Abstract
Aim
The aim was to assess the central nervous system (CNS) effects, pharmacokinetics and safety of GPI 5693, an inhibitor of a novel CNS-drug target, NAALADase which is being evaluated for the treatment of neuropathic pain.
Methods
This was a double-blind, placebo-controlled, exploratory study in healthy subjects receiving oral GPI 5693 single ascending doses of 100, 300, 750, 1125 mg with a placebo treatment randomly interspersed. An open-label, parallel extension examined the effects of food and sex on the pharmacokinetics of 750, 1125 and 1500 mg doses. Blood samples were collected for pharmacokinetic and biochemical/haematological safety analysis, vital signs, ECG and adverse event checks were performed regularly up to 48 h postdose. Postdose CNS effects were assessed using eye movements, adaptive tracking, electroencephalography (EEG), body sway and Visual Analogue Scales (VAS).
Results
CNS effects were mainly observed after the 1125 mg dose, showing a significant decrease of adaptive tracking performance, VAS alertness and VAS mood, and an increase of EEG occipital alpha and theta power. Gastro-intestinal (GI) adverse effects were frequent at higher doses. No clinically significant changes in vital signs or ECG were noted during any of the treatments. The therapeutically relevant concentration range (950–11 100 ng ml−1) as determined from animal experiments was already reached after the 300 mg dose. Cmax after the 300 mg and 750 mg dose was 2868 and 9266 ng ml−1 with a t1/2 of 2.54 and 4.78 h, respectively. Concomitant food intake (with the 750 mg and 1125 mg doses) reduced Cmax by approximately 66% and AUC by approximately 40%. With concomitant food intake, the dose-normalized Cmax also decreased significantly by −5.6 (CI: −2.6 to −8.7) ng ml−1 mg−1. The pharmacokinetic variability was largest after the 300 mg and 750 mg dose, resulting in a SD of approximately 50% of the Cmax.
Conclusion
NAALADase inhibition with GPI 5693 was safe and tolerable in healthy subjects. Plasma concentrations that were effective in the reversal of hyperalgesia in the chronic constrictive injury animal model of neuropathic pain were obtained at doses of 300, 750 and 1125 mg in the fasted state. Comcomitant food intake reduced Cmax and AUC. CNS effects and GI AEs increased in incidence over placebo only at the 1125 mg dose.
Keywords: NAALAD-ase-inhibitor, pharmacokinetics, central nervous system
Introduction
NAALADase (also termed glutamate carboxypeptidase II) is a membrane bound metalloprotease that hydrolyses the neuropeptide N-acetyl-aspartyl glutamate (NAAG) to N-acetyl-aspartate and glutamate. NAAG acts as an agonist at group II metabotropic glutamate receptors, and is also a mixed agonist/antagonist at the NMDA glutamate receptor. The functional relevance of NAALADase is unknown, but it is thought both to terminate the neurotransmitter activity of NAAG and to liberate glutamate from NAAG, which subsequently acts at the various glutamate receptor subtypes. NAALADase inhibition is thus primarily expected to cause indirect antiglutamatergic effects [1].
GPI 5693 ((R,S)-2-(3-mercaptopropyl) pentanedioic acid) is an orally bioavailable, selective and potent NAALADase inhibitor [2]. Rat models of peripheral neuropathy suggested that daily oral administration of NAALADase inhibitors can reduce neuropathic pain signs and simultaneously reduce the rate of decline in nerve conduction velocity [3]. Pathology results indicate that NAALADase inhibition also protects against diabetic-induced axonal atrophy and Wallerian degeneration. These data suggest a potential use for GPI 5693 as a treatment for neuropathic pain as well as retarding the progression of diabetic neuropathy in patients. The indirect antiglutamatergic effects of NAALADase inhibition in the central nervous system (CNS) also suggest a role for NAALADase inhibition in a host of diseases where glutamate may be involved [4, 5], but this has not yet been studied extensively.
Since the physiological role of NAALADase is unknown, potential CNS effects can only be predicted indirectly, e.g. from the various known effects of inhibitors of excitatory amino acids on the CNS. Currently, no behavioural toxicity has actually been observed in animal experiments. Since it may be possible to detect subtle CNS effects in humans that might not be observed in animals, it is important to carefully study the potential CNS effects of NAALADase-inhibition. Also, observed CNS effects may provide indications of the functional relevance of NAALADase inhibition, and provide methods to quantify these effects in future studies.
Drugs affecting glutamatergic neurotransmission can cause a general depression of CNS activity, including sedation [6, 7]. It is essential to know if significant CNS depression occurs, since this may limit applicability of the compound during prolonged treatment of neuropathy. There are many different methods to quantify CNS depression, and saccadic peak velocity is one of the most sensitive parameters [8, 9]. Effects on motor performance could also occur with inhibitors of excitatory neurotransmitters. Different motor effects can be quantified using smooth pursuit eye movements, adaptive tracking and body sway [10].
Some antiglutamatergic drugs have psychotomimetic properties. The illicit drug PCP (‘angel's dust’) and the related anaesthetic agent ketamine are direct NMDA receptor antagonists with psychotropic effects. It is unknown whether these effects are related to glutamatergic inhibition, but the subjective effects of subanaesthetic doses of ketamine can be quantified and related to central and peripheral drug concentrations, using specific questionnaires and visual analogue scales [11, 12]. Although GPI 5693 does not possess direct NMDA-antagonist activity, potential psychotomimetic effects were evaluated for safety.
In theory, the clinical effects of glutamatergic modulation could be measured most sensitively in the realms of attention, motor function and subjective changes. In addition, the pharmaco-electro-encephalogram (EEG) provides a sensitive general pharmacodynamic parameter of CNS penetration and activity [13]. Glutamatergic agents have caused concentration-related changes in EEG-activity in healthy subjects [14].
This study represents the first administration of a NAALADase-inhibitor in humans. It aimed to describe the pharmacokinetics and safety of GPI 5693, and to determine the central nervous system effects of NAALADase inhibition concentrations that showed efficacy in neuropathic animal models.
Methods
After approval of the protocol by the Ethical Review Board of the Leiden University Medical Centre, 25 healthy male and female subjects (age 18–45 years) were asked to participate in this exploratory study. After giving written informed consent, subjects received a full medical examination before entering the study, including a pregnancy test for female participants of childbearing potential. Alcohol and xanthine use was restricted to two units per day, from two days prior to the study day. Furthermore, subjects refrained from alcohol, caffeine and xanthine containing foods and beverages 12 h prior to the study days and during the study days. Female subjects were instructed to use barrier contraceptives. Concomitant medication other than paracetamol was not permitted during the study period.
This was a double-blinded, partially randomized, placebo-controlled, ascending dose study of GPI 5693, with minimum washout periods of 48 h. The study aimed to reach a maximum concentration (Cmax) and area under the concentration curve (AUC) at the concentrations showing reversal of hyperalgesia in experimental animal models.
The study was performed in panels, with interim analyses of pharmacokinetics and safety to adapt the design of consecutive panels. Four male subjects participating in Panel 1 received fasting oral GPI 5693 doses of 100 mg, 300 mg and 750 mg, with a placebo occasion randomly inserted. A fifth open-label study day was added to this panel to investigate the effects of food on the 750 mg dose. After an interim analysis, the selected doses for Panel 2 (which also consisted of four male subjects) were 300 mg, 750 mg, 1125 mg and placebo. Interim analyses of the first two panels provided indications for a food effect on GPI 5693 pharmacokinetics. The safety and tolerability of the drug in men allowed inclusion of women in a third open-label panel, investigating the tolerability and pharmacokinetics after single oral doses of 1125 and 1500 mg in fed state for both sexes. No CNS effects were measured during Panel 3 which consisted of six female and six male subjects.
The eye-movement recording, adaptive tracking, body sway and Visual Analogue Scales (VAS) were practised before the first study day to reduce learning effects during the study. GPI 5693 was administered as 100 mg and 375 mg capsules after overnight fasting. During all occasions of the first two panels without food interaction, EEG, eye movements, adaptive tracking, body sway and VAS were measured at baseline and at 30 min intervals for the first 2 h, and with increasing intervals until 8 h after drug administration. ECG, vital signs and adverse events were checked regularly and continuous two-lead ECG monitoring was performed for 4 h after drug administration. In the 24–48-h period after dosing, a fecal sample was taken to check for occult blood. Blood sampling for haematological and biochemical parameters was done at baseline and 24 h after dosing. Pharmacokinetic sampling was performed every 10 min for the first hour and with increasing intervals until 48 h after drug administration. GPI 5693 was measured by means of LC/MS/MS. Plasma GPI 5693 concentrations were linear from 1 to 2000 ng ml−1 and urine GPI 5693 concentrations were linear from 10 to 2000 ng ml−1. The average within assay CV was 2.5% and the average between assay CV was 1.6%.
Subjects remained at the study site until 24 h after each dosing. A poststudy check-up took place 48 h after their last study day including final follow-up of any adverse effects.
Electroencephalograms were recorded and analysed using CED software (Cambridge Electronics Design, Cambridge, UK), as described previously [10]. EEG recordings were made using silver-silver chloride electrodes, fixed with collodion at Fz, Cz, Pz and Oz, with the same common ground electrode as for the eye movement registration (international 10/20 system). The electrode resistances were kept below 5 kOhm. All recordings were done with the subjects’ eyes closed. EEG signals were obtained from leads Fz-Cz and Pz-Oz. The signals were amplified by use of a Nihon Kohden AB-621G bioelectric amplifier (Nihon Kohden Corporation, Tokyo, Japan) with a time constant of 0.3 s and a low pass filter at 100 Hz. Per session eight consecutive blocks of 8 s were recorded over a 2-min period. The sampling rate was 1024 Hz. Datablocks containing artefacts were identified by visual inspection and these were excluded from analysis. Fast Fourier transform analysis was performed to obtain the sum of amplitudes in the delta- (0.5–3.5 Hz), theta- (3.5–7.5 Hz), alpha- (7.5–11.5 Hz) and beta (11.5–30 Hz) frequency ranges. The total recording bandwidth was 0–50 Hz.
Recording and analysis of saccadic and smooth pursuit eye movements were done with a microcomputer-based system [15]. The equipment used for stimulus display, signal collection and amplification was from Nihon Kohden (Nihon Kohden Corporation, Tokyo, Japan). Saccadic eye movements were recorded for stimulus amplitudes of 15 degrees to either side. Interstimulus intervals varied randomly between 3 and 6 s, and 15 saccades were recorded. The average values of saccadic peak velocity, latency (reaction time) and inaccuracy were used as parameters. For smooth pursuit eye movements the target moved sinusoidal at frequencies range from 0.3 to 1.1 Hz, increasing by steps of 0.1 Hz. The amplitude of target displacement corresponds to 20 degrees eyeball rotations to both sides. Four cycles were recorded for each stimulus frequency.
The adaptive tracking test was performed as originally described by Borland and Nicholson [16], using customized equipment and software (Hobbs, 2000, Hertfordshire, UK). The average performance and the standard deviation of scores over a 10-min period were used for analysis. Adaptive tracking is a pursuit tracking task. A circle moves randomly about a screen. The subject is instructed to try to keep a dot inside the moving circle by operating a joystick. If this effort is successful, the speed of the moving circle increases. Conversely, the velocity is reduced if the test subject cannot maintain the dot inside the circle. Performance was scored after a fixed 10-min period. The adaptive tracking test is more sensitive to impairment of eye–hand coordination by drugs than compensatory pursuit tasks or other pursuit tracking tasks, such as the pursuit rotor. The adaptive tracking test has proven to be useful for measurement of CNS effects of alcohol [9], various psychoactive drugs and sleep deprivation [10].
The body sway meter allows measurement of body movements in a single plane, providing a measure of postural stability. Body sway was measured with an apparatus similar to the Wright ataxiameter [17]. With a string attached to the waist, all body movements over a period of time were integrated and expressed as mm sway on a digital display. The contribution of vision to postural control was eliminated by asking subjects to close their eyes. Subjects were instructed to wear the same pair of comfortable, low-heeled shoes on each session. Before starting a measurement, subjects were asked to stand still and comfortable, with their feet approximately 10 cm apart and their hands in a relaxed position alongside the body. Subjects were not allowed to talk during the measurement. The total period of body-sway measurement was 2 min
Visual Analogue Scales as originally described by Norris [18] were used to quantify subjective effects. From these measurements, three factors were derived as described by Bond and Lader [19], corresponding to alertness, mood and calmness. Psychedelic effects were monitored by visual analogue scales, translated from scales described by Bowdle et al. [20], since no validated version was available for the Dutch language and population.
All repeatedly measured dynamic variables were characterized using maximum change from pretreatment value (Emax) and using areas under the effect curve (AUECs). These AUECs were calculated using the linear trapezoidal rule and were divided by the corresponding time span resulting in a weighted average outcome. Nominal times were used. AUECs were calculated over the entire sampling period (12 h) and over the first 6 h because most of the response is expected to occur during this period.
Descriptive statistics were obtained for each pharmacodynamic parameter. Time course as well as derived parameters were summarized (using means, standard deviation (SD), min, median, max, n). Derived pharmacodynamic parameters were analysed untransformed. EEG parameters were analysed as percentage change from prevalue. These parameters were analysed, from the fasted occasions only, using analysis of variance (anova) accounting for subject and treatment.
Dose-independent parameters (Clearance, Cmax/dose, AUC0–inf/dose and tmax) were compared between the fasting and fed occasions using analysis of covariance with food and subject as factors, dose as covariate and including the dose–food interaction. This resulted in a predicted linear relationship between dose and the pharmacokinetic (PK) parameter for fed and fasted separately. This line was depicted in graphs of the individual PK parameters (Clearance, Cmax/dose, AUC0–inf/dose and tmax) plotted against dose, to visualize both dose dependency and potential differences between fasted and fed occasions. The line was calculated using the reported Least Square Means (at the average administered dose: 907.5 mg) along with the slopes on dose for the two groups (fed/fasted). In absence of a significant dose by food interaction, the anova model was recalculated assuming the interaction to be absent and in that case the resulting (parallel) lines are shown in the graphs.
Statistical calculations were performed using SPSS for Windows (SPSS, Inc., Chicago, IL) and SAS for Windows V8.1 (SAS Institute, Inc., Cary, NC, USA).
The pharmacokinetic parameters were calculated by standard noncompartmental analysis using the software package WinNonlin V3.1 (Pharsight, Inc., Mountainview, CA, USA).
Results
A total of 25 subjects were screened for this study. Twenty-one subjects received at least one dose of study medication: four males in Panel 1, five males in Panel 2 and 12 (six males, six females) in Panel 3. One subject dropped out after the first dose of study medication in Panel 2 for reasons unrelated to the study. All subjects who received at least one dose of study medication were included in the safety analysis. Subjects who participated in Panel 1 (occasion 1–4) and Panel 2 were included in the pharmacodynamic (CNS effect) and pharmacokinetic analysis if they had received at least one dose of study medication. The average (SD) weight of the participating subjects was 71 (10) kg, the height 178 (9) cm and age 23 (3) years.
The incidence of all adverse experiences judged as ‘treatment related’ are summarized in Table 1. Most of the treatment-related adverse events occurred in the WHO body system ‘Gastro-intestinal disorders’. After 53 administrations of active drug, 20 (38%) treatment related gastro-intestinal adverse events were reported. These consisted mainly of mild nausea, dyspepsia and reflux. Incidence of these adverse events was similar between placebo, 100 mg, 300 mg, and 750 mg. Incidence of these adverse events after the 1125 and 1500 mg dose was greater than placebo regardless of dosing in the fed or fasted state (approximately 50% of the subjects vs. incidence after placebo in 13% of the subjects). After concomitant food intake with the 750 mg dose, no gastro-intestinal adverse effects were reported. At the higher dose of 1125 mg the incidence was identical at the fed and fasted state.
Table 1.
Summary of treatment related adverse events
| Doses in mg (number of subjects) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Body systems(Preferred term) | 100 (4) | 300 (9) | 750 (8) | 750* (4) | 1125 (4) | 1125* (12) | 1500* (12) | Placebo (8) |
| Body as a whole – general disorders | 1 | 1 | 4 | |||||
| Fatigue | 1 | 3 | ||||||
| Temperature changed sensation | 1 | 1 | ||||||
| Central and peripheral nervous system disorders | 2 | 1 | 1 | 1 | ||||
| Dizziness | 1 | 1 | ||||||
| Headache | 2 | 1 | ||||||
| Gastro-intestinal disorders | 1 | 1 | 2 | 3 | 6 | 7 | 1 | |
| Abdominal pain | 1 | |||||||
| Diarrhoea | 1 | |||||||
| Dyspepsia | 1 | 1 | 1 | 2 | 2 | 2 | ||
| Eructation | 1 | |||||||
| Gastro-esophageal reflux | 2 | 1 | ||||||
| Nausea | 1 | 2 | 2 | 1 | ||||
| Psychiatric disorders | 1 | 1 | 3 | |||||
| Insomnia | 1 | |||||||
| Somnolence | 1 | 3 | ||||||
| Skin and appendages disorders | 1 | 1 | 1 | |||||
| Pruritis | 1 | |||||||
| Skin reaction localized | 1 | 1 | ||||||
| Vision disorders | 1 | |||||||
| Vision abnormal | 1 | |||||||
Dose administered with standardized meal.
Other frequently reported adverse events were somnolence and headache, which were mostly judged as not treatment related (91% and 86%, respectively). No clinically significant changes in vital signs or ECG were noted during any of the treatments. Three positive fecal occult blood tests were reported; twice after the 300 mg dose, once after the 1500 mg dose (fed state) and once after placebo.
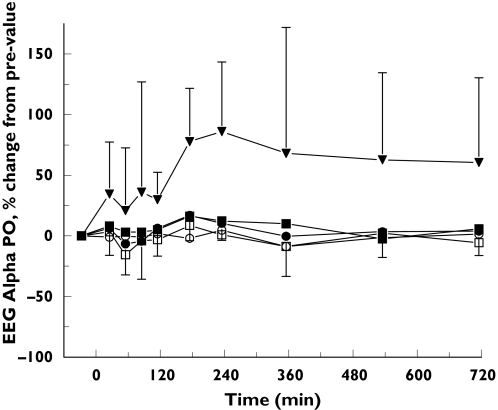
Compared with placebo, small but consistent statistically significant CNS effects were observed with the highest dose of 1125 mg. As indicated by the average time profile (Figure 1), EEG occipital alpha power AUC0−6 h increased (43.9 (95% confidence interval (CI): 4.8–83.1)%). Occipital theta power AUC0−6 h also increased after 1125 mg (17.1 (CI: 0.2–34.1)%).
Figure 1.
Average EEG alpha power: mean ± SD time-effect profiles of EEG alpha power for placebo (•), GPI 5693 100 mg (○), 300 mg (▪), 750 mg (□) and 1125 mg(▾)
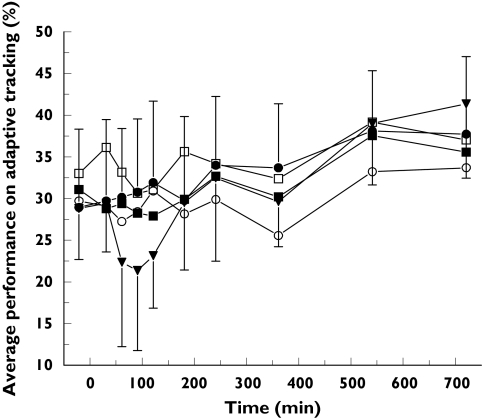
The effect of GPI 5693 on adaptive tracking performance is shown Figure 2. Adaptive tracking performance AUC0−6 h decreased after 1125 mg (−8.7 (CI: −14.0 to −3.5)%).
Figure 2.
Average adaptive tracking performance: mean ± SD time-effect profiles of adaptive tracking performance for placebo (•), GPI 5693 100 mg (○), 300 mg (▪), 750 mg (□) and 1125 mg (▾)
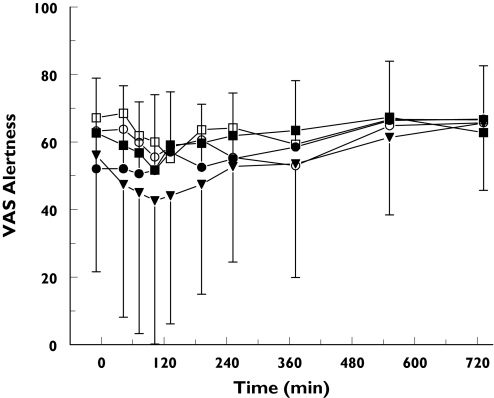
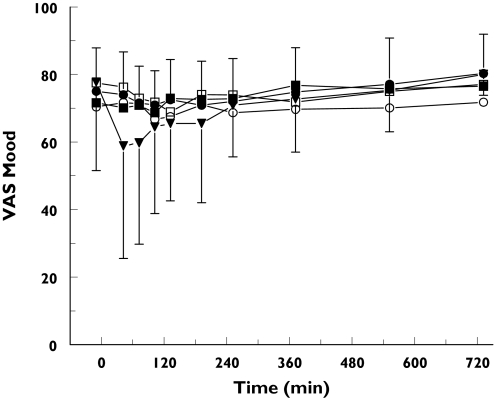
The average time profiles for the Bond and Lader visual analogue scales are presented in Figures 3 and 4. GPI 5693 1125 mg caused a reduction of the AUC0−6 h for VAS alertness and the VAS mood (−9.9 (CI: −19.2 to −0.6) mm and −8.6 (CI: −16.7 to −0.5) mm, respectively).
Figure 3.
Average Visual Analogue Scales alertness: mean ± SD time-effect profiles of visual analogue scales for alertness for placebo (•), GPI 5693 100 mg (○), 300 mg (▪), 750 mg (□) and 1125 mg (▾)
Figure 4.
Average Visual Analogue Scales mood: mean ± SD time-effect profiles of visual analogue scales for mood for placebo (•), GPI 5693 100 mg (○), 300 mg (▪), 750 mg (□) and 1125 mg (▾)
At lower dose levels, inconsistent VAS effects were observed which did not occur at higher doses and thus did not show a dose relationship. The AUC0−6 h for body sway after the 300 mg dose was decreased compared with placebo (−40.3 (CI: −66.6 to −14.0) mm min−2). The AUC0−6 h of the VAS scores for Meaning (‘I had the idea that events, objects, or other people had particular meaning that was specific for me’) and Anxious (‘I felt anxious’) were decreased after the 300 mg dose (−2.8 (CI: −4.8 to −0.8) mm and −1.3 (CI: −2.6 to −0.0) mm, respectively). 750 mg showed a decrease of the VAS score for Meaning (−2.2 (CI: −4.2 to −0.2) (mm).
No effects were seen on saccadic or smooth pursuit eye movements after any of the doses.
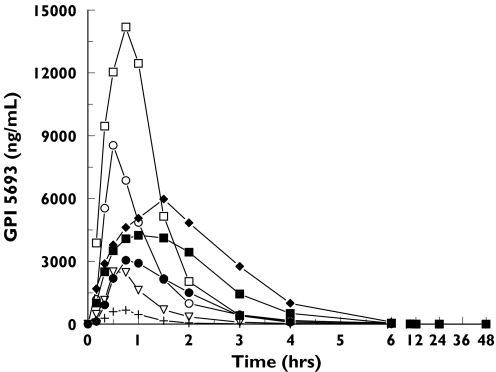
The pharmacokinetic parameters are presented in Table 2. The average concentration–time profiles of plasma GPI 5693 are presented in Figure 5. The therapeutically relevant concentration range (950–11 100 ng ml−1) as determined from animal experiments was already reached in the first panel after the 300 mg dose. The pharmacokinetic variability was largest after the 300 mg and 750 mg dose, resulting in a SD of approximately 50% of the Cmax. Although no formal testing was performed, and pharmacokinetics in females were only studied in the fed state, there was no indication of sex differences in pharmacokinetics (third panel).
Table 2.
GPI 5693 summary pharmacokinetic parameters
| Dose GPI5693 | n | Cmax (ng ml−1) | SD | tmax (min) | SD | AUCinf (h ng−1 ml−1) | SD | t1/2 (h) | SD |
|---|---|---|---|---|---|---|---|---|---|
| 100 mg | 4 | 723 | 201 | 38 | 9 | 684 | 205 | 0.87 | 0.02 |
| 300 mg | 9 | 2868 | 1384 | 42 | 10 | 2733 | 779 | 2.54 | 0.71 |
| 750 mg | 8 | 9266 | 4650 | 51 | 53 | 8855 | 2498 | 4.78 | 2.21 |
| 1125 mg | 4 | 15 742 | 3958 | 45 | 12 | 17 332 | 4542 | 6.05 | 0.70 |
| 750 mg fed | 4 | 3057 | 1046 | 45 | 0 | 5144 | 1239 | 8.83 | 7.03 |
| 1125 mg fed | 12 | 5309 | 2086 | 74 | 33 | 10 523 | 3292 | 6.88 | 6.71 |
| 1500 mg fed | 12 | 6822 | 2229 | 93 | 37 | 15 218 | 3708 | 8.62 | 5.48 |
Figure 5.
Average plasma concentrations of GPI 5693: mean ± SD time-concentration profiles after GPI 5693 100 mg (+), 300 mg (▿), 750 mg (○), 750 mg (fed) (□), 1125 mg (•), 1125 mg (fed) (▪), 1500 mg (fed) (♦)
With concomitant food intake, the Cmax decreased from 9266 to 3057 and from 15 742 to 5309 (ng ml−1) after the 750 mg and 1125 mg, respectively. Food intake also reduced the AUCinf from 8855 to 5144 and from 17 332 to 10 523 (h ng−1 ml−1) for the 750 mg and 1125 mg doses, respectively. These food effects were evaluated for these doses, where food and dose were used as anova-covariates. The dose-normalized Cmax decreased significantly by −5.6 (CI: −2.6 to −8.7) ng ml−1 mg−1. Food caused a significant reduction of the slope of the relationship between dose and AUC0–inf. Dose-normalized tmax showed no significant food effects.
The effects of food on pharmacokinetic variability were unclear. Although no formal testing was performed, food seemed to reduce the variability following the 750 mg dose, from a CV of 50% to a CV of 34% for Cmax and a CV of 28% to a CV of 24% for AUC0–inf. Following the 1125 mg dose however, food caused a slight increase in the CV for Cmax (from 25 to 39%) and for AUC0–inf (from 26 to 31%).
Discussion
The main aim of this exploratory study was to assess the single-dose pharmacokinetics and to evaluate safety and tolerability, including CNS effects of a newly developed NAALADase-inhibitor. In all, five dose levels were administered, of which three were also dosed with concomitant food intake.
The pharmacokinetics showed that therapeutically relevant plasma concentrations were reached in this study. Although there was some pharmacokinetic variability, this will presumably not cause major problems in the development of the drug, since the therapeutic range was broad and it appears that the upper end of this range can be achieved without dose-limiting adverse effects. There were clear effects of food on pharmacokinetics, mainly resulting in a lower Cmax and AUC, which should be taken into account when designing a dose schedule for further studies.
The main safety issues after single ascending dose administration of GPI 5693 were the relatively high incidence of gastro-intestinal side-effects, such as nausea and dyspepsia (generally mild and short lasting) which appeared to occur more often at higher dose levels. Three subjects presented with a positive feces occult blood test, but these were not necessarily related to GPI 5693. Firstly, there was no relation to dose or to repeated exposure. Secondly, subjects were not required to adhere to a strict diet and result could therefore have been false positive. Also, one subject had a positive feces occult blood test following placebo. NAALADase inhibition per se is not expected to cause gastrointestinal effects. The gastrointestinal effects of GPI 5693 could be due to the acidic nature of the compound and could be a limiting factor during prolonged treatment of neuropathic pain patients with this agent. Firstly, gastro-intestinal effects may be enhanced during repeated exposure with multiple dosing. Secondly, the associated autonomic neuropathy in diabetic patients often leads to changes in gastro-intestinal motility. A prolonged exposure of the gastric or duodenal epithelium to GPI 5693 may also increase the incidence of gastrointestinal adverse events. Furthermore, if the gut epithelium is affected by GPI 5693, pharmacokinetic properties of the drug may be altered.
Central nervous system AEs were also reported although the incidence was not different following placebo or active treatment. A relatively high number of somnolence and headache AEs were reported after both GPI 5693 and placebo dosing. These effects may be partly due to caffeine abstination and disruption of normal daily routines. At the highest tested dose (1125 mg) at which central nervous system tolerability was evaluated, some CNS effects were observed. The performance on adaptive tracking declined and the scores of the VAS alertness were significantly affected, even in the small group of four subjects. These effects could be associated with clinically relevant sedation by GPI 5693. The findings of these CNS effects warrant a more extensive CNS profiling of the drug with therapeutic dosages, focusing on sedation and on the possible development of tolerance to these effects after prolonged exposure in a multiple dose study. However, these effects also demonstrate CNS penetration of the drug. This may be important if the expansion of the area of development of this drug includes central neurodegenerative diseases. Pharmacokinetic-dynamic modelling was not performed because the effects were small and only consistently found at the highest dose level.
In summary, GPI 5693 was safe and generally well tolerated at plasma exposures that were effective in animal model of neuropathic pain. A clear food effect was observed on the pharmacokinetics, reducing Cmax and AUC. Furthermore a relatively high, dose-dependant incidence of gastro-intestinal adverse events was observed. Although no significant drug related CNS adverse events were reported, mild CNS effects were observed following the highest dose level (at which these were assessed). These findings warrant a multiple dose study in healthy subjects in a fed state, to further evaluate the pharmacokinetic properties and adverse events profile of GPI 5693, prior to proceeding to patient studies.
Acknowledgments
This study was sponsored by Guilford Pharmaceuticals Incorporated, Baltimore, MD, USA.
References
- 1.Jackson PF, Slusher BS. Design of NAALADase inhibitors: a novel neuroprotective strategy. Curr Med. 2001;8:949–57. doi: 10.2174/0929867013372797. [DOI] [PubMed] [Google Scholar]
- 2.Jackson PF, Tays KL, Maclin KM, Ko YS, Li W, Vitharana D, Tsukamoto T, Stoermer D, Lu XC, Wazniak K, Slusher BS. Design and pharmacological activity of phosphinic acid based NAALADase inhibitors. J Med Chem. 2001;44:4170–5. doi: 10.1021/jm0001774. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Slusher B, Murakawa Y, Wazniak K, Tsukamoto T, Jackson PF, Sima AA. GCPII (NAALADase) inhibition prevents long-term diabetic neuropathy in type 1 diabetic BB/Wor rats. J Neurol Sci. 2002;194:21–8. doi: 10.1016/s0022-510x(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 4.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–8. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 5.Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Hacubuni I, Bhaedwaj A, Traystman RJ, Robinson MD, Britton P, Lu XC, Tortella FC, Wazniak KM, Yudhoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 6.Wauquier A. EEG and neuropharmacology. In: Niedermeyer E, Lopez da Silva F, editors. Electroencephalography – Basic Principles, Clinical Applications, and Related Fields. 3. Baltimore: Williams & Wilkins; 1993. pp. 619–29. [Google Scholar]
- 7.Saletu B, Grünberger J, Anderer P, Linzmayer L, König P. On the cerebro-protective effects of caroverine, a calcium-channel blocker and antiglutaminergic drug: double-blind, placebo-controlled, EEG-mapping and psychometric studies under hypoxia. Br J Clin Pharmacol. 1996;41:89–99. doi: 10.1111/j.1365-2125.1996.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 8.van Steveninck AL. State University Leiden; 1993. Methods of assessment of central nervous system effects of drugs in man. Thesis. [Google Scholar]
- 9.Van Steveninck AL, Schoemaker RC, Pieters MSM, Kroon JM, Breimer DD, Cohen AF. A study comparing the sensitivities of adaptive tracking, eye movement analysis and visual analogue lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50:172–80. doi: 10.1038/clpt.1991.122. [DOI] [PubMed] [Google Scholar]
- 10.Van Steveninck AL, Van Berckel BNM, Schoemaker RC, Breimer DD, Van Gerven JMA, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:10–7. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 11.Hartvig P, Valtysson J, Lindner K-J, Knstensen J, Karlsten R, Bustafsson LL, Persson J, Svensson JO, Oye I, Antoni G. Central nervous system effects of subdissociative doses of (s)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58:165–73. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 12.Harborne GC, Watson FL, Healy DT, Groves L. The effects of sub-anaesthetic doses of ketamine on memory, cognitive performance and subjective experience in healthy volunteers. J Psychopharmacol. 1996;10:134–40. doi: 10.1177/026988119601000208. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AF, Ashby L, Crowley D, Land G, Peck AW. Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol. 1985;20:619–29. doi: 10.1111/j.1365-2125.1985.tb05120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muir KW, Grosset DG, Gamzu E, Lees KR. Pharmacological effects of the non-competitive NMDA antagonist CNS 1102 in normal volunteers. Br J Clin Pharmacol. 1994;38:33–8. doi: 10.1111/j.1365-2125.1994.tb04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Steveninck AL, Cohen AF, Ward T. A microcomputer based system for recording and analysis of smooth pursuit and saccadic eye movements. Br J Clin Pharmacol. 1989;27:712–3. [Google Scholar]
- 16.Borland RG, Nicholson AN. Visual motor coordination and dynamic visual acuity. Br J Clin Pharmacol. 1984;18:69S–72S. doi: 10.1111/j.1365-2125.1984.tb02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright BM. A simple mechanical ataxia-meter. J Physiol. 1971;218:27P–28P. [PubMed] [Google Scholar]
- 18.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–91. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- 19.Bond A, Lader M. Self-concepts in anxiety states. Br J Med Psychol. 1976;49:275–9. doi: 10.1111/j.2044-8341.1976.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 20.Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88:82–8. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]