Abstract
Aims
To study outpatient statin use after first acute myocardial infarction (AMI) in Denmark between 1995 and 2002 and to determine the predictors of statin use.
Methods
This is a nationwide population-based study using administrative registries. Patients with first AMI between 1995 and 2002 older than 30 years of age and alive 6 months after discharge (n = 45 219) were identified through the National Patient Registry. The statins purchased by these patients within 6 months after discharge were determined using the Registry of Medicinal Product Statistics, a nationwide prescription database.
Results
Statin use following AMI increased from 13% in 1995 to 61% in 2002. In 2002, 81% of patients aged 30–64 years used statins. Older patients used fewer statins, but use increased more among patients aged 75–84 years: from 1.3% to 43%. Use in elderly patients did not differ according to gender in 2000–02, but young men used more than younger women. In 2000–02, patients with diabetes (odds ratio (OR): 0.84; 95% confidence interval (CI): 0.74–0.95) and with heart failure (OR: 0.70; 95% CI: 0.64–0.76) were undertreated; this trend was present throughout the period.
Conclusions
In this nationwide study, younger patients after AMI had high statin use in 2002, but high-risk patients such as those with diabetes and heart failure were still being undertreated.
Keywords: acute myocardial infarction, secondary prevention, statins, epidemiology, drug prescription
Introduction
Compelling scientific evidence shows that statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) reduce the risk of recurrent coronary events and improve survival in patients after acute myocardial infarction (AMI) [1–4]. The benefits are clear in several subgroups studied, including by gender, age and the presence of diabetes [5–10].
Nevertheless, surprisingly few patients with coronary heart disease (CHD) use statins. The two European Action on Secondary Prevention by Intervention to Reduce Events (EUROASPIRE) surveys in 1995–96 and 1999–2000 reported that CHD patients 70 years or younger underuse statin across Europe [11]. Both studies involved selected hospitals willing to participate, and hence an even lower proportion could be expected in Europe as a whole. A study of medical practices in the USA with high-volume prescription rates of lipid-lowering drugs showed that 52% of CHD patients of all age groups were prescribed these drugs in 1998 [12].
Each available study indicates that underuse of statin is likely to be widespread. A nationwide study including all hospitals is necessary to avoid the selection bias that may occur if only specialized centres are included. Denmark has unique potential for studying the use of medication on a national scale. All prescription-based dispensing from pharmacies has been systematically registered for each individual since 1995 using a unique civil registration number. This number, used for various administrative purposes, is permanent and assigned to each resident of Denmark. It provides a link to registries allowing each patient's history of hospitalization and medicine use to be tracked longitudinally. In Denmark, statins for AMI patients are strongly subsidized prescription-based drugs, implying that virtually all statins are purchased from pharmacies in Denmark by prescription. We thus studied the outpatient use of statins following hospitalization with first AMI between 1995 and 2002.
Methods
The National Patient Registry contains administrative data for each hospitalization in Denmark since 1978, including all diagnoses and procedures [13]. We used this to establish all cases with AMI as primary or secondary diagnosis (International Classification of Diseases (ICD)-10, codes I21–I22) between 1 July 1995 and 30 June 2002. We only accepted inpatient hospitalizations of patients 30 years or older. To study first hospitalizations with AMI, we further traced the hospitalizations of the selected patients back to 1978, using the ICD-8 code 410 in the period 1978–94. To ensure a similar period of history, each case was only included if the particular patient had not been recorded with an AMI in the preceding 17 years. We studied statin use within 6 months after discharge among patients alive 6 months after discharge to ensure equal potential to buy statins. We allowed for sequential admission to more than one hospital when determining the discharge date. Information on patients’ vital status (dead or alive) was obtained from the Civil Registration System.
We divided the 73 hospitals providing acute care for AMI into three categories. Local community hospitals (n = 35) are typically small hospitals with departments of surgery and medicine and some cardiologists. Main regional hospitals (n = 33) are the central hospitals in Denmark's 16 health care regions with a range of medical specialities, including cardiology. Tertiary cardiac care centres (n = 5) are university-affiliated hospitals with capacity for invasive treatment.
The Registry of Medicinal Product Statistics is a national prescription database on all outpatient pharmacy-dispensed prescription drugs in Denmark. The Registry was established in 1994 to provide complete statistics on the use of drugs in Denmark and is updated monthly. The pharmacies are legally obliged to provide this information, and reimbursement to pharmacies is linked to reporting through the Registry. Data on prescriptions are registered for each patient via the civil registration number. Each prescription record contains the date of purchase, the dispensing pharmacy, the prescribing physician and detailed information on the drug dispensed (anatomical therapeutic classification (ATC) system name, dosage, package size and formulation). The indication for treatment and prescribed daily dose are not registered.
All prescribed statins (ATC: C10AA) purchased by the identified AMI patient population from 1995 to 2002 were obtained from the Registry of Medicinal Product Statistics. Patients were defined as statin users if at least one statin was purchased within 6 months after discharge. This time frame was chosen to account for the statin reimbursement rules in Denmark that endorse at least 3 months of dietary intervention before reimbursement. Antidiabetic drugs and loop diuretics were used to classify the patients in connection with admission; hence a shorter time frame was chosen for these drugs. Insulin or oral antidiabetic drugs (ATC: A10) were used as a proxy for the presence of diabetes, and loop diuretics (ATC: C03C) were used as a proxy for the presence of heart failure. For both drugs, at least one purchase within 3 months before or 1 month after discharge was required.
Predictors of statin use within 6 months after discharge were estimated for nonprior users (patients not using statins 6 months prior to admission). Since both calendar year and gender and calendar year and age interacted significantly, the study period was divided into three periods: 1995–97, 1998–99 and 2000–02. Patients were divided into six age groups: 30–44, 45–54, 55–64, 65–74, 75–84 and ≥85 years. Parameter estimates were adjusted for gender, use of antidiabetic drugs, use of loop diuretics and type of admitting hospital. Multiple logistic regression with generalized estimating equations was used to estimate the model parameters and standard error to account for the effects of clustering at the hospital level [14]. All statistical calculations were performed using SAS software version 8.2 (SAS Institute, Cary, NC, USA).
The Danish Data Protection Agency approved the study. Data were delivered with anonymized but unique personal identification numbers, enabling anonymous linkage between registries on an individual level. According to Danish law, the project did not require approval by the regional committee on scientific ethics.
Results
We identified 61 656 patients with first AMI from 1 July 1995 to 30 June 2002; 16 437 patients died within 6 months after discharge and were excluded. The final cohort consisted of 45 219 patients.
Table 1 shows the patient characteristics. During the 7-year period, the proportion of elderly patients discharged and alive more than 6 months after first AMI increased. One average, women were 71.1 years of age, and men 64.3 years. The proportion of men declined with increasing age; 81% of the patients aged 45–54 years were men vs. 36% among those ≥85 years. The proportion using statins prior to admission reached 7% in 2000–02; of these, more than 94% continued statin use after discharge.
Table 1.
Characteristics of first AMI patients in Denmark in 1995–97, 1998–99 and 2000–02
| 1995–97 | 1998–99 | 2000–02 | |
|---|---|---|---|
| n (% men)* | 15 263 (64.4) | 12 099 (64.8) | 17 857 (62.7) |
| Age groups, n (%) | |||
| 30–44 years | 711 (4.7) | 585 (4.8) | 818 (4.6) |
| 45–54 years* | 2 189 (14.3) | 1 741 (14.4) | 2 339 (13.1) |
| 55–64 years | 3 391 (22.2) | 2 789 (23.1) | 3 873 (21.7) |
| 65–74 years* | 4 424 (29.0) | 3 354 (27.7) | 4 806 (26.9) |
| 75–84 years* | 3 538 (23.2) | 2 795 (23.1) | 4 479 (25.1) |
| ≥85 years* | 1 010 (6.6) | 835 (6.9) | 1 542 (8.6) |
| Concomitant medication (%)† | |||
| Prior statins* | 2.0 | 4.0 | 7.0 |
| Antidiabetic drugs* | 8.9 | 9.5 | 10.7 |
| Loop diuretics | 35.4 | 34.6 | 35.0 |
| Admitting hospital type (%) | |||
| Local community hospital* | 25.1 | 23.1 | 20.9 |
| Main regional hospital* | 60.3 | 60.4 | 61.4 |
| Tertiary cardiac care centre* | 14.6 | 16.6 | 17.8 |
Test for trend in a logistic regression between groups: P < 0.05;
Statin: 6 months before admission. Antidiabetic drugs and loop diuretics: 3 months before and 1 month after admission.
The statins comprised 97.2% of the lipid-lowering drugs used as first choice within 6 months after discharge in 1995–97, 99.1% in 1998–99 and 99.7% in 2000–02.
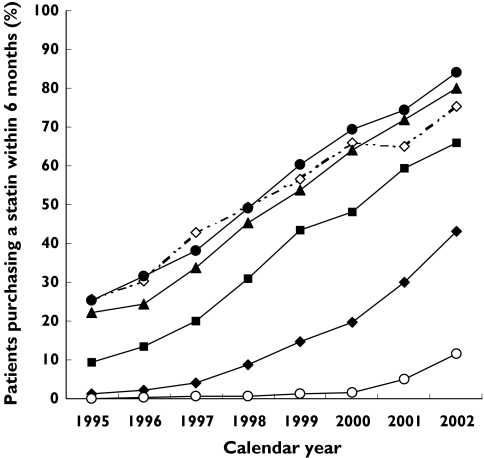
In 1995, 13% of all first AMI patients purchased a statin within 6 months after discharge; this number increased to 61% in 2002 (Figure 1). Patients increased statin use as follows: 30–64 years, from 24% in 1995 to 81% in 2002; 65–74 years, from 9% in 1995 to 66% in 2002; 75–84 years, from 1.3% in 1995 to 43% in 2002; and ≥85 years from 0% in 1995 to 12% in 2002.
Figure 1.
Proportion of first AMI patients who purchased a statin within 6 months after discharge in various age groups from 1995 to 2002. Age groups: 30–44 years (◊), 45–54 years (•), 55–64 years (▴), 65–74 years (▪), 75–84 years (♦), 85+ years (○)
Table 2 shows predictors of statin use among nonprior users. Statin use was strongly and inversely correlated with increasing age. For example, in 2000–02, patients aged 65–74 years were 45% (95% confidence interval (CI) 39–50%) less likely to use statin than the reference group of patients aged 55–64 years.
Table 2.
Predictors of statin use within 6 months after discharge for first AMI for nonprior statin users
| 1995–97 (n = 15 263) | 1998–99 (n = 12 099) | 2000–02 (n = 17 857) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||||
| Age | ||||||||||||
| 30–44 years | 1.42 | (1.16–1.72) | 1.13 | (0.92–1.38) | 0.89 | (0.77–1.03) | ||||||
| 45–54 years | 1.33 | (1.19–1.49) | 1.26 | (1.10–1.43) | 1.22 | (1.09–1.37) | ||||||
| 55–64 years | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||||
| 65–74 years | 0.46 | (0.41–0.52) | 0.59 | (0.52–0.67) | 0.55 | (0.50–0.61) | ||||||
| 75–84 years | 0.06 | (0.05–0.09) | 0.12 | (0.10–0.15) | 0.19 | (0.17–0.21) | ||||||
| ≥85 years | 0.005 | (0.001–0.02) | 0.01 | (0.002–0.02) | 0.02 | (0.02–0.03) | ||||||
| Gender* | ||||||||||||
| Female | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||||
| Male | 0.77 | (0.69–0.85) | 0.79 | (0.72–0.85) | 1.05 | (0.97–1.13) | ||||||
| Concomitant medication† | ||||||||||||
| Anti-diabetic | 0.69 | (0.57–0.85) | 0.77 | (0.65–0.91) | 0.84 | (0.74–0.95) | ||||||
| Loop diuretic | 0.72 | (0.63–0.82) | 0.78 | (0.71–0.86) | 0.70 | (0.64–0.76) | ||||||
| Hospital type‡ | ||||||||||||
| Local community hospital | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||||
| Main regional hospital | 1.16 | (0.92–1.46) | 1.09 | (0.87–1.37) | 1.02 | (0.82–1.26) | ||||||
| Tertiary cardiac care centre | 1.37 | (0.90–2.08) | 1.70 | (1.29–2.26) | 1.14 | (0.86–1.52) | ||||||
OR, odds ratio; CI confidence interval; OR are adjusted for all variables shown
Since gender and age interacted, they were analysed separately (Figure 2);
Non-user of the specific drug as reference;
Test that all three categories within type of hospital are equal: 1995–97, P = 0.21; 1998–99, P < 0.0006; 2000–02, P = 0.65.
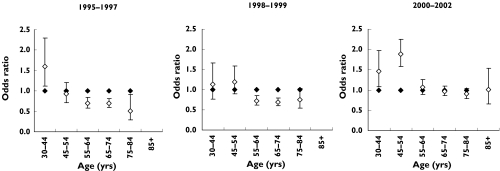
Figure 2 shows odds ratios for statin use by gender within each age group. Among patients 55 years or older, women were more likely to use statins than men in 1995–97 but not in 2000–02. Among patients younger than 55 years of age, the proportion of men using statins increased more rapidly than that of women during the period.
Figure 2.
Odds ratios for statin use within 6 months after discharge among men (women as reference) within each age group, adjusted for use of antidiabetic drugs, use of loop diuretics and type of admitting hospital. There were insufficient observations for the age group ≥85 years in 1995–97 and 1998–99 to calculate odds ratios. Female (♦), male (◊)
Patients using antidiabetic medicine or loop diuretics were less likely to use statins than patients not using these drugs (Table 2). Early in the period, patients discharged from tertiary cardiac care centres tended to use statins more often than patients discharged from local community hospitals; in the middle of the period this trend accelerated, but statin use at the end did not differ according to discharging hospital (Table 2).
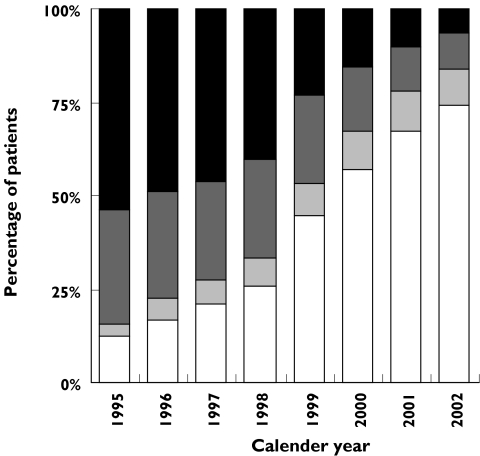
For new statin users (nonprior users who initiated statin treatment after admission), the time elapsed between discharge and the statin purchase decreased markedly during the period (Figure 3). The decrease was pronounced between 1998 and 1999, when the proportion of new users purchasing a statin within 1 week after discharge increased by 73%. A total of 74% of new users purchased their first statin within 1 week in 2002.
Figure 3.
Distribution of time elapsed before first statin purchase after discharge for all new statin users from 1995 to 2002. Time intervals: 3–6 months (▪), 1–3 months (▪), 1–4 weeks (▪), 1 week (□)
The tendency towards earlier statin initiation during the period also reflects the type of physician who prescribed the first statin. In 1995–97, 1594 of the 2379 new users (67%) purchased statin prescribed by hospital physicians and 774 (33%) by general practitioners. In 2000–02, 6576 of the 7943 new users (83%) purchased statin prescribed by hospital physicians and 1315 (17%) by general practitioners.
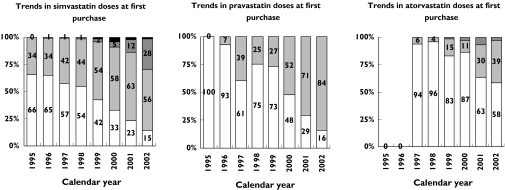
Among the new statin users, simvastatin, pravastatin and atorvastatin were the three most frequently used statins. Simvastatin constituted 87% of the first statin purchases in 1995 and 56% in 2002, pravastatin constituted 5% in 1995 and 35% in 2002 and atorvastatin constituted 6% in 1997 and 10% in 2002. The initial dose of these three statins increased during the period (Figure 4).
Figure 4.
Trends in doses of the three most frequently used statins at first purchase after AMI from 1995 to 2002. 80 mg (▪), 40 mg (▪), 20 mg (▪), 10 mg (□)
Discussion
Statin use increased significantly after first AMI among both men and women of all ages from 1995 to 2002, and the increase did not diminish at the end of the period. Among surviving patients with first AMI aged 30–64 years, 81% received statins within 6 months after discharge in 2002. Our study confirms and expands on previous work on the prescribing of statins for AMI patients [11, 15–17]. We demonstrated that the increase in statin use found in the EUROASPIRE I and II surveys as well as in studies in Denmark seems to have continued [11, 18]. The EUROASPIRE I and II surveys found that the percentage of AMI patients aged ≤70 years receiving statins at least 6 months after discharge increased from 17% in 1995–96 to 59% in 1999–2000. Both studies involved selected hospitals willing to participate. In comparison, our study involved all hospitals, thereby avoiding selection bias, and the proportion of patients aged ≤70 years using statins within 6 months from discharge was 23% in 1995–96, 58% in 1999–2000 and 78% in 2002.
The observed increase in statin use during the period coincides with the publication of several landmark statin trials on secondary prevention after CHD. These include the Scandinavian Simvastatin Survival Study in 1994 [1], the Cholesterol and Recurrent Events Trial in 1996 [2], the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study in 1998 [3] and the Heart Protection Study in 2002 [4]. All these trials showed that statins improved long-term morbidity and mortality after a CHD event and resulted in focus on statins and the implementation of guidelines recommending the use of statins. Further, AMI was redefined in 2000 in Denmark as well as in most of Europe, and this probably resulted in more focus on treatment guidelines. These circumstances presumably account for much of the observed increase in use.
The strength of our study is that it is a nationwide registry-based study of all hospitals in Denmark. Population-based record linkage studies are an efficient method of measuring drug utilization in actual populations, allowing both genders of all ages to be studied, unlike the large clinical statin trials, which have mainly focused on middle-aged men [5]. Our findings reflect the actual statin purchase among outpatients and not just the prescription patterns of physicians, thereby avoiding overestimating statin use when patients do not purchase their prescribed statin.
Subgroup analysis of several trials with statins conducted during the 1990s strongly suggested that therapy to lower concentrations of low-density lipoproteins (LDL) significantly reduces the risk of CHD in older people [5]. But the Heart Protection Study and the Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER) trial, both published in 2002, strongly justify intensive LDL-lowering therapy in older people with established cardiovascular disease, and the Adult Treatment Panel III of the National Cholesterol Education Program in the USA published revised guidelines in 2004 recommending LDL-lowering therapy with statins in older people with established cardiovascular disease [4, 8, 19]. In the PROSPER trial, 5804 patients (2565 with a history of vascular disease and 3239 with risk factors for vascular disease) aged 70–82 years with good cognitive functioning were randomized to pravastatin 40 mg or matching placebo daily for 3.2 years [8]. Pravastatin was well tolerated, and CHD mortality declined significantly. The PROSPER trial criteria indicate no dementia and an expected lifetime of at least 3 years before statin treatment is initiated among patients aged 70–82 years; evidence is still lacking for even older patients. In Denmark, 7% of people aged 65–84 years have dementia, and 76% of our patients aged 70–82 years in 1995–99 survived more than 3 years after the AMI [20]. Based solely on these two criteria, about 70% of patients aged 70–82 years would be eligible for statin treatment. In our study, 52% in this age group used statins in 2002. Age and the likelihood of using statins were inversely related, which other investigators have found [21], but we also found that patients 75 years or older experienced a 33-fold increase in use during the period, making them the age group with the highest relative increase in use.
Elderly women were more likely to receive statins than elderly men early in the period, even though evidence at that point did not support use among elderly women with AMI [22, 23]. At the end of the period, young men used more statins than young women, whereas elderly patients did not differ by gender. A possible reason is that it is men who predominantly experience AMI at younger ages, and hence most clinical trials have focused on men [5]. This has resulted in a lack of evidence that women benefit from statin therapy, and only recently have meta-analyses stated that lipid-lowering significantly reduces CHD mortality as secondary prevention among women [5, 10]. Today, statin therapy after AMI should not differ by gender; this was true for elderly people, but more focus is needed on younger women with AMI.
Our study included the paradox that high-risk patients receive less evidence-based therapy than low-risk patients [21]. AMI patients with (vs. without) diabetes have a substantially higher risk of further cardiovascular events and mortality and benefit significantly from statin therapy [6, 24]. Nevertheless, patients with (vs. without) diabetes used fewer statins after AMI, although this seemed to change in 2000–02, with a trend that young patients with (vs. without) diabetes used more statins (OR 1.31; 95% CI 0.61–2.78). AMI patients with (vs. without) presumed heart failure (using loop diuretics) used fewer statins, which concurs with reports from the USA [12]. Whether or not statins have beneficial effects in patients with ischaemic heart failure remains to be firmly established, but several studies have shown beneficial effects in this group. Horwich et al.[25] studied a cohort of 551 patients with systolic heart failure and found that statin therapy was associated with improved survival in both nonischaemic and ischaemic patients at 1-year follow-up. Subgroup analyses of the Cholesterol and Recurrent Events Trial revealed that pravastatin significantly reduces coronary events among patients with an ejection fraction between 26% and 40%[2]. A retrospective analysis of the Evaluation of Losartan in the Elderly II (ELITE II) trial found that patients with symptomatic heart failure who received statin therapy had significantly lower mortality than did patients not on statin therapy [26]. There seems to be great potential for improving statin therapy among heart failure patients.
Initiating early in-hospital statin therapy has been found to reduce mortality and increase statin compliance among CHD patients [27–30]. Although no guidelines in Denmark recommend early statin initiation among CHD patients and the reimbursement rules endorse 3 months of dietary intervention before reimbursement, the proportion of prescriptions dispensed by hospital physicians increased and the time elapsed between discharge and the first statin purchase decreased, indicating that in-hospital initiation increased during the period. The decrease in time elapsed was especially marked from 1999, when the statin reimbursement rules were eased such that the physician no longer needed to complete an individual reimbursement application.
The Danish Medicines Agency reports that 47% of all statin users in Denmark in 2002 used simvastatin, 13% pravastatin and 36% atorvastatin. This is in contrast to our findings that 56% used simvastatin, 35% used pravastatin and 10% used atorvastatin in 2002. We cannot explain this difference, but hospital physicians and general practitioners seem to differ in preference of type of statin. Since our data do not indicate whether the general practitioner chose statin type after recommendation from the hospital, we cannot meaningfully investigate whether hospital physicians and general practitioners differ in the type of statin used as first choice.
Among the simvastatin users, only 29% purchased 40 mg or more as the initial dose in 2002, the dose reducing mortality significantly in the Heart Protection Study [4]. However, 82% of pravastatin users purchased 40 mg as the initial dose in 2002, the dose reducing mortality in the LIPID study [3].
The validity of registry studies depends on the quality and completeness of data. The validity of the AMI diagnosis in the National Patient Registry has been found to be high, but this is not the case for the coding of the secondary diagnoses of diabetes and heart failure, so we chose to use antidiabetic drugs as a proxy for diabetes and loop diuretics as a proxy for heart failure [31]. Applying antidiabetic drug use as a proxy for diabetes covers at least 85% of patients with diabetes, and this method is highly valid [32]. In Denmark, 37% of AMI patients had signs of congestive heart failure [33]. Only 8.5% of our study population had a diagnosis of heart failure (ICD-10 I50) together with the AMI, whereas 35% of our population used loop diuretics 3 months before or 1 month after admission. Eighty-three per cent of the heart failure patients used loop diuretics. Thus, we find that using loop diuretics as a proxy for heart failure is acceptable, although it somewhat underestimated the number of heart failure patients.
The Registry of Medicinal Product Statistics relies on data collected at the pharmacy level in a similar automatic fashion nationwide. Denmark has several regional prescription databases receiving similar data from the pharmacies. These databases are very complete [34]. Further, the dispensed dose is highly concordant with the daily dose prescribed, which is important since the registry does not contain this information [15]. Experience from similar databases demonstrates that automated dispensing data are clearly superior to any data relying on patient recall, since they are collected when the drug is purchased and are therefore not influenced by patient recall bias [35]. We assume that the patients ingested the purchased statin: one reason is that patients in all cases pay about 25% of the price of the drug.
We have no information about the cholesterol level of each patient and therefore do not know how many patients would qualify for statin treatment. However, a study [16] showed that 76% of AMI patients discharged alive in Denmark in 1993–97 qualified for lipid-lowering treatment because total cholesterol exceeded 5.4 mmol l−1. This number is even higher today, since Denmark's guidelines from 1998 to 2002 recommended giving statins to CHD patients when total cholesterol exceeded 5.0 mmol l−1 and from 2002 when total cholesterol exceeds 3.5 mmol l−1. A trial of patients hospitalized for acute coronary syndrome [36] showed that reducing LDL to 1.6 mmol l−1 using 80 mg of atorvastatin vs. a reduction to 2.5 mmol l−1 using 40 mg of pravastatin further reduced the hazard ratio by 16% for the primary end-point, which was a composite of death from any cause and cardiovascular events. This trial (together with especially the Heart Protection Study has shown that practically all CHD patients benefit from statin therapy regardless of their initial cholesterol level [4].
In conclusion, this nationwide study of statin use among all first AMI outpatients in Denmark in 1995–2002 found that the proportion of patients aged 30–64 years who purchased a statin within 6 months after discharge reached a very high level in 2002. Given the clinical evidence of the benefit of statin therapy among elderly AMI patients, the statin use in this group was still low, although increasing rapidly [8]. Elderly patients did not differ by gender in 2000–02, but younger men were more likely to use statins than younger women. Patients with diabetes and heart failure patients are being undertreated, and more focus is needed on these high-risk AMI patients.
Acknowledgments
An unrestricted research grant from the Danish Pharmaceutical Association (grant number 31–03) supported this study.
References
- 1.Pedersen TR, Kjekshus J, Berg K, et al. Randomized Trial of Cholesterol-Lowering in 4444 Patients with Coronary-Heart-Disease – the Scandinavian Simvastatin Survival Study Group. Lancet. 1994;344(4S):1383–9. [PubMed] [Google Scholar]
- 2.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wunn CC, Davies BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 4.MRC/BHF. Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 5.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 6.MRC/BHF. Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 7.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study. Diabetes Care. 1997;20(4S):614–20. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. The Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 9.Williams MA, Fleg JL, Ades PA, Chaitman BR, Miller NH, Mohiuddin SM, Ockene IS, Taylor CB, Wenger NK. Secondary prevention of coronary heart disease in the elderly (with emphasis on patients ≥75 years of age): An American Heart Association Scientific Statement From the Council on Clinical Cardiology Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2002;105:1735–43. doi: 10.1161/01.cir.0000013074.73995.6c. [DOI] [PubMed] [Google Scholar]
- 10.Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291:2243–52. doi: 10.1001/jama.291.18.2243. [DOI] [PubMed] [Google Scholar]
- 11.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U, Sans S, Ambrosio GB, Amouyel P, Cokkinos D, Deckers JW, Erhardt L, Graham I, Gutzwiller F, Keber I, Keil U, Lehto S, Ostor E, Pajak A, Sans S, Simon J, Turk J, Wood D, Wood DA, Schofield B, Bowker T, Ingham J, McLennan N, Kotseva K, Gollapalli M, Valay R, De Backer G, De Bacquer D, James D, Mackness M, Simon J, Linhartova K, Hafman P, Mayer O, Rosolova H, Bocek F, Hrncarek M, Lupinek P, Marek T, Plaskova M, Skodova Z, Cifkova R, Lehto S, Lehto R, Kemppainen A, Koukkunen H, Luukkonen J, Puhakka M, Karkkainen K, Savolainen K, Amouyel P, Montaye M, Lemaire B, Danet S, Domanievicz R, Lenoir M, Beauchant S, Dusart A, Bonte F, Fievet N, Poissonnier L, Ledoux P, Marecaux N, Steclebout C, Keil U, Liese A, Broxtermann U, Heimbach M, Heidrich J, Kalic M, Breithardt G, Enbergs A, Kerber S, Scheld H, Roeder N, Kleine-Katthofer P, Assmann G, Ostor E, Borbas S, Podmaniczky M, Ruzsanyi T, Janosi A, Belatiny-Kenez A, Bradak A, Adam Z, Barczy G, Birtalan K, Gallai I, Ambrosio GB, Leprotti C, Zardini P, Rossi L, Gallo A, Tavella D, Stritoni P, Pedrocco A, Perissinotto F, Vanuzzo D, Pilotto L, Deckers JW, Post F, Jansen C, Veerhoek M, Boer A, Stockx E, van de Berg R, Remme WJ, van Vliet R, Vos J, van der Knaap M, Turk J, Keber I, Marn K, Salapura V, Skof E, Span E, Sans S, Paluzie G, Perez I, Puig T, Varas C, Balana L, Lopez FN, Sanz G, Ferrer C, de Luna AB, Caralps JM, Dominguez M, Monras P, Rey M. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. Lancet. 2001;357:995–1001. doi: 10.1016/s0140-6736(00)04235-5. [DOI] [PubMed] [Google Scholar]
- 12.Sueta CA, Massing MW, Chowdhury M, Biggs DP, Simpson RJ., Jr Undertreatment of hyperlipidemia in patients with coronary artery disease and heart failure. J Card Fail. 2003;9:36–41. doi: 10.1054/jcaf.2003.5. [DOI] [PubMed] [Google Scholar]
- 13.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–8. [PubMed] [Google Scholar]
- 14.Urbach DR, Baxter NN. Does it matter what a hospital is ‘high volume’ for? Specificity of hospital volume–outcome associations for surgical procedures: analysis of administrative data. BMJ, doi: 10.1136/bmj.38030.642963.AE (published 12 March 2004) [DOI] [PMC free article] [PubMed]
- 15.Kanstrup H, Lassen JF, Heickendorff L, Lauritzen T, Larsen ML. Quality of lipid-lowering therapy in patients with ischaemic heart disease: a register-based study in 3477 patients. J Intern Med. 2004;255:367–72. doi: 10.1111/j.1365-2796.2003.01299.x. [DOI] [PubMed] [Google Scholar]
- 16.Larsen J, Andersen M, Bjerrum L, Kragstrup J, Gram LF. Insufficient use of lipid-lowering drugs and measurement of serum cholesterol among patients with a history of myocardial infarction. J Cardiovasc Risk. 2003;10:61–4. doi: 10.1097/01.hjr.0000048740.87586.f8. [DOI] [PubMed] [Google Scholar]
- 17.Reid FDA, Cook DG, Whincup PH. Use of statins in the secondary prevention of coronary heart disease: is treatment equitable? Heart. 2002;88:15–9. doi: 10.1136/heart.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riahi S, Fonager K, Toft E, Hvilsted-Rasmussen Lars, Bendsen Jorgen, Paasker Johnsen Soren, Toft Sorensen Henrik. Use of lipid-lowering drugs during 1991–98 in Northern Jutland, Denmark. Br J Clin Pharmacol. 2001;52:307–11. doi: 10.1046/j.0306-5251.2001.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Merz CNB, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 20.Andersen K, Lolk A, Nielsen H, Kragh-Sorensen P. Prevalence and incidence of dementia in Denmark. The Odense study. Ugeskr Laeger. 2000;162:4386–90. [PubMed] [Google Scholar]
- 21.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291:1864–70. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 22.Feely J, McGettigan P, Kelly A. Growth in use of statins after trials is not targeted to most appropriate patients. Clin Pharmacol Therapeutics. 2000;67:438–41. doi: 10.1067/mcp.2000.105152. [DOI] [PubMed] [Google Scholar]
- 23.Savoie I, Kazanjian A. Utilization of lipid-lowering drugs in men and women: a reflection of the research evidence? J Clin Epidemiol. 2002;55:95–101. doi: 10.1016/s0895-4356(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 25.Horwich TB, Maclellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–8. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Segal R, Pitt B, Wilson PP, Sharma D, Bradstreet DC, Ikeda LS. Effects of HMG-COA reductase inhibitors (statins) in patients with heart failure (Abstract) European J Heart Failure. 2000;2(Suppl 1):96. [Google Scholar]
- 27.Fonarow GC, Ballantyne CM. In-hospital initiation of lipid-lowering therapy for patients with coronary heart disease: the time is now. Circulation. 2001;103:2768–70. doi: 10.1161/01.cir.103.23.2768. [DOI] [PubMed] [Google Scholar]
- 28.Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001;285:430–6. doi: 10.1001/jama.285.4.430. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 30.Muhlestein JB, Horne BD, Bair TL, Li Q, Madsen TE, Pearson RR, Anderson JL. Usefulness of in-hospital prescription of statin agents after angiographic diagnosis of coronary artery disease in improving continued compliance and reduced mortality. Am J Cardiol. 2001;87:257–61. doi: 10.1016/s0002-9149(00)01354-0. [DOI] [PubMed] [Google Scholar]
- 31.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56:124–30. doi: 10.1016/s0895-4356(02)00591-7. [DOI] [PubMed] [Google Scholar]
- 32.Drivsholm TB, Frederiksen K, de Fine ON, Odegaard B, Kristensen JK. The prevalence of diabetes in Denmark. Development of a method for a registry-based assessment. Ugeskr Laeger. 2003;165:2887–91. [PubMed] [Google Scholar]
- 33.Kober L, Torp-Pedersen C. Clinical characteristics and mortality of patients screened for entry into the Trandolapril Cardiac Evaluation (TRACE) study. Am J Cardiol. 1995;76:1–5. doi: 10.1016/s0002-9149(99)80791-7. [DOI] [PubMed] [Google Scholar]
- 34.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–8. [PubMed] [Google Scholar]
- 35.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 36.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]