Abstract
Aim
The aim of this study was to investigate the impact of a pharmacist-led pharmaceutical care programme, involving optimization of drug treatment and intensive education and self-monitoring of patients with heart failure (HF) within the United Arab Emirates (UAE), on a range of clinical and humanistic outcome measures.
Methods
The study was a randomized, controlled, longitudinal, prospective clinical trial at Al-Ain Hospital, Al-Ain, UAE. Patients were recruited from the general medical wards and from cardiology and medical outpatient clinics. HF patients who fulfilled the entrance criteria, and had no exclusion criteria present, were identified for inclusion in the study. After recruitment, patients were randomly assigned to one of two groups: intervention group or control group. Intervention patients received a structured pharmaceutical care service while control patients received traditional services. Patient follow-up took place when patients attended scheduled outpatient clinics (every 3 months). A total of 104 patients in each group completed the trial (12 months). The patients were generally suffering from mild to moderate HF (NYHA Class 1, 29.5%; Class 2, 50.5%; Class 3, 16%; and Class 4, 4%).
Results
Over the study period, intervention patients showed significant (P < 0.05) improvements in a range of summary outcome measures [AUC (95% confidence limits)] including exercise tolerance [2-min walk test: 1607.2 (1474.9, 1739.5) m·month in intervention patients vs. 1403.3 (1256.5, 1549.8) in control patients], forced vital capacity [31.6 (30.8, 32.4) l·month in the intervention patients vs. 27.8 (26.8, 28.9) in control patients], health-related quality of life, as measured by the Minnesota living with heart failure questionnaire [463.5 (433.2, 493.9) unit·month in intervention patients vs. 637.5 (597.2, 677.7) in control patients; a lower score in this measure indicates better health-related quality of life]. The number of individual patients who reported adherence to prescribed medications was higher (P < 0.05) in the intervention group (85 vs. 35), as was adherence to lifestyle advice (75 vs. 29) at the final assessment (12 months). There was a tendency to have a higher incidence of casualty department visits by intervention patients, but a lower rate of hospitalization.
Conclusions
The research provides clear evidence that the delivery of pharmaceutical care to patients with HF can lead to significant clinical and humanistic benefits.
Keywords: Heart Failure, clinical pharmacy, pharmaceutical care, health outcomes, RCT
Introduction
It has been estimated that the prevalence of heart failure (HF) doubles with each decade of ageing. In contrast to other cardiovascular diseases, the incidence of HF has increased over the past 20 years because more patients are surviving after acute myocardial infarction and the population is getting older [1]. Even with intensive medical interventions, mortality remains high in HF patients. Many patients with more severe forms of the disease are seriously disabled, requiring intensive follow-up and repeated hospitalizations [2]. An important goal, therefore, is the development of a holistic approach to HF management, including both pharmacological and nonpharmacological interventions that can improve the quality of life and control symptoms in these patients [3].
The primary means of improving disease management in patients with HF are via optimization of pharmacological therapy and improving patients’ willingness to comply with the prescribed treatments and lifestyle advice, all of which require significant cooperation with and participation of patients [4]. From a pharmacological perspective, the optimal use of ACE inhibitors, diuretics, β-blockers and vasodilators improve the heart failure patient's health-related quality of life and, in the case of ACE inhibitors, extend life [5]. Qualitative research has, however, revealed general patient confusion with regards to their medication and a lack of understanding of the HF condition [6–8]. Furthermore, the physical and mental lack of energy, which HF patients can feel, may cause such patients to believe that neither they nor their therapeutic management can influence their life situation and there is a risk that these patients become resigned to poor health [9]. In order to help HF patients get out of this vicious circle of limitation and resignation, it is important that healthcare providers, having ensured that therapy is optimized, teach patients self-care and describe the possibilities that exist in everyday life [10]. Education/counselling should focus on the benefits to be gained from adherence to prescribed medications and on helping the patient to develop a healthy diet (in particular restricted fluid and salt intake), to stop smoking (if applicable) and to become involved in an exercise programme. These latter lifestyle adjustments can be particularly challenging, since they may conflict with patient desires, traditions and culture [11]; however, effective counselling will help alleviate anxiety over performing daily activities that might provoke shortness of breath and patients can, for example, be encouraged to increase the distance walked over several months while monitoring their symptoms [12].
There is an imperative for hospital-based clinical pharmacists to become involved in the management of patients with chronic illness though the process of pharmaceutical care provision. Pharmaceutical care has been defined as the responsible provision of drug therapy for the purpose of achieving definite outcomes that improve a patient's quality of life [13]. It involves collaboration between healthcare professionals, working together with the patient in designing, implementing and monitoring a therapeutic plan together with patient education on their medications and disease state. A number of studies have shown benefits from clinical pharmacist involvement in the management of heart failure patients [14–18]. These studies, which were carried out in the USA, Canada, Australia and the UK, with one exception [15] have looked at specific aspects of heart failure patient care, e.g. prescribing of ACE inhibitors or home care, rather than a comprehensive approach to both interventions and clinical plus humanistic outcomes.
In the United Arab Emirates (UAE), the country in which the present study was performed, clinical pharmacy services are at an early stage of development. The aim of this study was to investigate the following hypothesis, as it relates to HF patients in the UAE, within a randomized controlled clinical trial: the introduction of a clinical pharmacy programme involving optimization of drug treatment and intensive education and self-monitoring of patients with congestive heart failure, either prior to hospital discharge or during hospital outpatient clinic appointments, will improve the outcome of therapy as determined by objective measures of disease control, quality of life and utilization of healthcare facilities.
Methods
Study design
The study was a randomized, controlled, longitudinal, prospective clinical trial. The study was approved by the Research Ethical Committee, Faculty of Medicine, Emirates University, UAE.
The study site was Al-Ain Hospital, Al-Ain, UAE, a 450-bed facility. Patients were recruited from the generalmedical wards and from cardiology and medical outpatient clinics. The research pharmacist (A.S.) made personal contact with each of the consultant physicians responsible for these various areas within the hospital, explaining the objectives of the study and how the study would be conducted (summary of the study protocol).
Sample size
In a similar study carried out in the UK by Varma et al. [15], statistically significant improvements were found for the Minnesota Living with Heart Failure (MLHF) questionnaire and for hospitalization rates as a result of a pharmaceutical care intervention. A sample size calculation based on variability of data for the MLHF questionnaire obtained in the latter study, indicated that to detect a 10-point difference in this measure (at P = 0.05 and a power of 80%) a sample size of 38 patients per group (intervention and control) was required. Additionally, a multidisciplinary study, which addressed home care of HF patients released from hospital, found significant improvements in a range of outcome measures with a total sample size of 200 patients [19]. Both studies covered a period of 12 months, the study period chosen for the present research. Based on these data, to ensure sufficient statistical power, a target sample size of 200 patients (100 control and 100 intervention) was selected for the present study.
Study subjects
The study entrance criteria were as follows: confirmed diagnosis of HF (by a hospital consultant), cognitive status [score >6 as assessed by the Clifton Assessments Procedures for the Elderly (CAPE) survey] and hospital consultant consent to patient entering trial. The exclusion criteria were: significant airways disease, e.g. chronic obstructive airways disease and severe mobility problems due to other causes, e.g. osteoarthritis [since both these parameters would influence forced vital capacity (FVC) and walk tests used as outcome measures in the study]. HF patients who fulfilled the entrance criteria, and who had no exclusion criteria present, were identified for inclusion in the study. Eligible patients were informed verbally about the study, provided with additional written information and if willing to participate were asked to sign a consent form. If they were unable to sign the consent form by themselves, their next of kin or their caregivers were asked to sign on their behalf.
After recruitment, patients were randomly assigned to one of two groups: intervention group or control group. The randomization was carried out using the minimization method described by Gore [20]. Both groups were matched as closely as possible, for the following parameters: severity of HF (NYHA Grade I–IV), renal function (serum creatinine ≥200 µmol l−1 or <200 µmol l−1), other concomitant illness and cognitive status (CAPE survey score).
Baseline measurements and assessments
Baseline measurements were performed by a research pharmacist (A.S.) with the exception of the 2-min walk test and the FVC test, which were performed by nursing staff or a pharmacy technician. They were blinded regarding the group to which individual patients had been assigned and received training on test administration. Nursing staff also helped in the collection of serum creatinine data, ensuring that these was placed in each patient's chart prior to randomization. Each patient's physician was asked to grade the degree of the heart failure according to the NYHA classification if the information was not present in the patient's chart. In addition to recording the matching parameters mentioned above, baseline assessment involved evaluation of each patient's health-related quality of life (the MLHF Questionnaire [21, 22] and the SF36 [23]). These tests were purposefully chosen, i.e. one disease-specific questionnaire and one generic questionnaire, as recommended by Sneed et al. [10]. The questionnaires were either self-completed by the patient, or interviewer-administered by the research pharmacist (according to a strict protocol on questionnaire administration), depending on the patient's needs. Arabic versions of the questionnaires were used when the patient was unable to understand the English version.
An objective measure of functional status and exercise tolerance was obtained using a 2-min walking test [23, 24]. Patients were told to walk at a brisk pace as far as they could within the allotted time. The distance covered in 2 min was measured, as described previously by Lipkin et al. [25]. To help minimize variations in the test results, the same physical area was used each time and standardized directions were given to patients as directed by Guyatt et al. [26]. The test was a challenge for some of the more debilitated patients, but an attempt to perform the test with each patient was undertaken and the results recorded. During the 2-min walk test, the times taken to walk 25 and 50 m were also recorded [27]. Baseline assessment also involved recording body weight, pulse and blood pressure (BP). These readings were noted from the patient's medical chart.
FVC was measured at baseline for each patient using a portable Vitalograph Compact® spirometer (Vitalograph Ltd., Buckingham, UK); this was used as a measure of pulmonary oedema [26]. In addition, each patient was interviewed by the research pharmacist to obtain demographic details and information on medication knowledge and usage and to record symptoms. This information was collected using a structured questionnaire, prepared and developed for this study based on previous work in Belfast [15]. In hospitalized patients, these baseline assessments were carried out prior to discharge, when the patient's condition had stabilized. Outpatients were assessed during a scheduled clinic visit.
Pharmaceutical care interventions
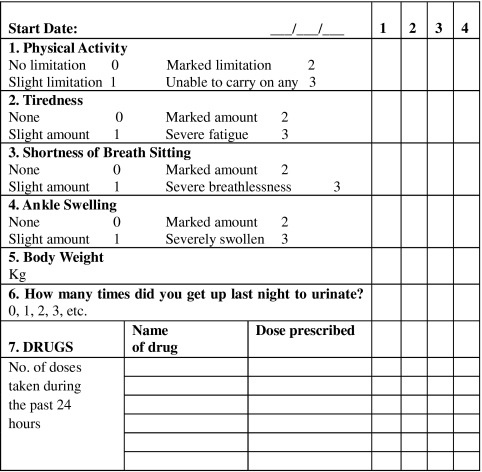
For all patients randomized to the intervention group, the research pharmacist had discussions with their physicians regarding drug therapy if rationalization of therapy or simplification of dosage regimens were deemed appropriate. This involved reference to the treatment algorithm used in a previous pharmaceutical care study in the UK [15]. Intervention patients were also educated (in a structured fashion) on HF, their prescribed medication and the management of HF symptoms by the research pharmacist. A printed booklet developed for this type of education programme [15] was used and each patient was given a copy to take home. The booklet contained information on HF, its symptoms, the aims of treatment, the types of medication used and their possible side-effects, diet and lifestyle changes, advice to stick to one brand of digoxin (it having a narrow therapeutic index) and information on the action to take if doses of medication were missed. Intervention group patients were also instructed on a self-monitoring programme (signs and symptoms of HF; compliance with prescribed medication) in which they were asked to become involved; a monitoring diary card (covering 1 month) was used for this purpose (Figure 1).
Figure 1.
Diary card for patient self-monitoring, covering a period of 1 month (first 4 days only shown)
Intervention patients were asked to complete their monitoring diary cards at home and to show them to their physicians when attending an appointment. The patients were asked to return their completed diary cards to the research pharmacist for review when they visited the hospital to receive medication refills. Reinforcement of the educational message was carried out by the pharmacist as deemed necessary. Copies that had been translated into Arabic were used when the patient could not grasp English thoroughly. The patients were also asked to record their weight daily in their diary card. An additional point for the intervention group patients was that they were instructed to take an extra dose of their diuretic and to contact their physician immediately if their weight increased by 3 kg over 48 h or if there was a marked deterioration in their HF signs/symptoms, in particular increased shortness of breath or increased ankle swelling. Intervention group patients were also asked to perform daily exercise (walking). Each intervention group patient's physician was contacted by telephone by the research pharmacist to discuss the self-monitoring programme that had been introduced.
Control group patients received traditional management, i.e. excluding counselling and education by the research pharmacist, self-monitoring, pharmacist liaison with physicians, etc. Both groups of patients were asked to return to a hospital outpatient clinic at their scheduled appointment intervals followed by the hospital (3-month intervals) to allow follow-up assessments of their HF to be performed.
Outcome measures
At the 3-monthly outpatient clinics, both groups of patients were assessed as per initial baseline assessments as follows: 2-min walk test (including time to walk 25 and 50 m), BP, body weight, pulse, FVC, quality of life questionnaires (MLHF questionnaire and the SF36), questionnaire on symptoms and knowledge of, and compliance with, prescribed medication and lifestyle advice. Medication knowledge was scored as a percentage value relating to the number of correct answers given to questions on name of prescribed medications, daily dosage, strength, purpose of each medication and significant side effects. A score of <50% was deemed to be poor knowledge. In relation to compliance with prescribed medications, patient self-report on missing doses or taking extra doses of their medication, without medical advice to do so, was considered noncompliance. Regarding compliance with lifestyle advice, questions on the following were asked to each patient: dietary modification and sodium restriction, limitation of or abstinence from alcohol, restricted fluid intake, not sleeping flat, taking mild to moderate exercise and smoking cessation (if appropriate). Each parameter was awarded one mark – if the patient received a score of ≥75%, they were graded as being compliant with lifestyle advice.
Data analysis
Data collected for patients were coded and entered into a spreadsheet using Microsoft Excel® software. Data cleaning was carried out by double-checking of all entries. Once data cleaning was completed, the data were exported into SPSS (SPSS Inc, Chicago, USA) for statistical analysis. Summary measures, i.e. area under the parameter–time curve, were used where possible in the data comparisons. Outcomes for intervention and control group patients were compared as follows: 2-min walk test (including time to walk 25 and 50 m), BP, pulse, and FVC results were compared using the area under the curve (AUC) method (independent samples t-test). In addition, the independent sample t-test was used in the statistical comparison of the mean values at 0, 3, 6, 9 and 12 months.
Quality of life questionnaire (MLHF questionnaire and the SF36) scores were compared using the AUC method (independent samples t-test). In addition, the Mann–Whitney U-test was used to compare mean scores at 0, 3, 6, 9 and 12 months. Responses from the questionnaire on symptoms were compared using the Mann–Whitney U-test or the Chi2-test as appropriate for data obtained at 0 and 12 months. The explore-descriptive test (SPSS) was used to measure the confidence intervals for each parameter. A P-value of <0.05 was considered statistically significant.
Results
A total of 221 HF patients (109 intervention; 112 control) were recruited into the study. Two patients in each group died during the study; in addition, three patients withdrew from the intervention group and six from the control group during the study, meaning that a total of 104 patients in each group completed the 12-month follow-up study. The patients were generally suffering from mild to moderate heart failure (NYHA Class 1, 29.5%; Class 2, 50.5%; Class 3, 16%; and Class 4, 4%). Demographic details of the patients who completed the study are summarized in Tables 1 and 2. It is clear from these data that the minimization technique led to good matching of the intervention and control patients.
Table 1.
The number and ages of the recruited patients who completed the study
| Intervention | Control | |||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| Number of patients recruited from wards | 16 | 18 | 34 | 21 | 25 | 46 |
| Number of patients recruited from outpatient clinics | 36 | 34 | 70 | 31 | 27 | 58 |
| The total number of patients in the study | 52 | 52 | 104 | 52 | 52 | 104 |
| Mean age (years) | 58.6 | 58.5 | 58.6 | 58.8 | 58.6 | 58.7 |
| Age group (< 50) | 19 | 17 | 36 | 18 | 18 | 36 |
| (50–65) | 25 | 19 | 44 | 22 | 21 | 43 |
| (> 65) | 8 | 16 | 24 | 12 | 13 | 25 |
Table 2.
Disease status of heart failure patients who completed the study
| Disease status | Intervention | Control |
|---|---|---|
| Ischaemic heart disease (total) | 54 | 49 |
| Hypertension (total) | 24 | 24 |
| Ischaemic heart disease and hypertension | 5 | 5 |
| Diabetes (total) | 18 | 20 |
| Diabetes and ischaemic heart disease | 3 | 7 |
| Diabetes and hypertension | 1 | 2 |
| Cardiomyopathy | 1 | 2 |
| Mitral valve disease | 15 | 21 |
Two-minute walk test
The results for the 2-min walk test are presented in Table 3. As can be seen from the table, the mean data for the intervention patients increased over time, while the results for the control patients stayed approximately constant. Statistical significance was reached for the improvement in intervention patient data at the 6-month, 9-month and 12-month follow-up periods (P < 0.05; df = 197). The AUC summary data for this parameter were also statistically significant (P < 0.05; df = 197). Three patients from the intervention group and six patients from the control group did not feel well enough to undertake the test at each interval. As mentioned in Methods, the time taken to walk 25 and 50 m was also recorded. These proved to be less sensitive measures, and although there were trends within the data towards improved performance in the intervention group, at only one time point (6 months for both the 25-m and the 50-m results) were there statistically significant differences between the two groups (25 m, P < 0.05, df = 173; 50 m, P > 0.05, df = 172); AUC data for the 25- and 50-m time tests did not reach statistical significance.
Table 3.
Mean distance walked (metres) in 2 min by the intervention group and control group patients at each of the assessment periods
| Group | Baseline (m) | 3 months (m) | 6 months (m) | 9 months (m) | 12 months (m) | AUC (m·month) |
|---|---|---|---|---|---|---|
| Intervention (95% confidence limits) | 124.0 (113.2, 134.8) | 128.8 (117.8, 139.8) | 136.1 (125.0, 147.2) | 138.5 (127.3, 149.7) | 140.2 (128.9, 151.5) | 1607.2 (1474.9, 1739.5) |
| Control (95% confidence limits) | 120.8 (108.2, 133.4) | 118.5 (106.1, 130.9) | 114.6 (102.4, 126.8) | 115.4 (103.4, 127.4) | 117.2 (105.2, 129.6) | 1403.2 (1256.5, 1549.8) |
| P-value | 0.702 | 0.212 | 0.011 | 0.006 | 0.001 | 0.043 |
Based on 101 intervention patients and 98 control patients at all time points (df = 197).
Forced vital capacity (FVC; l)
At baseline assessment, intervention group and control group patients exhibited approximately the same FVC data [mean (95% confidence limits)] of 2.4 l (2.2, 2.6) for the intervention patients and 2.3 l (2.2, 2.4) for the control patients. Over time the intervention patients showed improved performance in this test, with statistically significant improvements when compared with control patients at 6, 9 and 12 months. The AUC data for this parameter also indicated a statistically significant improvement (over control group data), i.e. 31.6 (30.8, 32.4) for the intervention group vs. 27.8 (26.8, 28.9) for the control group (P < 0.05, df = 206).
Blood pressure and pulse
There were no statistically significant differences in systolic BP, diastolic BP and pulse between the two groups at baseline; however, all parameters were improved (P < 0.05, df = 206) over time in the intervention patients from the 3-month assessment onwards. Mean data at 12 months for each of the parameters for intervention and control groups respectively were: systolic BP 122.1 (120.7, 123.5) vs. 127.1 (124.9, 129.4); diastolic BP 81.5 (80.3, 82.7) vs. 86.6 (84.6, 88.5); pulse 75.3 (74.6, 76.1) vs. 82.9 (81.5, 84.2). Summary AUC data were also improved in the intervention patients (P < 0.05, df = 206).
Quality of life questionnaires
Minnesota Living with Heart Failure Questionnaire (MLHFQ)
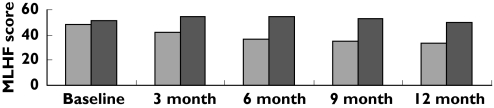
Data analysis using the nonparametric Mann–Whitney U-test revealed that although the data were equivalent at baseline, the intervention group patients tended to score lower on this questionnaire compared with control group patients throughout the remainder of the study period (P < 0.05), indicating that their heart failure was not preventing them from living as they wanted, to as great an extent as for control group patients (Figure 2). The summary measure (AUC) for this parameter was also statistically significantly lower in the intervention group [463.5 (433.2, 493.9)]vs. the control group [637.5 (597.2, 677.7)] (P < 0.05, df = 206).
Figure 2.
Mean Minnesota Living with Heart Failure questionnaire scores for intervention group and control group patients at each of the assessment periods. Intervention ( ), control (
), control ( )
)
SF36 questionnaire
Intervention group patients, in general, scored higher on this questionnaire in each of the eight domains compared with control group patients, indicating that the health-related quality of life of the intervention group patients was not affected by their health to as great an extent as for control group patients. The data for the different domains of the SF36 questionnaire are presented in Table 4. Summary measure data (AUC) indicated that intervention groups achieved significantly higher scores (P < 0.05, df = 206) for all domains except general health and physical functioning (P > 0.05, df = 206).
Table 4.
Summary of SF36 data for the different quality of life domains
| Baseline | 3 months | 6 months | 9 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | I | C | I | C | I | C | I | C | I | |
| Physical functioning | 49.79(44.9, 55.3) | 52.6(47.4, 57.4) | 49.5(44.8, 55.1) | 57.1*(51.9, 61.9) | 51.4(46.2, 56.7) | 59.2*(54.1, 63.8) | 55.6(50.0, 60.6) | 59.0(53.8, 64.2) | 52.8(47.4, 58.3) | 63.1*(58.1, 68.2) |
| Role, physical | 32.9(28.2, 38.2) | 43.2*(38.4, 48.0) | 38.7(33.0, 45.1) | 59.1*(54.0, 63.9) | 41.5(35.6, 47.3) | 61.7*(57.4, 66.1) | 50.8(44.5, 56.6) | 61.6*(56.4, 66.9) | 48.0(41.6, 54.5) | 69.9*(65.7, 74.2) |
| Bodily pain | 36.8(33.2, 41.1) | 40.7(37.2, 44.1) | 37.8(34.2, 42.1) | 47.1*(43.8, 50.3) | 43.8(39.8, 47.7) | 49.1(45.5, 52.7) | 48.6(44.7, 51.9) | 56.1*(52.2, 59.9) | 49.8(45.5, 45.1) | 62.7*(59.4, 65.4) |
| General health | 12.7(10.8, 14.8) | 12.1(10.8, 14.5) | 11.3(9.6, 13.2) | 10.2(8.6, 11.8) | 6.5(5.3, 7.7) | 6.4(5.6, 7.1) | 6.8(5.9, 7.8) | 8.1*(7.3, 8.9) | 8.0(6.9, 9.1) | 9.4*(8.6, 10.1) |
| Vitality | 45.3(42.2, 48.4) | 47.9(45.1, 50.7) | 43.7(40.9, 47.1) | 52.7*(50.0, 55.4) | 44.3(41.0, 47.5) | 56.6*(54.0, 59.2) | 50.9(47.8, 54.0) | 56.6*(53.1, 60.1) | 48.4(45.1, 51.7) | 63.1*(60.3, 65.8) |
| Social functioning | 55.0(50.5, 59.4) | 57.3(53.6, 61.0) | 54.4(49.8, 59.1) | 62.2*(58.7, 65.8) | 57.4(52.8, 62.1) | 68.6*(65.4, 71.9) | 62.4(58.2, 66.6) | 71.0*(67.1, 74.7) | 62.8(58.3, 67.4) | 76.9*(74.1, 79.8) |
| Role, emotional | 51.9(46.4, 57.1) | 58.6(54.8, 64.1) | 50.0(44.9, 55.3) | 61.8*(58.5, 66.2) | 52.1(46.8, 57.3) | 64.1*(60.2, 68.5) | 57.6(52.0, 62.4) | 67.6*(63.8, 72.7) | 55.9(50.3, 61.6) | 72.1*(67.9, 76.4) |
| Mental health | 51.8(48.9, 54.6) | 55.0(52.8, 57.1) | 49.8(47.0, 53.1) | 58.6*(56.1, 61.1) | 49.9(47.1, 52.6) | 61.6*(59.2, 64.0) | 54.9(51.9, 57.3) | 61.8*(58.7, 64.0) | 51.9(49.2, 54.6) | 67.0*(64.8, 69.2) |
C, Control group; I, intervention group.
P < 0.05.
HF symptoms
At baseline, the number of patients in the intervention group and the control group, respectively, who reported HF symptoms such as orthopnoea at rest (75 vs. 84), paroxysmal nocturnal dyspnoea (59 vs. 71), palpitation (98 vs. 100), chest pain at rest (57 vs. 65) and chest pain on exertion (90 vs. 93) was approximately the same. The number of intervention patients who reported these symptoms decreased over time, the 12-month data being as follows for intervention and control group patients, respectively: orthopnoea at rest (51 vs. 81), paroxysmal nocturnal dyspnoea (29 vs. 60), palpitation (76 vs. 101), chest pain at rest (32 vs. 57) and chest pain on exertion (88 vs. 95).
Questionnaire outcome measures on medication knowledge
Medication knowledge was assessed from the patient assessment questionnaire. Chi2-analysis was used to compare the differences in medication knowledge between the intervention group and the control group at baseline and after 12 months. At baseline the number of patients in the intervention group and the control group, respectively, whose medication knowledge was deemed poor was approximately the same (80 vs. 82); it was not statistically different (P > 0.05, df = 1). There was a significant improvement in the intervention group patients after 12 months (20 vs. 84; P < 0.05, df = 1).
Self-reported compliance with medications and lifestyle advice
Compliance with the prescribed medication and compliance with the recommended lifestyle adjustments were also assessed from the patient assessment questionnaire. The number of intervention group patients vs. control patients who exhibited self-reported compliance with the prescribed medicines (85 vs. 35) and lifestyle adjustment (75 vs. 29) was higher than in control group patients at 12 months (P < 0.05, df = 1). The baseline scores for these parameters were 33 vs. 32 and 22 vs. 23 respectively (P > 0.05, df = 1).
Hospital admissions and emergency visits
The patients’ records and the patient assessment questionnaire indicated that the intervention group patients (Table 5) had fewer hospital admissions when compared with the control group patients. These documents, however, indicated that the intervention group patients had more casualty clinic visits compared with the control group patients. The average cost per day on a medical ward in a private hospital in the UAE is about £150 and it is much higher than the average cost of a casualty visit, which approximates to £60. The mean length of stay in hospital due to HF for intervention group patients was 9.3 days, while that for control group patients was 10.2 days.
Table 5.
Hospital admissions and emergency visit cost for the control and the intervention patients over the 12-month study period
| Group | Number of casualty visits | Cost per visit | Sub-total cost | No. of hospital admissions | Mean length of stay (days) | Cost per day | Sub-total cost | Total cost |
|---|---|---|---|---|---|---|---|---|
| Intervention | 33 | £60 | £1980 | 22 | 9.3 | £150 | £30 690 | £32 670 |
| Control | 25 | £60 | £1500 | 36 | 10.2 | £150 | £55 080 | £56 580 |
It was not possible to differentiate how the different individual components of the pharmaceutical programme gave rise to the improvements noted above. All patients were receiving an ACE inhibitor at the start of the study, and as such, optimization of therapy pertained mainly to adjusting the dosage of this agent or changing to a selective angiotensin II agent if cough was a noted side effect. Although not specifically examined, it appears that improved compliance with medications and lifestyle advice were the keys to success of the pharmaceutical care intervention, rather than via pharmacological regimens being optimized. More changes to doses were, however, required in patients who were recruited from the hospital inpatient setting than from the outpatient clinic setting (ratio 1.5 : 1, hospital : outpatient) over the 12-month study period, probably reflecting more serious pathology in the former group of patients.
Discussion
Methodology used
All intervention approaches (e.g. HF education booklet) and data collection instruments were found to be fit for the purpose and presented no major difficulties during the research study. As has been found in other intervention studies in HF patients, the SF36, the MLHF questionnaire [10], the walk test [28] and pulmonary function testing [29] provided useful quantitative data for comparison purposes between the two patient groups. Sneed et al. [10] suggested the beneficial use of the MLHF questionnaire and SF36 questionnaires together – this benefit was confirmed in the present study, in that different aspects of health-related quality of life could be examined at each sampling interval. This multidimensional approach has been supported recently in a study by Westlake et al. [28]. The walk tests were generally easy to administer, although they could not be completed by all patients at all assessments due to a number of patients not being well enough to complete the tests. The pulmonary function tests were again easy to perform and provided details on a further aspect of HF control. The chart reviews and questionnaire administration were time-consuming to complete; however, they provided useful clinical and self-reported data that were not available from other sources. The research pharmacist delivered the pharmaceutical care programme and was also involved in some aspects of data collection. The possible bias that this could have introduced to the study was minimized, in those cases where the outcome questionnaires could not be self-completed by patients, by ensuring that a strict protocol for questionnaire administration was adhered to, as designated in the SF36 manual. Nursing staff and the pharmacy technician who were involved in carrying out the 2-min walk test and the FVC test were blinded to the group to which the patient belonged (control vs. intervention). Again, strict protocols for test administration were adhered to.
Main findings of the study
The analysis of the current study showed that there were significant improvements in the clinical and humanistic outcomes for the intervention group patients following the implementation of the pharmaceutical care programme when compared with the control group patients.
The findings revealed that the patients in the intervention group were able to walk a greater distance than the control group patients, in all assessment periods post baseline (Table 3) and positive trends, although generally not statistically significant, were also evident for the times taken to walk 25 m and 50 m. The summary measure (AUC) for the 2-min walk test also revealed a significant improvement when intervention patients' data were compared with control patients' data. Collectively, the results indicate that exercise tolerance was improving in the intervention group, while in the control group patients it was either remaining static or decreasing. This demonstrates a positive clinical response to pharmaceutical care provision, consistent with the conclusion that improved exercise capacity is a measure of success in the management of HF [30–32]. The research data revealed that there was a significant difference between the two groups with regard to BP (systolic and diastolic) and pulse, which was considered surprising as the majority of patients were receiving treatment with diuretics, ACE inhibitors and digoxin throughout the study period. These outcomes were probably due to the comprehensive education of patients and their families, the provision of dietary and other lifestyle advice, the review of medications and the intensive follow-up programme. Also, the decreases in diastolic and systolic BP together with the decreased pulse rate were likely to be due, at least in part, to improved compliance with prescribed drugs in the intervention group patients. The intervention group patients were advised to consume not more than 2 g of salt per day. Also, the patients in the intervention group were strongly advised to drink only moderate quantities of water and to drink not more than 30 ml per day of spirit [11, 33]. This advice may also have influenced the latter outcomes. Meyer [34] has commented that by analysis of heart rate, one could properly assess the autonomic tone and better appraise the control of sinus node functionality. Furthermore, a correlation between heart rate and atherosclerosis progression exists, in that an increase in heart rate of five beats per minute corresponds to an increase in the atherosclerosis progression score of 0.21 and an increase in the stenosis progression score of 0.27 [35]. This latter author emphasized the importance of decreasing heart rate in HF patients by adhering to drug regimens and lifestyle modifications.
The present findings revealed that there was also a successful trend for the intervention group patients to have higher FVC readings during receipt of the pharmaceutical care interventions. Pulmonary function testing was used in the Framingham Study as an indicator of worsening HF [36].
Data analyses of the scores obtained using the MLHF questionnaire indicated that the intervention group patients' heart failure did not affect their quality of life to as great an extent as in the control group patients (Figure 2). Also, analyses of SF36 scores demonstrated that the intervention group patients' quality of life had improved over time, while that of the control group patients remained at a relatively constant level (Table 4). HF and the related symptoms greatly affect the patients' ability to perform normal daily activities, thereby affecting their health-related quality of life [37]. The results of the present study therefore indicate that pharmaceutical care interventions can have a positive impact on how HF patients are able to cope with daily activities.
Researchers have estimated that the rate of noncompliance with prescribed medications ranges from 25% to 50%[38]. Noncompliance has been implicated as a major cause of unnecessary hospitalization of patients with HF [39]; it has been shown in one study that 27% of patients hospitalized for HF were re-hospitalized within 90 days [40], with most of these hospitalizations resulting from medication or dietary noncompliance. Better patient counselling and education are essential for improving outcomes, including patient compliance [33]. Patients in the intervention group received intensive education from the research pharmacist on their prescribed medicines, including dosage, side effects, and indications, and the need to adhere to both the prescribed medication regimen and lifestyle changes. This information was supported by a patient information booklet, prepared and developed for this study [15].
It has recently been reported that HF is the single most frequent cause of hospitalization in people aged ≥65 years [10]. Although the mean age of the patients participating in the present research was considerably less than 65 years (Table 1), the findings of the present study indicated a decrease in the number of hospital admissions in the intervention group patients when compared with the control group (Table 5). This was probably due to a whole range of positive factors that came about as a result of the pharmaceutical care interventions, not least improved compliance with the prescribed medications and lifestyle recommendations. Also, the intervention patients tended to seek help more frequently at the casualty department, perhaps indicating a better understanding of the need for early medical intervention in their heart failure management when symptoms were deteriorating. Although opportunity costs were not measured in the present study, the cost savings indicated in Table 5 are likely to more than cover the cost of pharmaceutical input.
The present study, which covered a period of 12 months, demonstrated significant improvements in a range of outcome measures with a total sample size of 208 HF patients. The UK study on which the present study was based [15] found that it was difficult to assess definitively the impact of the interventions due high patient drop-out rates, although there were improvements in a number of the measured parameters. Patient drop-out was not an issue in the present study, highlighting differences in patient approaches to pharmacy-led research in different settings. Also of note was the fact that the patients in the UK study were much older (mean age >70 years) than the patients in the present study (mean age <60 years), which undoubtedly had an effect on continued study participation. Age-related reasons for withdrawal in the UK study included difficulty in arranging transportation and becoming too confused to continue involvement [15]. These were clearly not issues in the present study. Ethnic differences in the two study populations were also likely to have an impact on both continued study participation and on adherence to advice (intervention patients) given by the pharmacist.
Conclusions
The present study was designed to measure the impact of pharmaceutical care on a wide range of clinical and humanistic outcomes related to the different aspects of health status in patients with HF. The broad range of data in the present work allowed a comprehensive assessment of the potential benefit of pharmaceutical care interventions on patient health status and gave some preliminary information on costs. Enhanced patient outcomes, as a result of pharmaceutical care delivery, were seen, as exemplified by improvements in:
Quality of life as measured by SF36 and MLHF questionnaires.
Exercise capacity (measured by 2-min walk test).
Pulmonary function, blood pressure and pulse.
Hospital admission rates (and overall hospital-based costs).
Self-reported compliance with the prescribed HF medication and lifestyle advice.
Acknowledgments
The authors thank all study participants and their hospital physicians for their participation in the study. The authors also thank Dr Gordon Cran, Department of Epidemiology and Public Health, Queen's University Belfast, for his statistical advice.
Competing interests: None declared.
References
- 1.Hobbs FDR. The scale of heart failure: diagnosis and management issues for primary care. Heart. 1999;82(Suppl. IV):8–10. doi: 10.1136/hrt.82.2008.iv8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahoney JS. An ethnographic approach to understanding the illness experiences of patients with congestive heart failure and their family members. Heart Lung. 2001;30:429–36. doi: 10.1067/mhl.2001.119832. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan MJ, Hawthorne MH. Nonpharmacological interventions in the treatment of heart failure. J Cardiovasc Nurs. 1996;10:47–57. doi: 10.1097/00005082-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Carlson B, Riegel B, Faan C, Moser D. Self-care abilities of patients with heart failure. Heart Lung. 2001;30:351–9. doi: 10.1067/mhl.2001.118611. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F. Evidence-based drug therapy for chronic heart failure. Eur Heart J. 2002;(Suppl. 4):D66–D72. [Google Scholar]
- 6.Varma S, McElnay JC, Hughes CM. A qualitative assessment of health-related issues of importance in elderly congestive heart failure patients. J Soc Admin Pharm. 2001;18:59–67. [Google Scholar]
- 7.Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Exploring the spiritual needs of people dying of lung cancer or heart failure: a prospective qualitative interview study of patients and their carers. BMJ. 2002;325:929–33. doi: 10.1136/bmj.325.7370.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers A, Addington-Hall JM, McCoy ASM, Edmonds PM, Abery AJ, Coats AJS, Gibbs JSR. A qualitative study of chronic heart failure patients' understanding of their symptoms and drug therapy. Eur J Heart Fail. 2002;4:283–7. doi: 10.1016/s1388-9842(01)00213-6. [DOI] [PubMed] [Google Scholar]
- 9.Martensson J, Karlsson J, Fridlund B. Male patients with CHF and their conception of the life situation. J Adv Nurs. 1997;25:579–86. doi: 10.1046/j.1365-2648.1997.1997025579.x. [DOI] [PubMed] [Google Scholar]
- 10.Sneed N, Paul S, Michel Y, VanBakel A, Hendrix G. Evaluation of three quality of life measurement tools in patients with chronic heart failure. Heart Lung. 2001;30:332–40. doi: 10.1067/mhl.2001.118303. [DOI] [PubMed] [Google Scholar]
- 11.Dracup K, Baker DW, Dunbar SB, Dacey RA, Brooks NH, Johnson JC, Oken C, Massie BM. Management of heart failure. Counselling, education, and lifestyle modifications. JAMA. 1994;272:1442–6. doi: 10.1001/jama.1994.03520180066037. [DOI] [PubMed] [Google Scholar]
- 12.Coats AJS. What causes symptoms in heart failure? Heart. 2001;86:574–8. doi: 10.1136/heart.86.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hepler CD, Strand L. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–43. [PubMed] [Google Scholar]
- 14.Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158:1067–72. doi: 10.1001/archinte.158.10.1067. [DOI] [PubMed] [Google Scholar]
- 15.Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of elderly congestive heart failure patients: Interventions and outcomes. Pharmacoth. 1999;19:860–9. doi: 10.1592/phco.19.10.860.31565. [DOI] [PubMed] [Google Scholar]
- 16.Gattis WA, Hasselbad V, Whellan DJ, O'Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team — Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159:1939–45. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- 17.Luzier AB, Forrest A, Feuerstein SG, Schentag JJ, Izzo JL. Containment of heart failure hospitalizations and cost by angiotensin-converting enzyme inhibitor dosage optimization. Am J Cardiol. 2000;86:519–23. doi: 10.1016/s0002-9149(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 18.Pearson GJ, Cooke C, Simmons WKT, Sketris I. Evaluation of the use of evidence-based angiotensin-converting enzyme inhibitor criteria for the treatment of congestive heart failure: opportunities for pharmacists to improve patient outcomes. J Clin Pharm Ther. 2001;26:351–61. doi: 10.1046/j.1365-2710.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- 19.Stewart S, Marley J, Horowitz J. Effects of a multidisciplinary, home based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomized controlled study. Lancet. 1999;354:1077–83. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 20.Gore S. Assessing clinical trials-restricted randomisation. BMJ. 1981;282:2114–7. doi: 10.1136/bmj.282.6282.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rector TS, Francis G, Cohn JN. Patients' self-assessment of their congestive heart failure. Heart Failure. 1987;1:192–6. [Google Scholar]
- 22.Rector TS, Spencer HK, Cohn JN. Patients' self-assessment of their congestive heart failure. Part 2. Heart Failure. 1987;1:198–209. [Google Scholar]
- 23.Bultand R, Pan J, Gross E, Woodcock A, Geddes O. Two-, six-, and twelve-minute walking test in respiratory disease. BMJ. 1982;284:1607–8. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dracup K, Walden J, Stevenson L, Brecht M. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;2:273–9. [PubMed] [Google Scholar]
- 25.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six-minute walking test for assessing exercise capacity in chronic heart failure. BMJ. 1986;292:653–5. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt G, Sullivan M, Fallen EL, Tihal H, Rideout E, HalCrow S, Nogradi S, Townsend M, Taylor DW. A controlled trial of digoxin in congestive heart failure. Am J Cardiol. 1988;61:371–5. doi: 10.1016/0002-9149(88)90947-2. [DOI] [PubMed] [Google Scholar]
- 27.Goodyer L, Ehsanullah M, Miskelly F. Validation of sub-maximal exercise test for the assessment of heart failure in the elderly. J Clin Exp Gerontol. 1990;12:253–8. [Google Scholar]
- 28.Westlake C, Dracup K, Creaser J, Livingston N, Heywood JT, Huiskes BL, Fonarow G, Hamitton M. Correlates of health-related quality of life in patients with heart failure. Heart Lung. 2002;31:85–93. doi: 10.1067/mhl.2002.122839. [DOI] [PubMed] [Google Scholar]
- 29.Schaufelberger M. Pulmonary diffusion capacity as prognostic marker in chronic heart failure. Eur Heart J. 2002;23:429–31. doi: 10.1053/euhj.2001.2960. [DOI] [PubMed] [Google Scholar]
- 30.Ziesche S, Cobb F, Cohn JN, Johnson G, Tristani F. For the V-HEFT VA Co-operative Studies Group. Hydralazine and isosorbide dinitrate combination improves exercise tolerance in heart failure. Circulation. 1993;87:56–64. [PubMed] [Google Scholar]
- 31.Willenheimer R, Erhardt L, Cline C, Rydberg E, Israelsson B. Exercise training in heart failure improves quality of life and exercise capacity. Eur Heart J. 1998;19:774–81. doi: 10.1053/euhj.1997.0853. [DOI] [PubMed] [Google Scholar]
- 32.Money-Kyrle AR, Timmis AD. Treatment paradigms in heart failure. Hospital Med. 2002;63:80–7. doi: 10.12968/hosp.2002.63.2.2084. [DOI] [PubMed] [Google Scholar]
- 33.Kosseim LM, Pifer AE, Zimmer R. Effective management of complex heart failure. Postgrad Med. 1999;105:17–24. [Google Scholar]
- 34.Meyer K. The revival of heart rate. Eur Heart J. 1999;20:853–4. doi: 10.1053/euhj.1999.1591. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari A, Rapezzi CF, Lombardi F. The revival of heart rate. Eur Heart J. 1999;20:853–4. doi: 10.1053/euhj.1999.1591. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, Hjortland M, Castellei W. Epidemiology and risk profile of cardiac failure. Cardiovasc Drug Ther. 1974;2:387–95. [PubMed] [Google Scholar]
- 37.Cohn SR, Mount MM, Tomas JJ, Mount LF. Existential well-being is an important determinant of quality of life. Cancer. 1996;77:567–86. doi: 10.1002/(SICI)1097-0142(19960201)77:3<576::AID-CNCR22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghali JK, Kadaki S, Cooper R, Ferlinz J. Precipitating factors leading to de-compensation of heart failure: trials among urban blacks. Arch Intern Med. 1988;148:2014–6. [PubMed] [Google Scholar]
- 40.Vinson J, Rich MW, Sperry JC, Shah AS, McNmara T. Early readmission of elderly patients with CHF. J Am Geriatr Soc. 1990;38:1290–5. doi: 10.1111/j.1532-5415.1990.tb03450.x. [DOI] [PubMed] [Google Scholar]