Abstract
Aims
The study was designed to evaluate the relative bioavailability of diclofenac in plasma, subcutaneous adipose and skeletal muscle tissue after repeated topical administration using MIKA Diclofenac Spray Gel (4%), a novel formulation, and after oral dosing using VOLTAREN® 50 mg enteric coated tablets.
Methods
Diclofenac (48 mg) was administered topically three times daily for 3 days onto a defined area of the thigh of 12 healthy males. After a 14-day wash out period, subjects were orally treated with 50 mg diclofenac three times daily for 3 days. In vivo microdialysis in subcutaneous and muscle tissues was performed immediately after the final doses from both treatments on day 4, and 48 h later. Plasma samples were taken simultaneously.
Results
The relative bioavailability of diclofenac in subcutaneous adipose and skeletal muscle tissue was substantially higher after topical compared with oral dosing (324% and 209%, respectively) whereas relative plasma bioavailability was 50-fold lower. Plasma Cmax values were approximately 250-fold lower after topical compared with oral drug administration (i.e. median values = 4.89 ng mL−1; 95% CI: 3.37–7.68 and 1240 ng mL−1; 95% CI: 787–1389 ng mL−1). Both treatments were well tolerated.
Conclusions
Owing to its favourable penetration characteristics and low systemic availability, MIKA Diclofenac Spray Gel 4% is a rational alternative to oral diclofenac formulations for the treatment of inflammatory soft tissue conditions.
Keywords: diclofenac, topical, spray gel, dermal penetration, microdialysis
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used throughout the world. Generally, they are well tolerated, but the occurrence of sometimes life-threatening adverse events limits their use and results in substantial morbidity, mortality and increasinghealth care costs [1]. To increase the effect–risk ratio of NSAIDs, topical formulations have been developed [2, 3].
The stratum corneum of the human skin, however, is known to be an effective and selective barrier to drug permeation [4, 5]. Therefore, to improve local drug delivery to deeper tissue layers, penetration enhancers and novel drug vehicles have been designed [6]. Recently, a new topical pharmaceutical form of diclofenac sodium, i.e. MIKA Diclofenac Spray Gel 4%, has been patented. The spray gel consists of water, isopropyl alcohol and propylene glycol as basic solvents and soy bean lecithin, ethanol, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, peppermint oil and ascorbyl palmitate as excipients. Sprayed onto to the skin, the solution changes its viscosity by evaporation of the alcoholic components and forms a low-viscous gel sticking to the skin. Penetration of diclofenac into deeper skin layers has been demonstrated in vitro using the spray gel formulation [7]. Based on the physico-chemical characteristics of the spray gel it was hypothesized that diclofenac tissue concentrations after spray gel application would be comparable if not higher than after administration of an established oral formulation [8].
The present study was designed to compare the bioavailibility of diclofenac with respect to subcutaneous adipose and skeletal muscle tissue after repeated topical and oral (VOLTAREN® 50 mg enteric coated tablet) administration of equivalent daily doses. Microdialysis, a minimally invasive technique for the measurement of unbound drug concentrations in target tissues was used. Microdialysis is recognized by regulatory authorities as a potential tool for bioequivalence evaluation of topical dermatological dosage forms [9, 10].
Materials and methods
The study was approved by the local Ethics Committee and was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the European Commission (EC-GCP guideline). Subjects were given a detailed description of the study and their written consent was obtained.
Study design
An open, prospective, single-centre, nonrandomized, fixed sequence Phase I study was performed.
Study population
12 healthy male Caucasians were studied, with a mean age (±SD) of 27.8 ± 4.1 years (range: 23–39 years), a mean body height of 1.83 ± 0.06 m (range: 1.75–1.94 m), a mean body weight of 77.2 ± 6.5 kg (range: 67.8–88.4 kg), and a mean BMI of 23.0 ± 1.8 (range: 20.0–25.4).
Study medication and experimental design
MIKA Diclofenac Spray Gel 4%, was supplied by MIKA PHARMA GmbH (Limburgerhof, Germany) in brown glass bottles containing 1 g diclofenac sodium in 25 g of solution. The bottles were fitted with a mechanical pump spray which delivered 8 mg diclofenac sodium in 200 µL solution per pump actuation. VOLTAREN® 50 mg enteric coated tablets (Novartis Pharma AG, Basel, Switzerland), were obtained from the local hospital pharmacy.
Subjects received the topical drug first, followed by the oral form, with a 14-day washout period between them. A dose of 48 mg diclofenac sodium (i.e. six spray actuations) was administered onto a shaved area of the thigh three times a day. Oral drug application was given at a dose of 50 mg three times a day at fixed time points irrespective of meals. In vivo microdialysis employing commercially available probes (CMA 10; CMA/Microdialysis AB, Stockholm, Sweden) was performed after the administration of the 10th dose (on day 4 of each regimen) after an overnight fast. Microdialysates and blood samples were collected every hour for 10 h post dosing and again at 48 h after the last dose, as described previously [3]. Probes were calibrated in vivo at the end of each study day according to a no net flux method [11]. All samples were stored at −80 °C prior to analysis.
Sample analysis
All drug analyses were conducted according to GLP guidelines. Diclofenac concentrations in plasma and microdialysates were determined by a validated LC-MS/MS method (Pharmakin SOP NMM_0037–0001, Version 1). After thawing, either 100 µL plasma (sampled after oral diclofenac administration, diluted to 1 mL with blank plasma) or 1 mL plasma (sampled after topical diclofenac administration) were mixed with 50 µL internal standard solution (containing 147 ng [2H6]-diclofenac). Acidification by addition of 500 µL 0.1 m HCl was followed by extraction with 6 mL cyclohexane: tert. butylmethyl ether (1 : 2 (v/v)), centrifugation, recovery of the organic layer, evaporation to dryness with nitrogen and dissolution of the dry residue in 250 µL mobile phase. Forty microlitres of microdialysate was mixed with 40 µL internal standard solution (containing 4.7 ng [2H6]-diclofenac) and 120 µL methanol. After sample work-up, 50 µL of plasma extract or microdialysate were injected into the LC-MS/MS.Separation of the analyte was achieved on a 5-µm Purospher RP18 column. Ammonium carbaminate solution 75 m m (adjusted to pH 4 by formic acid) was used as the mobile phase. Chromatography was performed in the isocratic mode and at room temperature. The column eluent was split and eluent at a flow of 100 µL min−1 was passed into the electrospray ionization source of a Micromass Quattro II triple quadrupole mass spectrometer (Micromass, Altrincham, UK). The nebulizing gas (nitrogen) flow was 25 L h−1 and the instrument was programmed for a scan dwell time of 500 ms. Diclofenac responses were measured in the positive ionization mode using multiple reaction monitoring. Mass transitions of m/z 296 > 214 for protonated diclofenac and m/z 302 > 219 for hexadeuterated diclofenac as internal standard were used to selectively monitor precursor ions and corresponding product ions. The lower limit of quantification of the assay was 0.15 ng mL−1. Within-day and between-day precision was 2.2% and 3.7% at 0.20 ng mL−1, 1.3% and 0.8% at 7.97 ng mL−1 and 0.4% and 0.4% at 79.71 ng mL−1.
Data analysis
AUC∞ (identical with AUC0−8 in steady state), AUC0−8 and Cmax data were determined using a noncompartmental approach (systattm, Version 10, SPSS Inc, Chicago, USA). Data were ln-transformed and relative diclofenac bioavailability ratios were determined by dividing AUC values after topical administration by those after oral dosing. Values for tissue/systemic circulation ratios were calculated from these bioavailibility ratios. Results were compared by one sample t-tests, and the type I error was fixed at α = 0.05.
Results
Figure 1 shows that plasma diclofenac concentrations were higher following oral drug administration. AUC and Cmax values are summarized in Table 1. The geometric mean steady state relative bioavailability ratio expressed as a percentage (95% CI) in plasma was 2.23% (1.55–3.20%; P < 0.0001) indicating oral diclofenac was about 50 times more systematically bioavailable than the topical form. In contrast, the relative bioavailability ratio was higher in subcutaneous and skeletal muscle tissue (324% (232−453%) and 209% (130−337%), respectively) after topical compared with oral drug administration, respectively (P < 0.0001 and P = 0.006).
Figure 1.
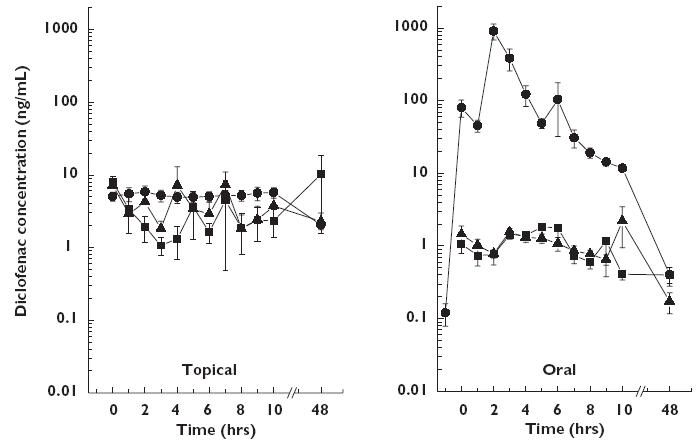
Mean concentration vs. time profiles of diclofenac in plasma (closed circles), subcutaneous adipose (open triangles) and skeletal muscle tissue (closed squares) after the final dose of a 3-day regimen of either MIKA Diclofenac Spray Gel 4% applied to the skin of the thigh (left panel) or with VOLTAREN® 50 mg enteric coated tablets given orally (right panel) in 12 healthy males. Results are presented as mean ± SE. Plasma (•), subcutaneous tissue (▴), skeletal muscle tissue (▪)
Table 1.
Main pharmacokinetic parameters for diclofenac obtained in plasma (total drug) and subcutaneous and skeletal muscle tissue (free drug) of the thigh in 12 healthy males after the final dose of a 3-day multiple dose regimen of either topical application of MIKA Diclofenac Spray Gel 4% or oral administration of VOLTAREN® 50 mg enteric coated tablets
| Topical | Oral | |||
|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | |
| Plasma (n = 12) | ||||
| AUC∞, AUCτ (ng h mL−1) | 32.8 | (22.7–52.9) | 1569.7 | (1255.8–1849.8) |
| Cmax (ng mL−1) | 4.9 | (3.4–7.7) | 1240.2 | (787.0–1388.9) |
| Subcutaneous tissue (n = 12) | ||||
| AUC∞, AUCτ (ng h mL−1) | 21.5 | (19.4–50.5) | 8.6 | (7.0–10.6) |
| Cmax (ng mL−1) | 13.1 | (9.3–33.6) | 1.9 | (1.6–2.5) |
| Skeletal muscle (n = 12) | ||||
| AUC∞, AUCτ (ng h mL−1) | 18.2 | (11.8–28.1) | 8.8 | (7.8–12.3) |
| Cmax (ng mL−1) | 12.3 | (6.2–22.0) | 2.6 | (2.0–4.0) |
AUC∞, AUCτ[ng h mL−1], Area under the plasma or tissue concentration vs. time curve of diclofenac approximated to infinity (AUC∞) or evaluated in the last dosage interval (0–8 h; AUCτ); Cmax[ng mL−1], Maximal plasma or tissue concentration.
After topical drug administration the mean bioavailability ratio between subcutaneous tissue and plasma was 78.1% (95% CI: 42.2–145%; P = 0.396), and that between muscle tissue and plasma was 52.2% (95% CI: 37.4–72.8%; P = 0.001). After oral treatment the corresponding bioavailability ratios were two orders of magnitude lower (0.536% (95% CI: 0.426–0.675%) for the subcutaneous tissue/plasma ratio and 0.556% (95% CI: 0.429–0.720%) for the muscle tissue/plasma ratio (P < 0.0001 for both ratios)).
No serious adverse events were reported. The spray gel was well tolerated except for slight erythema in 3 of 12 subjects after the third treatment day, most probably due to shaving the area of drug application.
Discussion
Repetitive treatment with the new spray gel formulation resulted in substantial dermal absorption of diclofenac. Unbound interstitial diclofenac concentrations in tissue layers underneath the application site were about 2.5 ng mL−1, and corresponding values for total concentration in plasma were about 4.1 ng mL−1. This is in accordance with unbound diclofenac plasma concentrations of approximately 0.004 ng mL−1, if a plasma protein binding of 99.9% is assumed [12]. Consequently, the present data indicate a steep tissue-to-plasma gradient of about 1000 for unbound diclofenac, demonstrating substantial skin penetration from the present formulation. The findings do not support the concept of local plasma-to-tissue rediffusion as described for other formulations of diclofenac [13]. Following oral administration, unbound diclofenac tissue concentrations were similar to those estimated for unbound drug in plasma. Thus, for the oral formulation, the plasma-to-tissue gradient of 1–2 indicates free plasma-to-tissue diffusion of unbound diclofenac.
Based on clinical studies demonstrating the efficacy of oral diclofenac at a daily dose of 150 mg [14, 15], our data suggest an effective target site concentration of 1.1 ng mL−1 for unbound diclofenac, and that effective concentrations equivalent or better than those obtained from oral dosing are attained following spray gel administration. Whereas tissue concentrations after oral dosing gradually declined after the last dose, those after repetitive topical drug administration remained relatively stable over time. Furthermore, after gel administration, the relative plasma bioavailability of diclofenac was approximately 50-fold lower than after oral dosing. Because maximum diclofenac plasma concentrations following spray administration were only 0.4% of those after the oral dose, systemic side-effects after topical administration should be negligible.
A consistent finding of in vivo transdermal studies is the considerable interindividual variability in the skin penetration of drugs. In the present work, tissue concentrations of diclofenac were far less variable following oral administration. The ‘double peaks’ in plasma seen in the concentration vs. time profiles were also reflected in tissue, indicating a relatively low experimental variability as described for other analytes in microdialysis experiments [16], and suggesting that the variability encountered after transdermal application is the true biological variability.
Maximal tissue concentrations following topical dosing showed relatively high interindividual variability. Substantial variability in plasma concentrations (of up to 400%) was also observed in some subjects, which indicates that transdermal penetration into the systemic circulation can also be variable. Differences and changes over time in (1) local blood flow (2) release kinetics of the drug from the stratum corneum (3) skin temperature and (4) leg movements and subsequent changes in local blood flow [17, 18] may account for this variability.
Following the topical drug administration, pharmacokinetic parameters have been reported to vary by orders of magnitude, a number of factors being responsible for this, including differences in types of subjects, and sites of drug application [19–22]. A recent review on diclofenac pharmacokinetics concluded that its topical delivery is largely dependent on the nature of the drug and vehicle, as well as skin integrity and hydration status [12].
In conclusion, owing to its favourable penetration characteristics and low systemic availability, MIKA Diclofenac Spray Gel is a rational alternative to oral diclofenac for the treatment of soft tissues injuries.
Acknowledgments
Competing interests: None declared.
References
- 1.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340(24):1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 2.Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–74. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Müller M, Rastelli C, Ferri P, Jansen B, Breiteneder H, Eichler HG. Transdermal penetration of diclofenac after multiple epicutaneous administration. J Rheumatol. 1998;25:1833–6. [PubMed] [Google Scholar]
- 4.Morgan CJ, Renwick AG, Friedmann PS. The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. Br J Dermatol. 2003;148(3):434–43. doi: 10.1046/j.1365-2133.2003.05163.x. [DOI] [PubMed] [Google Scholar]
- 5.Riess W, Schmid K, Botta L, Kobayashi K, Moppert J, Schneider W, Sioufi A, Strusberg A, Tomasi M. The percutaneous absorption of diclofenac. Arzneimittelforschung. 1986;36(7):1092–6. [PubMed] [Google Scholar]
- 6.Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316(7128):333–8. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artmann C. 1997. Verteilung und Deposition von Diclofenac. In-vivo-Untersuchungen zur Verteilung und Deposition auf der menschlichen Rückenhaut mit verschiedenen galenischen Zubereitungen von Diclofenac. Wissenschaftlicher Kurzbericht Nr.97003, 14.03.1997. MIKA Pharma data on file. [Google Scholar]
- 8.Link R. 2000. Placebo-controlled clinical multicentre study to verify efficacy and safety of Diclofenac Spray Gel 4 % MK in the treatment of ankle distortions, MIKA Pharma data on file.
- 9.Müller M. Science medicine, and the future: Microdialysis. BMJ. 2002;324(7337):588–91. doi: 10.1136/bmj.324.7337.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah VP. Topical drug products – microdialysis: regulatory perspectives. Int J Clin Pharmacol Ther. 2004;42(7):379–81. [Google Scholar]
- 11.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–31. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 12.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33(3):184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 13.Radermacher J, Jentsch D, Scholl MA, Lustinetz T, Frolich JC. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol. 1991;31(5):537–41. doi: 10.1111/j.1365-2125.1991.tb05576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacher J, Feldman D, Gerli R, Scott D, Hou SM, Uebelhart D, Rodger IW, Ozturk ZE, Etoricoxib OA Study Group. A comparison of the therapeutic efficacy and tolerability of etoricoxib and diclofenac in patients with osteoarthritis. Curr Med Res Opin. 2003;19(8):725–36. doi: 10.1185/030079903125002469. [DOI] [PubMed] [Google Scholar]
- 15.Arcangeli P, Andreotti L, Palazzini E. Effective treatment of osteoarthritis with a 150 mg prolonged-release formulation of diclofenac sodium. Riv Eur Sci Med Farmacol. 1996;18(5–6):217–23. [PubMed] [Google Scholar]
- 16.Stahle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49(24):1853–8. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 17.Guy RH, Hadgraft J, Bucks DA. Transdermal drug delivery and cutaneous metabolism. Xenobiotica. 1987;17(3):325–43. doi: 10.3109/00498258709043943. [DOI] [PubMed] [Google Scholar]
- 18.Borg N, Gotharson E, Benfeldt E, Groth L, Stahle L. Distribution to the skin of penciclovir after oral famciclovir administration in healthy volunteers: comparison of the suction blister technique and cutaneous microdialysis. Acta Derm Venereol. 1999;79(4):274–7. doi: 10.1080/000155599750010643. [DOI] [PubMed] [Google Scholar]
- 19.Tegeder I, Muth-Selbach U, Lotsch J, Rusing G, Oelkers R, Brune K, Meller S, Kelm GR, Sorgel F, Geisslinger G. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen administration. Clin Pharmacol Ther. 1999;65(4):357–68. doi: 10.1016/S0009-9236(99)70128-1. [DOI] [PubMed] [Google Scholar]
- 20.Benfeldt E, Serup J. Effect of barrier perturbation on cutaneous penetration of salicylic acid in hairless rats: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Arch Dermatol Res. 1999;291(9):517–26. doi: 10.1007/s004030050447. [DOI] [PubMed] [Google Scholar]
- 21.Schnetz E, Fartasch M. Microdialysis for the evaluation of penetration through the human skin barrier – a promising tool for future research? Eur J Pharm Sci. 2001;12(3):165–74. doi: 10.1016/s0928-0987(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 22.Wester RC, Maibach HI. Percutaneous absorption of drugs. Clin Pharmacokinet. 1992;23(4):253–66. doi: 10.2165/00003088-199223040-00002. [DOI] [PubMed] [Google Scholar]


