Abstract
Aims
The Aerodose® inhaler is a novel, palm-sized, breath actuated device which requires little patient coordination. This study compared the dose–response of salbutamol delivered by the Aerodose® Inhaler (Aerogen Inc., Mountain View, USA) vs Pari LC Plus jet nebulizer (Pari LC Plus; Pari GmbH, Starnberg, Germany) and Ventolin™ Evohaler™ HFA pMDI (Evohaler; Allen & Hanburys [GlaxoSmithkline], Uxbridge, UK).
Methods
Twenty-two moderate to severe asthmatic patients, mean (s.d.) age: 44.7 (9.4), FEV1: 58.1 (12.0), received 4 cumulative doubling doses of salbutamol in a randomised, investigator blind, balanced crossover design. Spirometry and systemic safety variables (heart rate, blood pressure, T wave amplitude, QTc interval and potassium) were measured at baseline and after each dose.
Results
Parallel regression analysis revealed that microgram relative potency ratios for the Aerodose® Inhaler to be five times more efficient for FEV1 than either the Pari LC Plus (0.202, 90% Cl: 0.189–0.216) or the Evohaler (0.202, 90% Cl: 0.189–0.216), while there was no difference between Pari LC Plus vs Evohaler. Similarly, Aerodose® Inhaler vs. Pari LC Plus showed approximately five-fold greater potency for all systemic parameters, except blood pressure. As compared to the Evohaler, Aerodose® Inhaler had equivalent potency for plasma potassium and T wave amplitude, but demonstrated greater potency for heart rate and QTc interval.
Conclusions
This study has indicated therefore, that Aerodose® Inhaler is approximately five times as efficient as the Pari LC Plus and Evohaler in relative lung delivery of salbutamol in moderate to severe asthmatics.
Keywords: asthma, Inhalation devices, salbutamol
Introduction
Inhaled short-acting β2-agonists, such as salbutamol, are the bronchodilator agents of choice for treating acute asthma symptoms and exacerbations [1]. Facilitating delivery direct to the airways, inhalation delivery maximizes the efficiency of the drug while minimizing systemic adverse effects.
Nebulizers and metered dose inhalers (MDIs) plus large volume spacers are the preferred delivery systems for use with high dose inhaled bronchodilators and are commonly used in patients with more severe asthma. However, both devices have their limitations. Firstly, conventional nebulizers are typically inefficient as they lose a substantial percentage of drug through dissipation into the atmosphere by delivering during both inspiration and expiration, in addition to leaving a large residual (0.5–1.0 ml) volume at the end of dosing [1]. Furthermore, a high degree of variability in particle size emitted by commonly used nebulizers also reduces the efficiency of lung deposition of aerosol [1–3]. Although they are less dependent on patient coordination and enable the delivery of higher doses during severe exacerbations than hand held inhalers, conventional nebulizers have been described as ‘expensive, time-consuming, and bulky’[1].
An alternative to nebulizers is the pressurized metered dose inhaler (pMDI) with or without a large volume spacer. Alone, the pMDI is small, portable, and relatively inexpensive, and is useful for both less severe exacerbations and as maintenance therapy [1]. However, the need for a high degree of patient coordination to enable optimum delivery, and the possibility of spontaneous bronchospasm [4] in response to the propellant, means that it is often not a therapeutic option for many patients [1]. Furthermore, even when optimum coordination is achieved, a high proportion of the dose administered by pMDI is deposited in the oropharynx, rather than the lungs. Improved delivery with a reduction in oropharynx deposition and the likelihood of spontaneous bronchoconstriction can be achieved with the addition of a spacer device. However, these devices are typically bulky and still require some patient coordination.
A novel inhaler, the Aerodose® Inhaler, is currently being developed for the delivery of aerosolized medications. The Aerodose® Inhaler is a small, silent, palm sized battery powered device (Figure 1). Overcoming many of the limitations of the conventional nebulizers and pMDIs, aerosolization via the Aerodose® Inhaler is triggered by an inspiratory flow of −12 l min−1 where upon the high-frequency oscillation of a curved metal aperture plate (AeroGen's Aerosol Generator) emits particles of 3–5 µm mass median aerodynamic diameter (MMAD). Aerosolization continues for a preset time limit provided that the inspiratory flow through the device is maintained above −12 l min−1[10]. This low threshold, breath actuated delivery system, enables the device to be used during tidal breathing, without coordination and with minimal loss of dose.
Figure 1.
Picture of Aerodose® Inhaler
The primary objective of our study was to compare the efficacy of salbutamol delivered by Aerodose® Inhaler, as compared with delivery by a conventional nebulizer (Pari LC Plus) and a pMDI (Ventolin Evohaler), in patients with moderate to severe persistent asthma. Secondary objectives were to assess the safety and tolerability of salbutamol delivered via Aerodose® Inhaler, as compared with the conventional nebulizer and pMDI. Patients with moderate to severe persistent asthma were selected as they are most likely to require high dose salbutamol via a nebulizer.
Device efficiency was assessed using pharmacodynamic measures of salbutamol response, including spirometry (forced expiratory volume in 1 s [FEV1], forced vital capacity [FVC], forced expiratory fraction from 25 to 75 % [FEF25–75] and peak expiratory flow rate [PEFR]) [5–8] plasma potassium (K+) [7–9], blood pressure, heart rate [8, 9], QTc interval and T wave amplitude [7].
Methods
Patients
The study was of a single-centre, investigator-blinded, randomized, three-way crossover design. Twenty-four patients (non-smokers of which 10 were males) with at least a 12 month history of moderate to severe persistent asthma, mean (s.d.) age: 44.7 (9.3) years, FEV1 57.8 (12.4)% predicted and 26.8 (8.9)% increase in FEV1 following 400 µg inhaled salbutamol (via Diskus) were screened. All patients had vital signs (blood pressure and heart rate) and chest X-ray within normal limits, no clinically important abnormalities of haematology and serum biochemistry and 12-lead ECG, no history of other lung disease which would affect the bioavailability of salbutamol, and were able to tolerate temporary withholding of bronchodilator therapy prior to all visits. Approval was obtained from the Tayside committee for Medical Ethics prior to the study. Patients provided signed consent prior to any invasive screening procedures being performed and a pregnancy test was performed on women of child-bearing potential at screening and at each study visit. Twenty-two patients (9 male), mean (s.d.) age: 44.7 (9.4) years, FEV1: 58.1 (12.0)% predicted, and 26.6 (8.9)% reversibility in FEV1 post 400 µg salbutamol completed the study per protocol.
Treatments
The in vitro relative device efficiency was used to determine the putative dose ratios of salbutamol sulphate used for Aerodose® Inhaler as compared to the Pari LC Plus and Ventolin Evohaler. For the Pari-LC Plus and the Aerodose® Inhaler, 2.5 ml 0.1% w/v salbutamol sulphate (i.e. 2.5 mg) as Ventolin Nebules (Allen & Hanburys [GlaxoSmithkline], Uxbridge, UK) were diluted to a final 3 ml volume by the addition of 0.5 ml 0.9% NaCl. For a 2.5 mg nominal dose (in 3 ml volume) of salbutamol sulphate from the Pari LC plus, assuming a residual volume of 1 ml (12), for the remaining 2 ml assuming an inhaled fraction of 25% [10], the inhaled drug volume via the Pari LC Plus would be 0.5 ml. In comparison, 88% of the emitted volume from the Aerodose® Inhaler assuming a residual volume of only 0.02 ml (12), along with an inhaled fraction of 88%, the inhaled drug volume via the Aerodose would be 2.62 ml [10, 11]. The respirable fraction of the inhaled drug volume for both devices is similar at approximately 80%.
To ensure that a comparable maximum delivered dose was given via the Aerodose inhaler vs the Pari LC Plus, a putative dose ratio of 0.2 was used, i.e. for 3 ml (2.5 mg salbutmaol sulphate) via the Pari LC Plus, 0.6 ml (0.5 mg salbutamol sulphate) was given via the Aerodose® Inhaler. Eight, 100 µg actuations of salbutamol from the Evohaler was selected as the highest cumulative dose, as this is regarded to be approximately equivalent to 2.5 mg salbutamol administered from a nebuliser [12]. Therefore the putative dose ratio for salbutamol sulphate from the Aerodose® Inhaler vs the Ventolin Evohaler was 0.6.
Doses equivalent to a cumulative dose of 2.5 mg salbutamol administered by the Pari LC Plus, were delivered by the Evohaler and the Aerodose® Inhaler immediately prior to dosing and used as a stock solution for the cumulative doses. At screening, the duration of time required for patients to inhale 3 ml sterile water via the Pari LC Plus was determined. It was anticipated that the duration to deliver all doses via both the Evohaler and the Aerodose® Inhaler would be considerably shorter than for the Pari LC Plus. To ensure that the end of dosing coincided for all treatments enabling the pharmacodynamic responses to be compared, the start of dosing for each of the Aerodose® Inhaler and Evohaler doses was delayed so that the end of dosing coincided with the end of the duration required for dosing via the Pari LC Plus, or the dosing duration. All patients showed adequate technique with all devices at screening and prior to dosing on each treatment day.
Individual and cumulative doses are shown in Table 1. For the Aerodose® Inhaler doses were administered as varying volumes stock salbutamol sulphate solution (i.e. 2.5 mg in 3 ml). Volumes were pipetted into the reservoir using a guiding plug with dedicated Gilson micropipette and sterile pipette tip (Gilson Inc. Middleton, Wisconsin USA). The guiding plug was replaced with an actuation plug immediately prior to dosing. Patients were directed to breath in via the integral mouthpiece at tidal volume, remove the device from the mouth and exhale. This maneuvre was repeated until the end of dosing was indicated by a light on the device. All devices passed in house in vitro device testing prior to use in the study [13].
Table 1.
Individual (actual) and cumulative (Σ) doses administered via Aerodose® Inhaler, Pari LC Plus nebuliser and Ventolin Evohaler pMDI. Volume per dose for the Aerodose® Inhaler and Pari LC Plus nebuliser, and puffs/dose for the Ventolin Evohaler pMDI are also shown
| Aerodose® Inhaler | Pari LC Plus Nebulizer | Ventolin™ Evohaler™ (pMDI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose | Actual (μg) | Σ (µg) | Volume (ml) | Actual (µg) | Σ (µg) | Volume (ml) | Actual (µg) | Σ | Puffs |
| 1st | 62.25 | 62.25 | 0.075 | 312.5 | 312.5 | 3.0 | 100 | 100 | 1 |
| 2nd | 62.25 | 124.5 | 0.075 | 312.5 | 625 | 3.0 | 100 | 200 | 1 |
| 3rd | 124.5 | 249 | 0.150 | 625 | 1250 | 3.0 | 200 | 400 | 2 |
| 4th | 249 | 498 | 0.300 | 1250 | 2500 | 3.0 | 400 | 800 | 4 |
For the Pari-LC Plus, compressed air, generated by the Pari TurboBOY (Pari GmbH, Starnberg, Germany) compressor at a flow rate of 5 l min−1 was used as the driving gas to generate salbutamol aerosols. Patients used a mouthpiece and breathed at tidal volume until nebulization was complete as indicated by the presence of sputtering.
100 µg salbutamol ex-valve per actuation was given via the Ventolin Evohaler, a HFA-containing metered dose inhaler. At the same time as actuating the device, patients were instructed to inhale from residual volume to total lung capacity followed by a 10 s breath-hold. In a room separate from the patient and immediately prior to each dose, inhalers were shaken and actuated 4 times to ensure maximal output [14]. A 30 s pause was given between the end of breath hold and the next actuation, as per the manufacturer's guidelines.
In order to ensure blinding of the study, the overall duration of dosing was maintained the same for all devices. The time required to deliver 3 mls via the Pari LC plus to sputtering was determined at screening. To accommodate for the much shorter duration of dosing for the Evohaler and AeroDose, the start of the dosing for each device was delayed so the end of dosing coincided with the end of the dosing duration. For example, the Pari LC plus dosing duration determined at screening was 10 min. At the lowest dose for each device, the time required to give the dose via the AeroDose or Evohaler was 1 min and <30 s, respectively. Therefore the start of dosing for the AeroDose or Evohaler would be 9 min and 9.5 min, respectively, into the dosing duration for the Pari LC Plus.
Measurements
To ensure that outcome measurements were conducted in a blinded fashion, dosing was conducted in a separate room and by persons independent from those performing the outcome measures. For an individual subject, the baseline measurements for all visits were conducted within a 2 h window, and each treatment was separated by a minimum of 48 h.
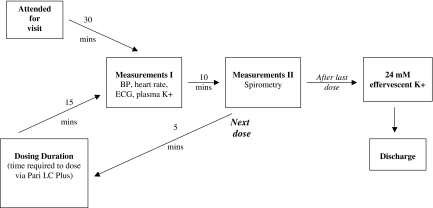
A schematic of the visit schedule is shown in Figure 2. At each visit, after a 30-min period of supine rest, systemic endpoints: blood pressure and heart rate measured using a Dinamap 1846SX (Critikon Inc, Tampa FL, USA), and lead II ECG (Hewlett Packard Inc 78351 A monitor and printer, Palo Alto, California, USA), were recorded and a 5 ml blood sample for plasma potassium were taken. Efficacy endpoints (standing spirometry, conducted according to American Thoracic Society criteria, using a Vitalograph Compact II spirometer [Vitalograph Ltd, Buckingham, UK]) were conducted within a further 10 min interval. The lowest dose of salbutamol was administered if the FEV1 was within 15% of screening and/or first treatment visit.
Figure 2.
Schematic of Visit Schedule. Blood pressure, lead II ECG, and potassium are indicated as BP, ECG and K+, respectively
Fifteen and 25 min following the end of each dose, systemic and efficacy endpoints were measured. The next dose was given 30 min after the end of the dosing duration (i.e. 5 min after second set of measurements). On each visit day, after the measurements had been taken following the last dose, 24 mmol effervescent potassium was administered to off-set the possible salbutamol induced potassium depletion. Patients were retained in the unit until T wave amplitude had returned to at least 50% of baseline and heart rate was ≤25 beats per minute over baseline.
The RR (s) and QT (ms) intervals were measured from the mean of three consecutive complexes. The heart rate was calculated from the RR interval and the formula of Bazett was used to correct the QT interval for heart rate (QTc). Plasma potassium was analysed by flame photometry using an Instrumentation Laboratory 943 analyser (Instrumentation Laboratory Ltd, Warrington, UK).
Statistical Analysis
The primary endpoint was FEV1, with secondary endpoints being FVC, FEF25–75, PEFR, K+, heart rate, QTc interval and T wave amplitude. A log dose–response comparison for all four doses of the three delivery devices was performed by parallel regression analysis using the Finney bioassay method to generate relative potency ratios (for the nominal dose of salbutamol sulphate) and 90% confidence intervals. Prior to constructing fitted lines from the parallel regression and an assessment of an overall dose–response relationship, an absence of deviation from parallelism and an absence of deviation from overlap were ensured. All statistical tests were performed with a significance level of 5% (α = 0.05). The relative potencies are the results of fitting a two-way analysis of variance model of doses and patients, and then modelling the doses on a Log10 scale. The analysis of variance model used for statistical analysis included patient, visit, treatment (or inhaler) and dose. Additional sets of analyses included pairwise comparisons between the inhalers using Tukey's test and the dose effects adjusted for the baseline, the latter analysis using Dunnett's paired comparison with a control.
Results
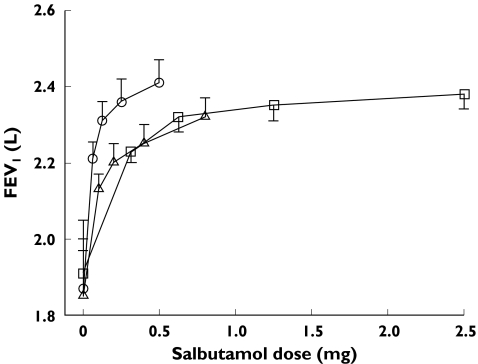
There were no significant differences among the baseline data at any visit for any of the efficacy or systemic variables (Table 2). A statistically significant change from baseline in FEV1 was found at all dose levels for all three devices (Figure 2). The FEV1 dose–response curves demonstrate that at least the two highest doses with each device are on or near the plateau of the dose–response curve.
Table 2.
Mean (standard deviation) baselines prior to each randomised treatment for efficacy and systemic endpoints. There were no significant differences for any endpoints
| Aerodose® Inhaler | Pari LC Plus Nebulizer | Ventolin Evohaler | ||||
|---|---|---|---|---|---|---|
| Airway endpoints | ||||||
| FEV1 (L) | 1.87 (0.61) | 1.91 (0.70) | 1.85 (0.61) | |||
| FEF25–75 (L/ s) | 1.14 (0.46) | 1.17 (0.58) | 1.14 (0.53) | |||
| Systemic endpoints | ||||||
| K+ (mmol/l) | 3.98 (0.18) | 3.97 (0.30) | 3.89 (0.22) | |||
| T wave amplitude (mV) | 0.30 (0.20) | 0.33 (0.18) | 0.26 (0.14) | |||
| QTc Interval (ms) | 384.6 (24.5) | 385.5 (22.7) | 397.6 (20.6) | |||
| Heart Rate (bpm) | 70 (8.2) | 68 (10.3) | 71 (8.79) | |||
| Systolic BP (mmHg) | 129 (13.7) | 132 (19.0) | 132 (16.3) | |||
| Diastolic BP (mmHg) | 79 (7.4) | 78 (11.3) | 80 (12.4) | |||
Figure 2 shows log salbutamol dose–response curves for efficacy endpoints, FEV1 and FEF25–75 and systemic endpoints, K+ and heart rate. Comparable increases in FEV1 from baseline were shown for all three devices at each dose level. The log salbutamol-FEV1 dose–response curves were parallel suggesting linear efficiency for all devices. However, the dose–response curve for Aerodose® Inhaler was shifted to the left indicative of greater response in FEV1 for a given dose. Estimation of the dose of salbutamol required to be delivered via Pari LC Plus and Evohaler to generate the same response as 100 µg salbutamol via Aerodose® Inhaler (Table 3) for FEV1 showed the Aerodose® Inhaler to be approximately five times as efficient at delivering salbutamol as either the Pari LC Plus or the Evohaler, while no difference in efficiency was found between the Pari LC Plus and Evohaler. Comparable differences were found between devices for FEF25–75 (see Table 3), FVC and PEFR (data not shown).
Table 3.
Estimated doses (90% confidence intervals) required by Pari LC Plus and Evohaler to generate an equivalent response to 100 µg. 5 g salbutamol via for Aerodose® Inhaler for efficacy and systemic endpoints. Estimated dose required by Evohaler to generate an equivalent response to 100 µg. 5 g salbutamol via Pari LC Plus is also shown
| Measure | Dose via Pari equivalent to 100 µg via Aerodose® Inhaler | Dose via Evohaler equivalent to 100 µg via Aerodose® Inhaler | Dose via Evohaler equivalent to 100µg via Pari |
|---|---|---|---|
| FEV1 (L) | 495 (526–714) | 495 (526–714) | 100 (93–107) |
| FEF25–75 (L/ s) | 366 (323–417) | 366 (323–417) | 100 (99–101) |
| K+ (mmol/l) | 278 (256–303) | 100 (93–109) | 36 (33–39) |
| Heart Rate (bpm) | 1235 (1000–1667) | 270 (222–345) | 22 (17–26) |
| QTc Interval (ms) | 559 (500–625) | 270 (244–303) | 48 (43–53) |
| T wave Amplitude (mV) | 735 (667–833) | 100 (90–111) | 14 (12–15) |
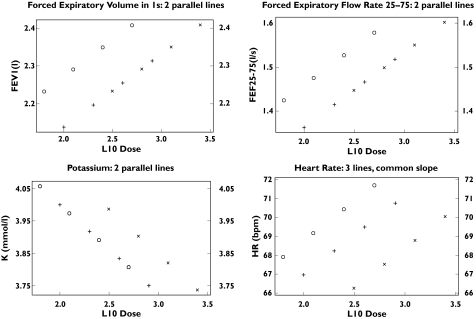
Dose–response curves were generated for plasma potassium, T wave amplitude, QTc interval and heart rate enabling the estimation of equivalent doses required to produce a response equivalent to 100 µg via Aerodose® Inhaler (Table 3). For all devices, absolute changes from baseline even after the highest dose were of a small magnitude and not clinically relevant (Table 4). For both systolic and diastolic blood pressure it was not possible to generate parallel dose–response curves and therefore no dose equivalencies could be determined. The dose–response curves for all systemic endpoints for the Aerodose® Inhaler were shifted to the left of the Pari LC Plus (Figure 3) as reflected in the significantly higher estimated salbutamol doses required by the Pari LC Plus to produce the same response as 100µg salbutamol via the Aerodose® Inhaler (Table 3). Estimated equivalent doses (Table 3) for the Evohaler were comparable with the Aerodose® Inhaler for potassium (Figure 3) and T wave amplitude, but were significantly higher for heart rate and QTc interval. Estimated equivalent doses were also significantly lower for the Evohaler as compared to the Pari LC Plus for all endpoints (Table 3).
Table 4.
Mean (standard deviation) absolute change from baseline for systemic endpoints following highest dose of salbutamol for each device. There was no significant differences among the three devices for any variable
| Measure | Aerodose® Inhaler | Pari | Evohaler |
|---|---|---|---|
| K+ (mmol/l) | −0.23 (0.27) | −0.28 (0.30) | −0.14 (0.31) |
| T wave amplitude (mV) | −0.08 (0.14) | −0.09 (0.11) | −0.03 (0.08) |
| QTc Interval (ms) | 13.3 (15.8) | 13.9 (23.7) | −3.4 (17.0) |
| Heart Rate (bpm) | 2.7 (8.3) | 2.5 (6.9) | −0.4 (7.4) |
| Systolic BP (mmHg) | 2.5 (10.5) | 1.1 (12.9) | 2.2 (12.9) |
| Diastolic BP (mmHg) | −1.4 (6.1) | 0.9 (8.3) | −0.5 (7.7) |
Figure 3.
Mean salbutamol dose–response for forced expiratory volume in one second (FEV1). The change from baseline was statistically significant (P < 0.0001) at each dose level for all three devices (Aerodose® Inhaler, ○, Pari LC Plus, □, and Ventolin Evohaler, ▵)
Discussion
The lung deposition of inhaled β2 adrenoceptor agonists, has been shown to be directly related to both clinical efficacy (bronchodilator endpoints) and systemic potency (including K+, QTc interval, T wave amplitude and heart rate) [5] in cumulative dose–response studies [6–9, 15]. In addition, changes in K+ have been shown to highly correlate with plasma salbutamol, suggesting that changes in K+ could be used as potential surrogate to evaluate the relative lung delivery of salbutamol from different inhaler devices [7]. At one fifth of the dose of the Pari-LC Plus, we have shown here that the Aerodose® Inahler has equal efficacy with no significant difference in systemic endpoints as the Pari LC Plus suggesting that the Aerodose® Inhaler is five times as efficient as the Pari LC Plus. We have shown that although 0.62 of the cumulative nominal dose administered by the Evohaler was given by the Aerodose® Inhaler, in terms of efficacy variables, the Aerodose® Inhaler was also five times as efficient as the Evohaler. These results are in line with in vitro data estimating the percentage respirable fraction to be 57% for the Aerodose® Inhaler [11] compared to 12.8% for the Pari LC Plus [16]. The comparability in terms of efficacy between the Pari LC Plus and the Evohaler shown here is in agreement with previous data by Zainudin et al.[17] and Eiser et al.[18], although this later study was performed in COPD and not asthma patients.
This is the first time that the Aerodose® Inhaler has been used in an asthmatic population, although the aerosol generator technology has been incorporated into the FDA cleared AeroNeb™ portable nebulizer system. We suggest that the greater efficiency of the Aerodose® Inhaler as compared to the Pari LC Plus shown here is related to the higher emitted volume during inhalation and lower residual volume rather than a greater proportion of particles within the 3–5 µm range. Indeed the emitted dose during inhalation via the Aerodose® Inhaler has been shown to be almost 88% of the dose as compared to 25% via the Pari LC Plus [10, 11], while the residual volume for the Aerodose® Inhaler was only 0.1 ml, one-fifteenth of the residual volume of the Pari LC Plus (12). Indeed with the Pari LC plus the solution in the chamber becomes relatively more concentrated with time due to vapourization. The proportions of particles emitted within the 3–5 µm range were comparable between the Aerodose® Inhaler Aerosol Generator (31%) [13] and Pari LC Plus (30%) [19]. In terms of the Evohaler as compared to the Pari LC Plus, confirming our results, Fowler and Lipworth reported, in a meta-analysis, that a 1200 µg nominal dose of salbutamol via a Sidesteam nebulizer (Medicaid, Pagham, UK) was approximately equivalent to 400 µg via HFA pMDI in terms of salbutamol bioavailability, a ratio of approximately 3 : 1 [20]. Furthermore, similar to Fink & Dhand [12], we have shown the Pari LC Plus and the Ventolin Evohaler to be equivalent at all doses for most variables. We did, however, observe an unexplainable dose dependent increase in heart rate with the Evohaler which was not observed with the Pari LC Plus and which we were unable to explain.
Figure 4.
Fitted lines from parallel regression analysis for log 10 salbutamol sulphate dose (μg) response for airway endpoints: forced expiratory volume in one second (FEEV1) and forced expiratory flow rate 25–75 (FEE25–75) and systemic endpoints: serum potassium (K+) and heart rate. The antilog of log 10 2.0 = 100 μg 2.5 = 316 μg, 3.0 = 1000 μg, 3.5 = 3162 μg Regression lines are shown for the Aerodose® inhaler (Aero, o), Pari LC Plus (Pari, x) and Ventolin Evohaler (Evo, +)
Comparison of conventional nebulizers in vitro, has shown that although the Pari LC Plus is superior to other jet nebulizers it is still a relatively inefficient device. Barry & O’Callaghan [19] showed that when using the Pari LC Plus with the TurboBOY compressor, it took 6.1 (1.3) minutes to deliver the dose to sputtering, which was shorter than the Pari LC Star with TurboBOY, Sidestream, Ventstream (Medicaid, Pagham, UK) and the Cirrus (Intersurgical Ltd, Wokingham, UK). However, the Pari LC Plus produced less particles of <5 µm (49%) compared to the other devices (∼70%), although the output from the Pari LC Plus was significantly greater than Ventstream, and Cirrus, but not the Sidestream. Similar results were shown by Devadason et al.[10] for salbutamol and Barry and O’Callaghan [21] for budesonide. Quieter and less bulky than jet nebulizers, a higher percentage of efficiently aerosolized drug has been reported for ultrasonic nebulizers (17.3 ± 6.7%) than for jet nebulizers (9.7 ± 9.6%) [22], although these devices are still inefficient as compared to the Aerodose® Inhaler (57%) [11]. However to date, no data are available on the relative comparability of nebulizer devices in adult asthmatics in vivo.
In summary, this study has indicated therefore, that Aerodose® Inhaler is approximately five times as efficient as the Pari LC Plus and Evohaler in relative lung delivery of salbutamol in moderate to severe asthmatics.
Acknowledgments
Conflict of Interest: Dr Erika J. Sims has received funding from AeroGen Inc. to attend an international meeting. At the time the study was performed, Ms Karla Taylor was employed by AeroGen Inc & Dr Robert Fishman is currently employed by AeroGen Inc & both hold shares in the company. Professor Brian J. Lipworth received funding from AeroGen Inc. to perform this study. Mrs Wendy Cockburn has no conflicts of interest.
References
- 1.National Institutes of Health. National Asthma Education and Prevention Programme. Expert Panel Report II. Guidelines for the Diagnosis and Management of Asthma. 1997. Publication no. 97–4053, Bethesda, MD 97. A.D. Ref Type: Generic.
- 2.Mason JW, Miller WC. Abbreviated aerosol therapy for improved efficiency. Aerosol Med. 1998;11:127–31. doi: 10.1089/jam.1998.11.127. [DOI] [PubMed] [Google Scholar]
- 3.Hultquist C, Wollmer P, Eklundh G, et al. Effect of inhaled terbutaline sulphate in relation to its deposition in the lungs. Pulm Pharmacol. 1992;5:127–32. doi: 10.1016/0952-0600(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 4.Young LY. Vancouver, Washington: Applied Therapeutics, Inc; 1996. Handbook of Applied Therapeutics. [Google Scholar]
- 5.Lipworth BJ, Newnham DM, Clark RA, et al. Comparison of the relative airways and systemic potencies of inhaled fenoterol and salbutamol in asthmatic patients. Thorax. 1995;50:54–61. doi: 10.1136/thx.50.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlman DS, Chervinsky P, Laforce C, et al. A comparison of salmeterol with salbutamol in a treatment of mild to moderate asthma. N Engl J Med. 1992;327:1420–5. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- 7.Fowler SJ, Lipworth BJ. Pharmacokinetics and systemic beta2-adrenoceptor-mediated responses to inhaled salbutamol. Br J Clin Pharmacol. 2001;51(4):359–62. doi: 10.1046/j.1365-2125.2001.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremner P, Burgess C, Beasley R, et al. Nebulized fenoterol causes greater cardiovascular and hypokalaemic effects than equivalent bronchodilator doses of salbutamol in asthmatics. Respir Med. 1992;86(5):419–23. doi: 10.1016/s0954-6111(06)80009-0. [DOI] [PubMed] [Google Scholar]
- 9.Lipworth BJ, McDevitt DG. Beta-adrenoceptor responses to inhaled salbutamol in normal subjects. Eur J Clin Pharmacol. 1989;36:239–45. doi: 10.1007/BF00558154. [DOI] [PubMed] [Google Scholar]
- 10.Devadason SG, Everard ML, Linto JM, et al. Comparison of drug delivery from conventional versus ‘Venturi’ nebulizers. Eur Respir J. 1997;10(11):2479–83. doi: 10.1183/09031936.97.10112479. [DOI] [PubMed] [Google Scholar]
- 11.Simon M, Mar D, Uster P, et al. Vito evaluation. Aerosol characteristics of the AeroDose® inhaler with salbutamol sulfate: an. Chest 120 2001, 225S : Ref. Type: (Abstract) [Google Scholar]
- 12.Fink J, Dhand R. Bronchodilator resuscitation in the emergency department. Part 2 of 2: dosing strategies. Respir Care. 2000;45(5):497–512. [PubMed] [Google Scholar]
- 13. Data held on file. AeroGen Inc., Sunnyvale, CA 94089 USA 2002: Ref Type: Data File.
- 14.Everard ML, Devadason SG, Summers QA, et al. Factors affecting total and ‘respirable’ dose delivered by a salbutamol metered dose inhaler. Thorax. 1995;50(7):746–9. doi: 10.1136/thx.50.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman SP, Pitcairn GR, Hooper G, et al. Efficient drug delivery to the lungs from a continuously operated open- vent nebulizer and low pressure compressor system. Eur Respir J. 1994;7(6):1177–81. [PubMed] [Google Scholar]
- 17.Zainudin BM, Biddiscombe M, Tolfree SE, et al. Comparison of bronchodilator responses and deposition patterns of salbutamol inhaled from a pressurised metered dose inhaler, as a dry powder, and as a nebulised solution. Thorax. 1990;45(6):469–73. doi: 10.1136/thx.45.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiser N, Angus K, McHale S. The role of domiciliary nebulizers in managing patients with severe COPD. Respir Med. 2001;95(4):265–74. doi: 10.1053/rmed.2001.1032. [DOI] [PubMed] [Google Scholar]
- 19.Barry PW, O'Callaghan C. An in vitro analysis of the output of salbutamol from different nebulizers. Eur Respir J. 1999;13(5):1164–9. doi: 10.1034/j.1399-3003.1999.13e37.x. [DOI] [PubMed] [Google Scholar]
- 20.Fowler SJ, Lipworth BJ. Therapeutic equivalence of inhaled salbutamol. Thorax. 2000;55(4):347–8. doi: 10.1136/thorax.55.4.345e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry PW, O'Callaghan C. The output of budesonide from nebulizers. J Allergy Clin Immunol. 1998;102(2):321–2. doi: 10.1016/s0091-6749(98)70103-4. [DOI] [PubMed] [Google Scholar]
- 22.Faurisson F, Dessanges JF, Grimfeld A, et al. Nebulizer performance: AFLM study. Association Francaise de Lutte contre la Mucoviscidose. Respiration. 1995;62(Suppl 1):13–8. doi: 10.1159/000196488. [DOI] [PubMed] [Google Scholar]