Abstract
Aims
To obtain in vivo evidence for the involvement of cytochrome P450 (CYP) 3A4 in the metabolism of brotizolam.
Methods
Fourteen healthy male volunteers received erythromycin 1200 mg day−1 or placebo for 7 days in a double-blind randomized crossover manner. On the 6th day they received a single oral 0.5-mg dose of brotizolam, and blood samplings were performed for 24 h.
Results
Erythromycin treatment significantly increased the peak plasma concentration (P < 0.05), total area under the plasma concentration-time curve (P < 0.01), and elimination half-life (P < 0.01) of brotizolam.
Conclusions
The present study provides in vivo evidence for the involvement of CYP3A4 in brotizolam metabolism.
Keywords: brotizolam, CYP3A4, erythromycin, metabolism
Introduction
Brotizolam is a triazolothienodiazepine, and widely used in the treatment of insomnia [1]. This drug undergoes extensive metabolism, and the main metabolite is α-hydroxybrotizolam and the second main metabolite is 6-hydroxybrotizolam in human urine [1]. These metabolites are less active than the parent compound, and it is unlikely that they contribute to the clinical effects [1].
Cytochrome P450 (CYP) 3A4 is one of the most important human drug metabolizing enzymes, and is involved in the metabolism of numerous drugs [2]. Several benzodiazepines such as alprazolam [3–5], midazolam [6], and triazolam [7] are metabolized predominantly by CYP3A4. The authors have recently clarified that etizolam, a close structural analogue of brotizolam, is also metabolized by this enzyme [8]. Regarding the metabolism of brotizolam, one in vitro study [9] has suggested the involvement of CYP3A4, but this has not been confirmed in vivo.
In the present study, to obtain in vivo evidence for the involvement of CYP3A4 in brotizolam metabolism, the effect of erythromycin, an inhibitor of CYP3A4 [2], on the single oral dose pharmacokinetics of brotizolam was examined.
Methods
The subjects were 14 healthy male volunteers. The mean (95% CI) of age was 28.1 (24.9, 31.4) years, and that of body weight was 69.8 (63.4, 76.2) kg. The study protocol was approved by the Ethics Committees of Yamagata University School of Medicine and Hirosaki University School of Medicine, and each subject gave his written informed consent to participate.
The study was conducted in a double-blind randomized crossover manner, with at least a 4-week washout period between the two phases. The subjects were randomly allocated to one of the two treatment sequences, placebo-erythromycin or erythromycin-placebo. Two 200 mg tablets of erythromycin (Erythrocin, Dainippon Pharmaceutical, Osaka, Japan) or matched placebo were given orally at 08.00 h, 12.00 h and 20.00 h for 7 days. After an overnight fast at 09.00 h of the 6th day, two 0.25 mg tablets of brotizolam (Lendormin, Nippon Boehringer Ingelheim, Kawanishi, Japan) were given orally with 100 ml of tap water. No food was allowed for 3 h. Blood samples (10 ml each) were collected into heparinized tubes before and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after brotizolam dosing. Plasma was separated and stored frozen until analyzed. At the same time as the blood samplings, psychomotor function was assessed using the Digit Symbol Substitution Test (DSST) adapted from the Wechsler Adult Intelligence Scale [10] in 90 s, and the Stanford Sleepiness Scale (SSS) [11].
Plasma concentration of brotizolam was measured in duplicate by the column-switching HPLC method of etizolam [12], with some modifications. The lowest limit of detection was 0.3 ng ml−1, and the interassay coefficient of variation was 5.2% at the concentration of 0.5 ng ml−1.
The elimination rate constant (λz) was estimated by the linear regression analysis of the terminal log-linear plasma concentration-time curve. The elimination t1/2 was calculated by 0.693/λz. The area under the plasma concentration-time curve (AUC) from 0 to 24 h (AUC(0, 24 h)) was calculated by the trapezoidal rule. The AUC from 0 h to infinity (AUC(0, ∞)), or total AUC, was calculated by AUC(0, 24 h) + C24/λz, in which C24 is the plasma concentration at 24 h. The peak plasma concentration (Cmax) and time to Cmax (tmax) were determined graphically.
For the DSST and SSS, the area under the score-time curve from 0 to 24 h (AUSC(0, 24 h)) was calculated.
Statistical analyses were performed by the Wilcoxon paired test, and a P value of 0.05 or less was considered significant.
Results
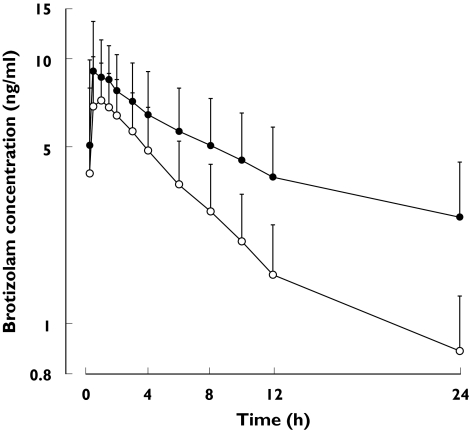
The mean plasma concentration-time data of brotizolam during treatment with placebo or erythromycin are shown in Figure 1.
Figure 1.
Mean plasma concentration-time data of brotizolam after a single oral 0.5 mg dose during the treatment with placebo (○) or erythromycin (•). The bracketed bars indicate standard deviations
Erythromycin treatment significantly increased the Cmax (P < 0.05), total AUC (P < 0.01), and elimination t1/2 (P < 0.01) of brotizolam (Table 1).
Table 1.
Pharmacokinetic and pharmacodynamic parameters of brotizolam after a single oral 0.5 mg dose of brotizolam
| Placebo | Erythromycin | |
|---|---|---|
| Pharmacokinetic parameters | ||
| Cmax (ng ml−1) | 8.2 (6.8, 9.6) | 10.0 (8.6, 11.4) |
| tmax (h) | 0.9 (0.6, 1.3) | 0.8 (0.5, 1.1)* |
| Total AUC (ng ml−1 h) | 79.2 (60.1, 98.4) | 199.9 (163.8, 236.0)** |
| Elimination t1/2 (h) | 9.4 (7.5, 11.3) | 20.7 (16.1, 25.3)** |
| Pharmacodynamic parameters | ||
| AUSC(0, 24 h) of DSST | 2111 (1912, 2310) | 2159 (1969, 2350) |
| AUSC(0, 24 h) of SSS | 58.6 (47.9, 69.3) | 65.4 (56.4, 74.5) |
The values on the table are means (95% CI). Cmax: peak plasma concentration, tmax: time to Cmax, AUC: area under the plasma concentration-time curve, t1/2: half-life, AUSC: area under the score-time curve, DSST: Digit Symbol Substitution Test, SSS: Stanford Sleepiness Scale
P < 0.05
P < 0.01, compared with placebo.
The AUSC(0,24 h) of DSST and SSS were not significantly different between the two phases (Table 1).
Discussion
To examine the effect of an inhibitor of CYP3A4 on the pharmacokinetics of a drug is a useful measure to prove the involvement of this enzyme in the metabolism of that drug. Some change(s) in the pharmacokinetic parameters indicative of metabolic inhibition suggest(s) the involvement of CYP3A4 in the metabolism. The macrolide antibiotic erythromycin is a potent inhibitor of CYP3A4 [2], and widely used for this purpose [3, 6, 7].
In the present study, erythromycin treatment significantly increased the Cmax, AUC, and t1/2 of brotizolam. These results indicate that erythromycin inhibits the metabolism of brotizolam in humans. Therefore, this study provides in vivo evidence for the involvement of CYP3A4 in the metabolism of brotizolam.
The degree of increase in the t1/2 of brotizolam (2.2-fold) was very similar to that of alprazolam (2.5-fold [3]), midazolam (2.4-fold [6]), and triazolam (2.3-fold [7]), which are metabolized predominantly by CYP3A4. However, the degree of increase in the Cmax (1.2-fold) and AUC (2.5-fold) of brotizolam were apparently smaller than those of midazolam (2.7-fold, 4.4-fold [6]) and triazolam (1.8-fold, 3.8-fold [7]), and close to those of alprazolam (no change, 2.5-fold [3]). CYP3A4 is present not only in the liver but also in the intestine, and contributes to first-pass metabolism in many drugs [2]. Midazolam and triazolam undergo extensive first-pass metabolism resulting in the low bioavailability of 30–40% [13]. Meanwhile, the bioavailability of alprazolam is 90% [14] and that of brotizolam is 70% [1], suggesting that these drugs receive modest first-pass metabolism. Therefore, it is likely that the impact of erythromycin on the Cmax and AUC is proportional to the extent of first-pass metabolism of the drug tested.
Erythromycin treatment induced no significant change in the pharmacodynamic parameters of brotizolam, in line with the result of alprazolam [3]. On the other hand, in the studies on midazolam [6] and triazolam [7], psychomotor function was significantly impaired by erythromycin. This pharmacodynamic difference appears to be explained by the differences in changes in the Cmax and AUC of these drugs.
Acknowledgments
Competing interests: None declared.
References
- 1.Langley MS, Clissold SP. Brotizolam. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an hypnotic. Drugs. 1988;35:104–22. doi: 10.2165/00003495-198835020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson GR. Cytochrome P4503A (CYP3A9) metabolism: prediction of in vivo activity in humans. J Pharmacokin Biopharm. 1996;24:475–90. doi: 10.1007/BF02353475. [DOI] [PubMed] [Google Scholar]
- 3.Yasui N, Otani K, Kaneko S, Ohkubo T, Osanai T, Sugawara K, Chiba K, Ishizaki T. A kinetic and dynamic study of oral alprazolam with and without erythromycin in humans: in vivo evidence for the involvement of CYP3A4 in alprazolam metabolism. Clin Pharmacol Ther. 1996;59:514–9. doi: 10.1016/S0009-9236(96)90179-4. [DOI] [PubMed] [Google Scholar]
- 4.Yasui N, Kondo T, Otani K, Furukori H, Kaneko S, Ohkubo T, Nagasaki T, Sugawara K. Effect of itraconazole on the single oral dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology. 1996;139:269–73. doi: 10.1007/s002130050715. [DOI] [PubMed] [Google Scholar]
- 5.Furukori H, Otani K, Yasui N, Kondo T, Kaneko S, Shimoyama R, Ohkubo T, Nagasaki T, Sugawara K. Effect of carbamazepine on the single oral dose pharmacokinetics and pharmacodynamics of alprazolam. Neuropsychopharmacology. 1998;18:364–9. doi: 10.1016/S0893-133X(97)00166-8. [DOI] [PubMed] [Google Scholar]
- 6.Olkkola KT, Aranko K, Luurila H, Hiller A, Saarnivaara L, Himberg JJ, Neuvonen PJ. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt DJ, von Moltke LL, Harmatz JS, Counihan M, Graf JA, Durol AL, Mertzanis P, Duan SX, Wright CE, Shader RI. Inhibition of triazolam clearance by macrolide antimicrobial agents: in vitro correlates and dynamic consequences. Clin Pharmacol Ther. 1998;64:278–85. doi: 10.1016/S0009-9236(98)90176-X. [DOI] [PubMed] [Google Scholar]
- 8.Araki K, Yasui-Furukori N, Fukasawa T, et al. Inhibition of the metabolism of etizolam by itraconazole in humans: evidence for the involvement of CYP3A4 in etizolam metabolism. Eur J Clin Pharmacol. 2004 doi: 10.1007/s00228-004-0789-1. in press. [DOI] [PubMed] [Google Scholar]
- 9.Senda C, Kishimoto W, Sakai K, Nagakura A, Igarashi T. Identification of human cytochrome P450 isoforms involved in the metabolism of brotizolam. Xenobiotica. 1997;27:913–22. doi: 10.1080/004982597240082. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler D. Harcourt Brace Jovanovich. New York NY: 1996. Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- 11.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 12.Hikida K, Inoue Y, Nouchi E, Ohkura Y. Determination of etizolam in human serum or plasma using automated column-switching high-performance liquid chromatography. Jpn J Clin Chem. 1990;19:354–9. [Google Scholar]
- 13.Garzone PD, Kroboth PD. Pharmacokinetics of the newer benzodiazepines. Clin Pharmacokinet. 1989;16:337–64. doi: 10.2165/00003088-198916060-00002. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam: therapeutic implications. Clin Pharmacokinet. 1993;24:453–71. doi: 10.2165/00003088-199324060-00003. [DOI] [PubMed] [Google Scholar]