Abstract
Aims
Interaction of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction with organic nitrates could lead to severe hypotension. NMI 861 is a combination of 7.7 mg yohimbine tartrate and 6 g l-arginine glutamate. A similar oral combination, which contains the same amount of yohimbine and l-arginine, has been shown to improve erectile function in previous studies.
Methods
In two placebo-controlled, randomized, double-blind, two-way crossover design studies we aimed to assess first the pharmacokinetics and pharmacodynamics of a single oral dose of NMI 861 administered in 16 healthy male subjects, and then the pharmacodynamics of orally administered NMI 861 in combination with intravenous nitroglycerine (GTN) in 12 healthy male subjects. Systolic (SBP) and diastolic (DBP) blood pressures, pulse rate and adverse events were measured in each study.
Results
NMI 861 was well tolerated by all subjects with no significant adverse reactions reported. For l-arginine, mean Cmax ± SEM (range) was 42 ± 2.2 (28–63) µg ml−1 and tmax (range) was 0.88 (0.50–1.5) h. AUC and t1/2 were not calculated for l-arginine because of the presence of endogenous concentrations and the contribution from food sources. For yohimbine, mean Cmax was 42 ± 11 (2.8–128) ng ml−1; tmax was 0.57 (0.25–1.0) h; mean AUC(0,8 h) was 65 ± 24 (5.4–332), ng ml−1 h and t1/2 was 1.0 ± 0.34 (0.40–6.0) h. There was a small but significant difference in the mean change from baseline for SBP from 0 to 6 h after NMI 861 treatment compared with placebo (0.8 ± 1.4 vs−4.1 ± 2.1 mmHg, respectively; 95% Cl 0.0, 9.8 mmHg (P = 0.047)). There was no significant difference in SBP between treatments for the studied periods 6–12 h and 12–24 h. There was no significant difference in DBP or pulse between NMI 861 and placebo treatments for the three studied time periods. In the study designed to investigate the interaction of organic nitrate with NMI 861, subjects were infused intravenously with increasing doses of GTN (15 min each dose) at 2.5, 5, 10, 20 and 40 µg min−1 starting 40 min after a single oral dose of either NMI 861 or placebo. There was no significant difference in the hypotensive response induced by GTN between the NMI 861 and placebo treatments. The mean maximum changes from baseline during GTN infusion for subjects administered with either NMI 861 or placebo were a decrease of 16.9 ± 3.4 vs 13.6 ± 2.4 mmHg (mean difference between treatments −3.3 mmHg, 95% CI −12.7, 6.0 mmHg (P = 0.460)) for SBP, a decrease of 14.7 ± 2.0 vs 14.0 ± 2.0 mmHg for DBP (mean difference −0.7 mmHg, 95% CI −8.2, 6.8 mmHg (P = 0.835)), and an increase of 11.8 ± 1.9 vs 14.1 ± 2.4 beats min−1 for pulse, respectively (mean difference −2.3 beats min−1, 95% Cl −9.3, 4.5 beats min−1 (P = 0.464)).
Conclusions
Acute oral administration of NMI 861 was found to be well tolerated and bioavailable in healthy male subjects and no significant hypotensive interaction with intravenous GTN was detected at the doses investigated.
Keywords: NMI 861, yohimbine, l-arginine, male erectile dysfunction, nitric oxide, nitroglycerine, sildenafil
Introduction
Male erectile dysfunction has been defined as the persistent inability to attain and maintain an erection adequate to permit satisfactory sexual intercourse. This condition is common with 25% of males aged 45–70 years reporting moderate erectile dysfunction and 10% reporting severe erectile dysfunction [1]. Vascular disease and diabetes are well recognized as the leading causes of organic erectile dysfunction and a significant proportion of these patients have either overt or covert coronary artery disease. Furthermore, male erectile dysfunction and cardiovascular disease share many of the same risk factors, namely hypertension, diabetes, hyperlipidaemia and smoking [1]. Accordingly, many patients with male erectile dysfunction may be treated with antianginal drugs including organic nitrates. Therefore, any interaction of treatments for male erectile dysfunction with organic nitrates could lead to serious adverse events.
Yohimbine, a selective α2-adrenergic receptor antagonist, may have some benefit in the treatment of male erectile dysfunction [2], but its efficacy is modest at best when given alone (for review, see Tam et al.[3]). Nitric oxide (NO), which is synthesized from the amino acid l-arginine, is essential for normal erections [4]. Therefore, drugs acting on the l-arginine-NO pathway are attractive as potential treatments for male erectile dysfunction. For this reason l-arginine has been combined with yohimbine as a treatment for this condition. In double-blind, placebo-controlled studies, an on-demand oral preparation of an l-arginine/yohimbine combination significantly improved erectile function in men with mild-to-moderate disease [5, 6], whereas neither l-arginine alone nor yohimbine alone was significantly better than placebo. Furthermore, a similar yohimbine and l-arginine combination has been found to increase substantially vaginal pulse amplitude responses to sexual visual stimulation compared with placebo in postmenopausal women with female sexual arousal disorder [7].
Sildenafil citrate (Viagra®) is a selective inhibitor of phosphodiesterase type 5, which with sexual stimulation leads to cGMP accumulation and relaxation of the smooth muscles of the corpus cavernosa, resulting in penile erection [8]. Sildenafil alone has vasodilating properties and amplifies the hypotensive effect of nitrates [9, 10]. For this reason sildenafil and other members of this class of drug are contraindicated in patients treated with nitrates.
We report the pharmacokinetic and pharmacodynamic properties of an orally administered preparation of yohimbine and l-arginine combination (NMI 861) given alone, and test its possible interaction with intravenous nitroglycerine (GTN) in healthy male subjects.
Methods
Both studies were approved by the Local Research Ethics Committee at Glasgow, UK and all subjects provided written informed consent.
Study 1 – Pharmacokinetic, pharmacodynamic and tolerance study of NMI 861
This was a placebo-controlled, randomized, double-blind, two-way crossover design study of the pharmacokinetics and pharmacodynamics of orally administered NMI 861 in 12 healthy male subjects aged 18–35 years inclusive, free of any acute or chronic disease and within 15% of ideal body weight. Subjects were excluded if they were known to have used yohimbine, yohimbe, or l-arginine, had known sensitivity to these compounds, suffered chronic illness or acute illness in the previous 30 days, had a clinically significant abnormality following review of clinical laboratory data, had a clinically significant ECG abnormality, had a history or evidence of alcohol or drug abuse within the previous 12 months, had donated blood or blood products within the previous 90 days, smoked in excess of 5 cigarettes day−1, consumed xanthine-containing beverages in excess of two servings day−1, used prescription medication in the previous 30 days or over-the-counter medication in the previous 5 days, suffered any condition that would interfere with their ability to provide informed consent, were medical or nursing students, and had participated in a study of an investigational drug or device within the previous 90 days. Clinical assessments included laboratory tests, ECG, and monitoring of vital signs and adverse events. Subjects who fulfilled the inclusion and exclusion criteria were randomized to receive a single oral dose of NMI 861 containing 7.7 mg yohimbine tartrate (equivalent to 6 mg yohimbine HCl) and 6 g l-arginine glutamate (equivalent to 3.25 g l-arginine base) followed 1 week later by a single dose of placebo, or placebo followed 1 week later by a single oral dose of NMI 861.
Subjects fasted from midnight on the nights before the study days and eligibility was reassessed by history, a urine drug screen, and an alcohol breath test at 08.30 h on the morning of the study. Subjects who were still eligible were given 250 ml fruit juice to drink and placed on a bed with their upper body maintained at 70 ° and a cannula was then inserted in an antecubital fossa vein.
Prior to 09.00 h, a venous blood sample was collected and at approximately 09.00 h, each subject was administered NMI 861 or placebo. Vital sign monitoring, performed with subjects in the sitting position, commenced at 10 min prior to dosing, using a Dynamap® blood pressure monitor, and continued at 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 h after dosing. Additional blood samples for pharmacokinetic analysis were collected at 0.25, 0.5, 0.45, 1.0, 1.5, 2, 3, 4, 6, 8, 12 and 24 h post dosing. After 12 h, subjects were allowed to go home wearing Spacelab® ambulatory blood pressure monitors, which were set to measure pulse and blood pressure every hour through the night. Subjects returned at 08.00 h the following morning and eligibility for the study was reassessed by history, a urine drug screen, and an alcohol breath test. At 09.00 h the final blood sample was collected and vital signs were measured.
Study 2 – Pharmacodynamic interaction study of NMI 861 and intravenous GTN infusion
The study had a randomized, placebo-controlled, double-blind, crossover design. The pharmacodynamic effects of intravenous GTN infusion in the presence of orally administered NMI 861 in 16 healthy male subjects between 18 and 35 years old were investigated. The exclusion criteria were identical to those in Study 1 except that in addition, systolic blood pressure (SBP) had to be <100 mmHg or subjects had to have a history of symptoms consistent with hypotension. Clinical assessments included laboratory tests, ECG, and monitoring of vital signs and adverse events. Subjects who fulfilled the inclusion and exclusion criteria were randomized to receive GTN infusion after the oral administration of a single dose of NMI 861 containing 7.7 mg yohimbine tartrate (equivalent to 6 mg yohimbine HCl) and 6 g l-arginine glutamate (equivalent to 3.25 g l-arginine base) followed 1 week later by GTN infusion in the presence of placebo, or GTN infusion in the presence of placebo followed 1 week later by GTN infusion after the oral administration of a single dose of NMI 861.
Subjects fasted from midnight on the night before the study days and eligibility was reassessed by history, a urine drug screen, and an alcohol breath test at 08.00 h the following morning. Subjects who were still eligible were placed on a bed with their upper body maintained at 70 ° and a cannula was inserted in an antecubital fossa vein. Twenty minutes prior to oral administration of NMI 861, an infusion of normal saline to keep open the cannula was commenced. Pulse and blood pressure were recorded every 5 min from 10 min prior to administration of NMI 861 until 300 min post administration, using a Dynamap® blood pressure monitor. Forty minutes after the oral administration of NMI 861, GTN (0.5 mg ml−1 in 5% dextrose) was administered as a stepwise intravenous infusion starting at a rate of 2.5 µg min−1 and doubling every 15 min to a maximum dose of 40 µg min−1 or until the subject experienced symptomatic hypotension or a sustained decrease in SBP of >25 mmHg on repeat testing. Baseline blood pressure was calculated as the mean of the three measurements immediately prior to commencement of GTN infusion, i.e. 30–40 min after administration of NMI 861 or placebo. In the event of symptomatic hypotension or a sustained drop in SBP >25 mmHg, the GTN infusion was discontinued.
Drug analysis
Both yohimbine and l-arginine were analyzed by HPLC, based on previously published methods [11, 12]. For yohimbine the limits of detection and quantification, using the conditions described, were 0.5 ng ml−1 and 1.0 ng ml−1, respectively. The interassay variation over the calibration range 0.5–100 ng ml−1 was 5.3%. For arginine the limits of detection and quantification, using the conditions described, were 1.0 µg ml−1 and 5.0 µg ml−1, respectively. The interassay coefficient of variation over the calibration range 5–100 µg ml−1 was 4.2%.
Data analysis
SAS 6.12 and trapezoidal techniques were utilized to calculate the area under the curve (AUC) and other related pharmacokinetic parameters. Pharmacokinetic results are expressed as mean ± SEM and range. Phamacodynamic results are expressed as mean ± SEM. Results from a previous study suggested that a sample size of 10 subjects would provide 84% power to detect a 15 mmHg change in SBP from baseline at the 5% level of significance.
Frequency of side-effects between treatments was compared by McNemar's test. In the first study, the mean change from baseline of blood pressure and pulse were analyzed for the first 6 h, second 6 h and the remaining 12 h, separately. Comparison between the two groups at each of the three periods was performed using a paired t-test. Mean baseline SBP, diastolic blood pressure (DBP) and pulse and mean maximum change from baseline for SBP, DBP and pulse were compared by a paired t-test. In the second study, changes in blood pressure and pulse were calculated by comparison with mean values at 30–40 min after administration of NMI 861 or matched placebo, i.e. immediately before the start of GTN infusion. The mean values for the change from baseline for SBP, DBP and pulse during each dose of GTN and the maximum change during the infusion were calculated. Comparison between treatments for each of the GTN doses was by a pairedt-test as was the comparison of mean baseline values and mean maximum changes. Statistical significance was set at P < 0.05.
Results
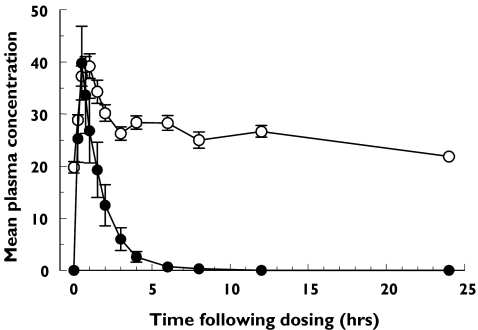
Owing to the presence of endogenous l-arginine, it was not possible to detect an increase in l-arginine concentrations after dosing compared with background concentrations at different times of the day. Therefore, AUC and t½ were not calculated for l-arginine. However, there was a consistent rise and decline in l-arginine concentration at the first 3 h after dosing as compared with the predose concentration suggesting that l-arginine was absorbed. The endogenous plasma l-arginine concentration during fasting immediately prior to dosing was 19.8 ± 1.1 µg ml−1 with a range of 12.7–27.7 µg ml−1. Upon oral administration of NMI 861, maximal plasma l-arginine concentration [Cmax (range)] increased to 42 ± 2.2 (28–63]µg ml−1 and its time to maximal concentration [tmax (range)] was 0.88 (0.50–1.5) h post dosing. As shown in Figure 1, the plasma l-arginine concentration did not further decline between 3 and 12 h suggesting the mean endogenous l-arginine concentration during nonfasting conditions was between 26.3 and 28.4 µg ml−1 with a range of 17.6–46.7 µg ml−1 in this study. For yohimbine, Cmax was variable with a mean (range) of 42 ± 11 (2.8–128) ng ml−1, tmax was 0.57 (0.24–1.0) h and half life (t1/2) was 1.0 ± 0.34 (0.40–6.0) h (Figure 1). Mean AUC(0, 8 h) (range) for yohimbine was 65 ± 24 (5.4–332), ng ml−1 h and tmax was similar for both compounds.
Figure 1.
Plasma concentrations of yohimbine and l-arginine following the oral administration of a single dose of NMI 861. Values represent the mean ± SEM data from 16 subjects. Yohimbine (µg/mL) (•), l-arginine (µg/mL) (○)
No subject from either placebo or NMI 861 groups reported any side-effects and in no subjects were there any clinically important changes in ECG and laboratory tests (haematology, serum chemistry and urinalysis) during the study.
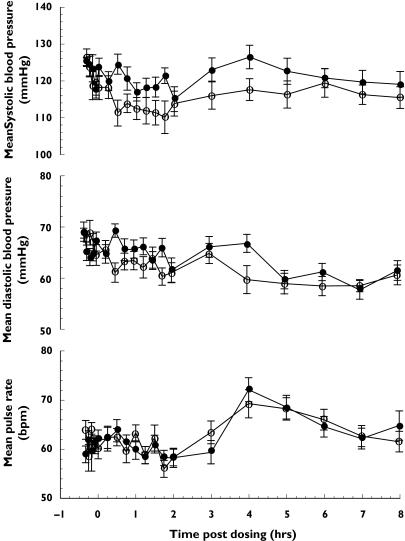
There was a small but significant difference in the mean change from baseline for SBP between NMI 861 and placebo treatments from 0 to 6 h only (Table 1). The mean SBP did not decrease from baseline after the administration of NMI 861, but SBP decreased for the first 2 h after the administration of placebo (Figure 2). There was no significant difference in SBP for the periods 6–12 h and 12–24 h for the two treatments. There was no significant difference in mean DBP or pulse rate between NMI 861 and placebo treatments for the periods of 0–6 h, 6–12 h and 12–24 h (Table 1). There was also no significant difference in maximum increase or decrease in SBP, DBP and pulse rate from baseline between NMI 861 and placebo treatments.
Table 1.
Haemodynamic effects of a single oral dose of NMI 861 vs placebo in healthy subjects
| Mean change from baseline ± SEM (P value) and mean difference between treatments (95% Cl) | |||
|---|---|---|---|
| 0–6 h | 6–12 h | 12–24 h | |
| Mean change in systolic blood pressure (mmHg) | |||
| Placebo | −4.1 ± 2.1 | 2.1 ± 2.4 | 0.3 ± 2.0 |
| NMI 861 | 0.8 ± 1.4 | 4.0 ± 1.5 | −0.2 ± 2.1 |
| (P = 0.047) | (P = 0.516) | (P = 0.859) | |
| 4.9 (0.0, 9.8) | 1.9 (−4.2, 8.0) | −0.5 (−6.6, 5.6) | |
| Mean change in diastolic blood pressure (mmHg) | |||
| Placebo | −3.6 ± 1.1 | −2.3 ± 1.5 | 2.2 ± 0.9 |
| NMI 861 | −0.8 ± 1.2 | −2.7 ± 1.2 | 0.8 ± 2.1 |
| (P = 0.138) | (P = 0.829) | (P = 0.537) | |
| 2.8 (−1.0, 6.6) | −0.4 (−4.5, 3.4) | −1.4 (−6.2, 3.4) | |
| Mean change in pulse rate (beats min−1 | |||
| Placebo | 1.8 ± 1.1 | 5.0 ± 1.9 | 5.8 ± 1.6 |
| NMI 861 | 1.8 ± 1.4 | 3.7 ± 2.0 | 4.1 ± 2.6 |
| (P = 0.995) | (P = 0.602) | (P = 0.621) | |
| 0.0 (−3.3, 3.3) | −1.3 (−6.6, 4.0) | −1.7 (−8.9, 5.5) | |
n = 16 subjects. P values were determined by paired t-test
Figure 2.
Haemodynamic effects of a single oral dose of NMI 861 vs placebo. Values represent the mean ± SEM data from 16 subjects. Placebo (○), NMI 861 (•)
Headache was common in both groups receiving GTN infusion. Ten out of 16 subjects receiving NMI 861 and GTN infusion reported mild to moderate headache. Six out of 16 subjects receiving placebo and GTN infusion reported headache, one of these being described as severe and requiring paracetamol. This difference between the groups was not significant. One subject receiving NMI 861 and GTN treatment reported feeling flushed. During the placebo and GTN treatment, one subject reported feeling light headed, one subject reported feeling flushed and one subject reported feeling hot and nauseous. This difference between the groups was not significant. No subjects recorded any clinically important change in ECG and laboratory tests during the study.
In no subject, either from placebo or NMI 861 treatment, was there a sustained 25 mmHg or greater drop in SBP, a sustained drop below SBP of 100 mmHg or symptomatic hypotension at any point before, during or after the GTN infusion. All subjects completed the protocol.
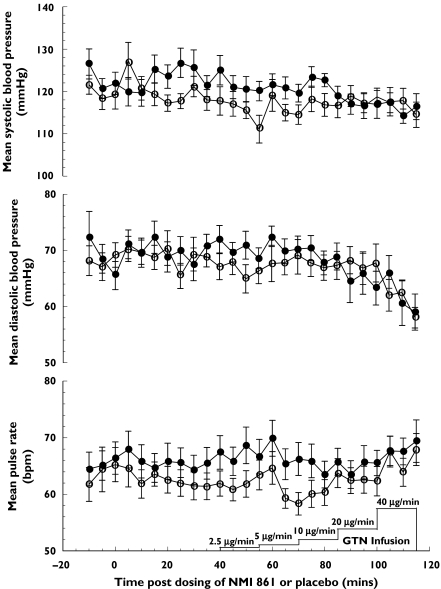
Baseline SBP, DBP and pulse, calculated as a mean of measurements at 30–40 min following NMI 861 or placebo administration, were not significantly different between treatments (Table 2). There was no significant difference between the NMI 861 and placebo treatments during the infusion of all the GTN doses of 2.5, 5, 10, 20 and 40 µg min−1 (Table 2 and Figure 3). The mean maximum changes from baseline during GTN infusion between subjects administered with either NMI 861 or placebo were not significantly different for SBP, DBP and pulse (Table 2).
Table 2.
Haemodynamic effects of intravenous GTN infusion starting 40 min after the oral administration of NMI 861 or placebo to healthy subjects
| Mean change from baseline* ± SEM (P value) and mean difference between treatments with 95% Cl GTN infusion (µg min−1)† | |||||||
|---|---|---|---|---|---|---|---|
| Mean baseline* ± SEM (P value) and mean difference with 95% Cl | 2.5 | 5 | 10 | 20 | 40 | Mean maximum change from baseline* ± SEM (P value) and mean difference with 95% CI | |
| Systolic blood pressure (mmHg) | |||||||
| Placebo | 119.0 ± 2.1 | −4.5 ± 1.6 | −2.4 ± 1.4 | −1.9 ± 1.3 | −1.4 ± 1.9 | −2.4 ± 2.1 | −13.6 ± 2.4 |
| NMI 861 | 123.4 ± 2.8 | −2.9 ± 2.3 | −2.8 ± 2.7 | −1.7 ± 3.1 | −5.7 ± 3.3 | −7.4 ± 3.0 | −16.9 ± 3.4 |
| (P = 0.106) | (P = 0.528) | (P = 0.893) | (P = 0.971) | (P = 0.329) | (P = 0.198) | (P = 0.460) | |
| 4.4 (−1.1, 9.9) | 1.6 (−3.8, 7.0) | −0.4 (−6.6, 5.8) | 0.2 (−8.1, 8.3) | −4.3 (−13.6, 5.0) | −5.0 (−13.0, 3.0) | −3.3 (−12.7, 6.1) | |
| Diastolic blood pressure (mmHg) | |||||||
| Placebo | 68.5 ± 2.2 | −2.1 ± 1.1 | −0.4 ± 1.3 | −1.2 ± 1.0 | −0.9 ± 1.4 | −7.7 ± 2.5 | −14.0 ± 2.0 |
| NMI 861 | 70.1 ± 2.3 | −0.4 ± 1.4 | 0.8 ± 1.2 | −1.1 ± 1.7 | −4.9 ± 1.9 | −8.2 ± 2.1 | −14.7 ± 2.0 |
| (P = 0.478) | (P = 0.418) | (P = 0.384) | (P = 0.963) | (P = 0.144) | (P = 0.898) | (P = 0.835) | |
| 1.6 (−3.3, 6.5) | 1.8 (−1.2, 2.4) | 1.1 (−1.7, 3.9) | 0.1 (−5.1, 5.2) | −4.0 (−9.7, 1.7) | −0.5 (−8.9, 7.9) | −0.7 (−8.2, 6.8) | |
| Pulse rate (beats min−1) | |||||||
| Placebo | 61.6 ± 2.2 | 0.6 ± 0.9 | −0.5 ± 1.2 | 0.0 ± 1.7 | 1.0 ± 1.4 | 5.1 ± 2.3 | 14.1 ± 2.4 |
| NMI 861 | 65.8 ± 2.5 | 1.3 ± 1.2 | 1.4 ± 1.5 | −0.7 ± 1.4 | −0.9 ± 1.4 | 2.5 ± 1.3 | 11.8 ± 1.9 |
| (P = 0.134) | (P = 0.718) | (P = 0.396) | (P = 0.792) | (P = 0.458) | (P = 0.373) | (P = 0.464) | |
| 4.1 (−1.5, 9.7) | 0.7 (−3.4, 4.8) | 1.9 (−2.9, 6.7) | −0.7 (−6.1, 4.7) | −1.9 (−7.4, 3.6) | −2.6 (−8.7, 3.5) | −2.4 (−9.3, 4.5) | |
n = 12 subjects.
Each dose of GTN was infused for 15 min before the dose was doubled.
P values were determined by paired t-test.
Figure 3.
Haemodynamic effects of infused GTN following the oral administration of a single dose of NMI 861 or placebo. Values represent the mean ± SEM data from 12 subjects. Placebo (○), NMI 861 (•)
Discussion
Orally administered NMI 861 was well tolerated in all subjects, both alone and in combination with intravenous GTN. No adverse events were reported after administration of placebo or NMI 861 alone and no clinically important change in ECG and laboratory tests was recorded for any subject in both studies. Common adverse effects of headache, light headedness and flushing after initiation of GTN infusion in both NMI 861 and placebo treatments were attributable to the GTN.
The pharmacokinetic parameters for yohimbine after the oral administration of NMI 861 were similar to those reported for oral administration of yohimbine HCl to fasted healthy subjects by other investigators (for review see Tam et al.[3]. The Cmax of yohimbine was highly variable and its terminal half-life was short. Peak plasma concentrations of yohimbine and l-arginine occurred at a mean of 0.57 and 0.88 h post dosing, respectively. This is consistent with the use of NMI 861 as an oral, on demand treatment for male erectile dysfunction between 0.5 and 1 h before intercourse [5, 6].
l-arginine, a substrate for nitric oxide synthases, when administered alone acutely by intravenous infusion (500 mg kg−1 over 30 min) decreases blood pressure in both normotensive and hypertensive subjects [13]. l-arginine administered orally at 9–18 g day−1 for 14 days to hypertensive haemodialysis patients or chronically for 2 months to hypertensive kidney transplant patients resulted in a decrease in both SBP and DBP [14]. The dose of l-arginine in NMI 861 (6 g) is less than that used in the study of Kelly et al.[14].
The effects of NMI 861 contrast with the vasodilating effect of sildenafil, which given alone produces mean maximum decreases in SBP and DBP of 8 mmHg and 6 mmHg, respectively [9]. Furthermore, sildenafil interacts with nitrates to amplify their hypotensive effect [10]. In a study using the same number of subjects and similar doses of intravenous GTN to those used in our study, all 12 healthy male subjects experienced decreases in SBP of greater than 25 mmHg or symptomatic hypotension when pretreated with 25 mg sildenafil three times daily for 4 days [10]. We detected no such interaction between NMI 861 and nitrate in the present study. None of the NMI 861-treated subjects had to interrupt the GTN infusion. In the study by Webb et al.[10], all sildenafil-treated subjects had to interrupt GTN infusion because of an excessive hypotensive response. There are important methodological differences between the study by Webb et al. and the present study. Thus, sildenafil was administered 60 min prior to GTN infusion whereas in this study NMI 861 was administered 40 min prior to intravenous GTN infusion. Furthermore, the dose of intravenous GTN was doubled every 5 min in the sildenafil study compared with every 15 min in this study [10]. The slower GTN dose escalation was used to ensure that no symptomatic hypotension was observed before the administration of the next higher dose of GTN. As a result of the slower GTN dose escalation, the mean systemic exposures of yohimbine and l-arginine were 46% and 85% of the concentrations at Cmax, respectively, at the beginning of the infusion of the highest dose of GTN. In our study, GTN produced hypotension of a similar magnitude in both NMI 861- and placebo-treated subjects. It has been clearly demonstrated by previous studies that sildenafil has a clinically relevant interaction with nitrates, whereas no such interaction was detected with NMI 861 in the current study. The interaction between sildenafil and GTN is easily rationalized given that nitrates release NO which relaxes the smooth muscle by activation of soluble guanylate cyclase, raising intracellular cGMP concentrations. Sildenafil impairs cGMP metabolism via phosphodiesterase type 5 inhibition resulting in cGMP accumulation, thereby amplifying the hypotensive effect of nitrates. This interaction is clinically important in that male erectile dysfunction and coronary artery disease often coexist and that many subjects with male erectile dysfunction are either treated or have the potential to require treatment with nitrates.
The combination of yohimbine and l-arginine has been shown to be bioavailable, safe and well tolerated. In contrast to sildenafil and other phosphodiesterase type 5 inhibitors, there is no evidence of a clinically significant adverse pharmacodynamic interaction between intravenous nitrates and yohimbine and l-arginine in healthy subjects under the experimental conditions in which the study was conducted.
Acknowledgments
This study was funded by a grant from NitroMed, Inc.
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159:433–6. doi: 10.1016/s0022-5347(01)63942-9. [DOI] [PubMed] [Google Scholar]
- 3.Tam SW, Worcel M, Wyllie M. Yohimbine: a clinical review. Pharmacol Ther. 2001;91:215–43. doi: 10.1016/s0163-7258(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovascular Pharmacol. 1999;34:879–86. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Lebret T, Herve JM, Gorny P, Worcel M, Botto H. Efficacy and safety of a novel combination of l-arginine glutamate and yohimbine hydrochloride: a new oral therapy for erectile dysfunction. Eur Urol. 2002;41:608–13. doi: 10.1016/s0302-2838(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 6.Padma-Nathan H. Hemodynamic effects of the eral administration of a combination of arginine and yohimbine measured by color duplex ultrasonography in men with erectile dysfunction (ED) Third Meeting of the European Society for Impotence Research (ESIR) 2000:28. [Google Scholar]
- 7.Meston CM, Worcel M. The effects of yohimbine plus 1-arginine glutamate on sexual arousal in postmenopausal women with sexual arousal disorder. Arch Sexual Behav. 2002;31:323–32. doi: 10.1023/a:1016220225392. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–404. doi: 10.1056/NEJM199805143382001. Sildenafil Study Group.[see comment] [erratum appears in N Engl J Medical 1998 July 2; 339 (1): 59]. [DOI] [PubMed] [Google Scholar]
- 9.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 10.Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol. 1999;83:21C–28C. doi: 10.1016/s0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 11.Lindroth P, Mopper K. High performance liquid chromatography determination of subpicomole amounts of amino acids by precolumn fluorescence derivation with o-phthaldialdehyde. Anal Chem. 1979;51:1667–74. [Google Scholar]
- 12.Owen JA, Nakatsu SL, Condra M, Surridge DH, Fenemore J, Morales A. Sub-nanogram analysis of yohimbine and related compounds by high-performance liquid chromatography. J Chromatogr A. 1985;342:333–40. doi: 10.1016/s0378-4347(00)84524-7. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Oshima T, Ozono R, Matsuura H, Kambe M, Kajiyama G. Effect of 1-arginine infusion on systemic and renal hemodynamics in hypertensive patients. Am J Hypertension. 1999;12(Part 1):t–15. doi: 10.1016/s0895-7061(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 14.Kelly BS, Alexander JW, Dreyer D, Greenberg NA, Erickson A, Whiting JF, et al. Oral arginine improves blood pressure in renal transplant and hemodialysis patients. J Parenteral Enteral Nutrition. 2001;25:194–202. doi: 10.1177/0148607101025004194. [DOI] [PubMed] [Google Scholar]