appropriate paediatric formulations which gave rise to off-label prescribing. Less than 15% admitted to specific concerns about the risk of side-effects, unevaluated efficacy and issues surrounding informed consent.
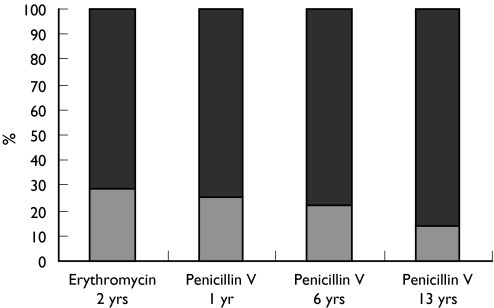
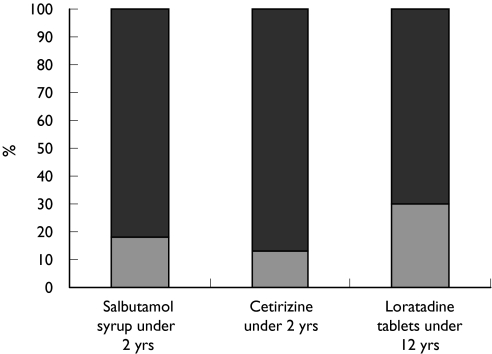
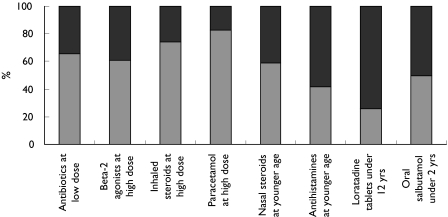
To assess the knowledge and attitudes of GPs towards the complex of reasons why medicines are most commonly prescribed off-label, respondents were asked to indicate if they were aware of the five given examples of, and reasons for, off-label prescribing and whether these would cause them concern. Approximately 80% of respondents stated that they were aware of the age and dose recommendations for prescribing erythromycin, penicillin V, salbutamol syrup, cetirizine and loratidine, despite these being commonly prescribed off-label in primary care [1, 2] (Figures 1 and 2). When doctors were asked to comment on eight examples of the most common medicines and reasons involved in off-label prescribing, the majority expressed concern and claimed to be aware of the importance of each example. The levels of concern expressed by respondents varied with the medicine and the reason for it being off-label, with the highest level of concern (81%) for paracetamol prescribed at higher than recommended dose, followed by inhaled steroids at higher than recommended dose (73%), antibiotics at lower than recommended dose (62%), β2-agonists at higher than recommended dose (60%), while least concern was expressed about the use of loratidine tablets in children below the recommended age (Figure 3). The reasons given for concern were lack of efficacy and development of antimicrobial resistance for antibiotics prescribed at lower than recommended dose; the possibility of side-effects and or toxicity when β2-agonists, inhaled steroids, and paracetamol are prescribed at higher than recommended doses; and side-effects or toxicity when nasal steroids, antihistamines and oral salbutamol are prescribed to a younger age group than recommended.
Figure 1.
GP knowledge of the SPC recommendations for age related dose increments for erythromycin and phenoxymethylpenicillin (penicillin V). Yes ( ), no (
), no ( )
)
Figure 2.
GP knowledge of age restrictions for commonly prescribed medicines and formulations. Yes ( ), no (
), no ( )
)
Figure 3.
GP levels of concern towards 8 examples of medicines commonly prescribed off-label. Yes ( ), no (
), no ( )
)
When asked to rank the most important ways by which off-label prescribing in primary care could be reduced, 97% of respondents placed clear and consistent labelling followed by the development of appropriate paediatric formulations as more important than fostering clinical trials in children (Table 2).
Table 2.
Importance of suggestions to reduce off-label prescribing (number of GPs (%))
| ++ | + | / | − | −− | Median | |
|---|---|---|---|---|---|---|
| Increased paediatric clinical trials for new drugs | 64 (32.8) | 90 (46.2) | 35 (17.9) | 5 (2.6) | 1 (0.5) | + |
| Paediatric clinical trials for existing drugs | 59 (29.9) | 92 (46.7) | 41 (20.8) | 4 (2.0) | 1 (0.5) | + |
| Appropriate formulations for young children | 103 (51.5) | 91 (45.5) | 6 (3.0) | 0 (0.0) | 0 (0.0) | ++ |
| More consistent and clearer dosage information | 124 (62.0) | 71 (35.5) | 5 (2.5) | 0 (0.0) | 0 (0.0) | ++ |
++ very important, + important,/moderate, – unimportant, –– very unimportant.
Discussion
Approximately one-third of GPs expressed a specific interest in paediatrics and consequently had more paediatric patients than their colleagues, however, they were no more experienced with, or knowledgeable about, off-label medicines than their colleagues.
The BNF was reported as the most commonly consulted source of information for paediatric prescribing followed by personal experience, while the Summary of Product Characteristics (SPC) and local formularies were the least commonly used; no respondent admitted to using any of the available paediatric formularies (Table 1). For most medicines the BNF follows the SPC recommendations [12, 13] and use is unlikely to result in off-label prescribing, however, the same cannot be said for the frequent use of personal experience, previous patient prescription notes and colleague experience, all of which may result in inadvertent off-label prescribing. Age and dosage recommendations vary widely even between medicines in the same class [12] making prescribing within the recommendations from memory or previous notes a less secure process than perceived by many of the respondents. This situation is further exacerbated by the current lack of consistency between prescribing guidelines and SPC recommendations together with variable age bandings for dosage regimens. The majority of GPs (73.6%) claimed to be reasonably familiar with the concept of off-label prescribing, although less than half were aware that off-label prescribing is common in general practice, indicating a significant lack of information concerning the extent and nature of off-label prescribing in primary care. When asked specifically about those medicines which are most commonly prescribed off-label in primary care [1, 14] (Figures 1 and 2), almost 80% of respondents claimed to be fully aware of the age and dose recommendations which gave rise to such off-label prescribing.
The most common reason given for off-label prescribing, by those respondents who admitted to knowingly doing so, was prescribing below the recommended age. This contrasts with surveys of actual practice in which the most common pattern observed was prescribing at lower or higher than the recommended dose [1, 2, 14]. Concern and knowledge about those medicines which are most commonly prescribed off-label in primary care [1, 2] was expressed by the majority of GPs indicating either a significant difference between perceived knowledge, experience, and actions or that the minority of GPs who admit to being unconcerned by this practice account for virtually all of off-label prescribing.
When asked to rate suggestions aimed at reducing the levels of off-label prescribing in primary care (Table 2), the development of appropriate formulations for young children and more consistent and clearer dosage information were the most popular options and ranked more highly than increasing the number of clinical trials in children (Table 2). These two areas were so highly rated by prescribers that it might be appropriate to consider addressing them in addition to proposals to increase and foster paediatric clinical trials [11].
In conclusion, off-label prescribing of medicines to children is a familiar concept to the majority of GPs although only a smaller number are aware that it is commonplace in primary care. The disparity between perceived (age related) and actual (dose related) reasons for off-label prescribing [1, 2] may have arisen because of a reliance on personal experience, colleague experience or previous patient prescription notes as a guide for future prescribing. In addition to the proposals for increasing paediatric clinical trials, consideration should be given to improving the consistency and clarity of prescribing guidelines and formulations available for young children. GPs are concerned about off-label prescribing, rate suggestions for reducing this practice as important and are likely to welcome initiatives aimed at improving prescribing for children.
Acknowledgments
JSM had the idea for the study and framed the questions with SE-D. SE-D extracted and analysed the data and SE-D, PJH and JSM wrote the paper with contributions from MWT. MWT is guarantor of the study. PJH has current grants from Glaxo Wellcome, and Merck, Sharp & Dohme. He has also performed consultancies for Merck Sharp & Dohme, Astra Zeneca and Glaxo Wellcome. JSM has current grant support from Glaxo Wellcome, SmithBeecham, Merck Sharp & Dohme, Roche Pharmaceuticals, Hesperion.
None of the contributors/authors have any conflicts of interest which might affect the publication of this study.
References
- 1.Ekins-Daukes S, Helms PJ, Simpson CR, Taylor MW, McLay JS. Off-label prescribing to children in primary care. Retrospective observational study. Eur J Clin Pharmacol. 2004;60:349–53. doi: 10.1007/s00228-004-0752-1. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off-label prescribing of drugs in general practice. Arch Dis Child. 2000;83(6):498–501. doi: 10.1136/adc.83.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner S, Longworth A, Nunn A, Choonara I. Unlicensed and off-label drug use in paediatric wards: prospective study. BMJ. 1998;316:343–5. doi: 10.1136/bmj.316.7128.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, Knoeppel C, Seybeth H, Pandolfini C, Raffaelli MR, Rochi F, Bonati M, Jong G, de Hoog M, van den Anker J. Survey of unlicensed and off-label drug use in paediatric wards in European countries. BMJ. 2000;320(7227):79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88(9):965–8. doi: 10.1080/08035259950168469. [DOI] [PubMed] [Google Scholar]
- 6.‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children's hospital. Pediatrics. 2001;108(5):1089–93. doi: 10.1542/peds.108.5.1089. [DOI] [PubMed] [Google Scholar]
- 7.Schirm E, Tobi H, De Jong-Van Den Berg LT. Unlicensed and off-label drug use by children in the community: cross sectional study. BMJ. 2002;324(7349):1312–3. doi: 10.1136/bmj.324.7349.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.British Pharmaceutical Association and the Association of the British Pharmaceutical Industry. Licensing Medicines for Children. London: Royal College of Paediatrics and Child Health; 1996. [Google Scholar]
- 9.Royal College of Paediatrics and Child Health. Medicines for Children. London: RCPCH Publications Limited; 1999. [Google Scholar]
- 10.Yamey G. MP introduces bill to protect children from unlicensed medicines. BMJ. 2000;320:208. [Google Scholar]
- 11.European Commission. DG Enterprises. Unit F2-Paediatric Initiative. Brussels. Better Medicines for Children: Summary of European Commission's Consultation Document. 2002. p. 28. February.
- 12.British Medical Association, Royal Pharmaceutical Society of Great Britain. British National Formulary. 40 2000.
- 13.Association of the British Pharmaceutical Industry. Compendium of Data Sheets and Summaries of Product Characteristics. London: Datapharm Publications Limited; 1999. [Google Scholar]
- 14.Ekins-Daukes S, McLay JS, Taylor MW, Simpson CR, Helms PJ. Antibiotic prescribing for children. Too much and too little? Retrospective observational study in primary care. Br J Clin Pharmacol. 2003;56:92–5. doi: 10.1046/j.1365-2125.2003.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]