Short abstract
Parasitic worms have largely been overlooked by medicine, but attitudes are changing with the realisation that they can seriously affect child development and that treatment is easy and cheap
Parasitic worms do not usually interest doctors because, although worms can cause severe clinical disease, they usually have insidious effects on growth and development that rarely cause attendance at health centres. Yet it is precisely these chronic effects, affecting more than two billion people with lifelong infections, that have forced the public health community to reassess the importance of these infections. And recognition of the simplicity, safety, low cost, and efficacy of treatment has now resulted in major global initiatives to achieve control.
Methods
Information for this review came from Medline and hand searches of published literature, correspondence with experts in the subject, and the personal experiences of the authors.
Size of the problem
Parasitic worms may be the commonest cause of chronic infection in humans. In many low income countries it is more common to be infected than not. Indeed, a child growing up in an endemic community can expect be infected soon after weaning, and to be infected and constantly reinfected for the rest of her or his life.
There are about 20 major helminth infections of humans, and all have some public health significance,1 but among the commonest of all human infections are the geohelminthiases. Recent global estimates indicate that more than a quarter of the world's population are infected with one or more of the most common of these parasites—the roundworm, Ascaris lumbricoides; the hookworms, Necator americanus and Ancylostoma duodenale; and the whipworm, Trichuris trichiura.2 About 85% of the 200 million people with schistosomiasis live on the African continent,3 70 million people have haematuria associated with Schistosoma haematobium infection, and the two commonest species together contribute to the deaths of more than a quarter of a million people a year from complicated nephrosis and portal hypertension.4 Hookworm infection is the leading cause of pathological blood loss in tropical and subtropical regions.5 Some 44 million pregnancies are currently complicated by maternal hookworm infection,6 placing both mothers and children at higher risk of death during pregnancy and delivery.
Summary points
The most common chronic infections globally are caused by intestinal nematodes and schistosomes
Worm infections impair child development by constraining growth, learning, and school attendance
Treatment costs less than $1 a year and can be delivered safely and effectively by people who are not health professionals, including teachers
A global Partnership for Parasite Control has been launched to intensify control efforts and reach 75% of all children in affected countries by 2010
All worm infections are not equal
The distribution of helminths among hosts is overdispersed: that is, while most hosts harbour few or no worms, a few harbour many parasites.7 This distribution has clinical consequences for hosts, as it is mainly the intensity of infection that determines the severity of morbidity.8,9 Infection with Trichuris trichiura and Ascaris lumbricoides typically reaches maximum intensity at 5-10 years of age, after which it declines to a lower level that then persists throughout adulthood (figure).1 A similar profile is apparent for Schistosoma infections, but with maximum intensity attained at a slightly later age, usually 10-14 years. A different profile is apparent for hookworm infections, with maximum intensity usually not attained until 20-25 years.8
Figure 1.
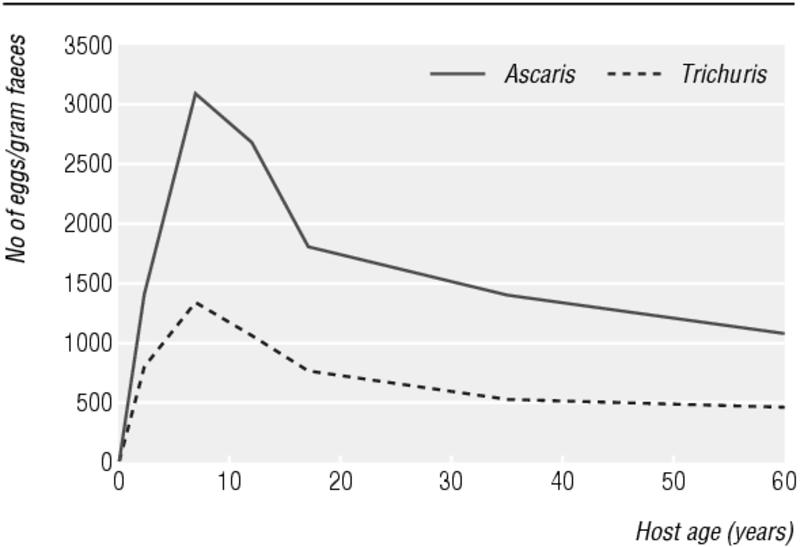
Intensity of infection with Ascaris lumbricoides and Trichuris trichiura by age of human host (adapted from Warren et al 19931)
Children of school age are thus particularly at risk from the clinical manifestations of disease. Indeed, it has been estimated that, for children aged 5-14 years in low income countries, intestinal worms account for 12% of the total disease burden. About 20% of disability adjusted life years (DALYs) lost due to communicable disease among school children are a direct result of intestinal nematodes.10 In 1999 the World Health Organization estimated that these infections represented more than 40% of the disease burden from all tropical diseases excluding malaria.
Effects of worm infections on child growth and development
By far the most common effect on health is a subtle and insidious constraint on normal physical development, resulting in children failing to achieve their genetic potential for growth and having the clinical consequences of iron deficiency anaemia and other nutritional deficiencies. Heavy hookworm burdens have long been recognised as an important cause of iron deficiency anaemia.11 Intense whipworm infection in children may result in trichuris dysentery syndrome, the classic signs of which include growth retardation and anaemia,12 and heavy burdens of both roundworm and whipworm are associated with protein energy malnutrition.13
Medium and large scale control programmes have clearly shown that morbidity from these infections can be significantly reduced through repeated and regular treatment with single dose anthelmintics delivered through school health programmes or other ongoing health or education programmes.14,15 Several controlled trials have shown that treatment has a positive effect on growth, although the extent of benefit gained has varied.16-18
Learning and education
Given the prevalence of high intensity infection in schoolchildren, it is particularly worrying that these infections can adversely affect cognition and educational achievement.19 The mechanism by which mental processes are affected is uncertain, but evidence suggests that the mechanism is indirect, perhaps mediated through iron deficiency anaemia and undernutrition.20
Whether helminth infections directly cause cognitive deficits is a matter of debate. In one analysis, the majority of 40 studies showed an association between geohelminthic infection and impaired cognitive or educational abilities,21 but few of these studies were well designed. Studies often show an effect in the most heavily infected children, but with such mixed results it has been difficult to reach any strong conclusions as to whether helminthiases directly contribute to impaired cognitive functioning.16
Recently, more comprehensive studies have used new measures of learning ability to detect the impact of infection. In Tanzania schoolchildren most heavily infected with worms achieved significantly lower scores in some tests of cognitive ability,22 but eradication treatment did not result in an immediate improvement in the children's ability to conduct these tests. However, when children were both treated and taught how to do cognitive tests they performed significantly better than children who were taught but not treated.23 These results suggest that children with chronic infection, and the constrained development that results from this, will need not only improved health but also a good education to catch up.
School based deworming programmes also boost school participation. In Kenya, such a programme reduced school absenteeism by a quarter, with the largest gains among the youngest children.24 Perhaps even more importantly, this study showed that those children who had not been treated benefited from the generally lowered transmission rate in the schools. The overall outcome was that an extra year of schooling could be obtained for an investment of some $4 (£2.50, €2.80) per child per year—a remarkable return on investment.
Treatment
Treatment of a child with praziquantel for schistosomiasis currently costs about 20 cents. A single dose treatment for soil transmitted helminth infections with any of the four anthelmintics on the WHO list of essential drugs (albendazole, levamisole, mebendazole, and pyrantel) costs less than 3 cents. WHO has calculated that the cost of intervention, including delivery, where schistosomiasis and soil transmitted helminth infections are endemic is typically less than $1 and can be as low as 30 cents per child per year, while treatment of soil transmitted helminth infections alone costs as little as 10 cents per child per year.25
Many of the technical problems associated with large scale treatment campaigns have been addressed and solved. For example, teachers and other people who are not health professionals can distribute anthelmintic drugs effectively to school age children with minimal training.26,27 Community treatment may be simplified by the use of a “dose pole”—which determines the correct dose from a child's height.28-30 Praziquantel treatment should also be offered to pregnant women with schistosomiasis, as the benefits of such treatment greatly outweigh any reported adverse effects.31 Mebendazole, albendazole, and other anthelmintics have also recently been approved for use in children over 12 months old.31
Not only children can benefit from treatment. In Sierra Leone anthelmintic treatment had an additive effect when combined with iron and folate supplements to control maternal anaemia during pregnancy.5 In Sri Lanka the same combined intervention improved the health of mothers, with a beneficial effect on their birth outcome without any increased risk of malformations.32,33
Support for treatment programmes
Delivering treatment through the education sector
In April 2000, at the Dakar World Education Forum, a partnership was launched by Unesco, Unicef, WHO, Education International, and the World Bank to help countries in “Focusing Resources on Effective School Health,” including support for the distribution of anthelmintics through schools.34 This “FRESH” partnership aims to improve the health and nutrition of school-children as a contribution to the global “Education for All” efforts to ensure universal access to basic education. To date, more than 20 projects targeting 45 million children of school age have been supported in Africa.
Supporting schistosomiasis control
In July 2002 the Bill and Melinda Gates Foundation gave a $30m grant to Imperial College, London, to support a schistosomiasis control initiative. This initiative is working with partners to ensure that treatment is available for young people, especially schoolchildren, and women and all those at particular risk through their work. The initiative aims to provide 35 million doses for both schistosomiasis and intestinal nematode infection to some 20 million people in Africa over the next five years, and to assist countries in making the transition to self sustained programmes.
A new partnership to boost global efforts
The 54th World Health Assembly resolution set a global target of scaling up intervention to regularly treat 75% of school age children at risk (398 million) by 2010. To achieve this, WHO has developed a broad partnership that promotes the incorporation of deworming into existing institutions and programmes, for both the education and health sectors. The Partnership for Parasite Control was launched in 2001 with the aim of mobilising resources and promoting new synergy among public and private efforts for the control of soil transmitted helminths and schistosomiasis at global and national levels. Working with the World Food Programme and the World Bank, WHO in 2001 trained representatives of the ministries of health and education of 21 countries, and deworming programmes have begun in 19 of the 41 African countries where infections are endemic.
The dangers of drug resistance
Expanding treatment programmes increases the drug pressure on parasite populations. The evidence for the emergence of drug resistance in human helminthes is equivocal, despite the long term widespread use of anthelmintics. Nevertheless, the risk of drug resistance is real. To prevent or delay the emergence of resistance treatment should be targeted only to high risk groups such as schoolchildren, which ensures gene flow among a worm population without drug pressure, and delivered infrequently, typically no more frequently than every 6 months. Monitoring and surveillance of drug efficacy is being built into operational programmes.35
Competing interests: None declared.
References
- 1.Warren KS, Bundy DAP, Anderson RM, Davis AR, Henderson DA, Jamison DT, et al. Helminth infection. In: Jamison DT, Mosley WH, Measham AR, Bobadilla JL, eds. Disease control priorities in developing countries. Oxford: Oxford University Press, 1993: 131-60.
- 2.Chan MS, Medley GF, Jamison D, Bundy DAP. The evaluation of potential global morbidity due to intestinal nematode infections. Parasitology 1994;109: 373-87. [DOI] [PubMed] [Google Scholar]
- 3.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Tropica 2000;77: 41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Werf MJ, De Vlas SJ, Brooker S, Looman CWN, Nagelkerke NJD, Habbema JDF, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Tropica 2003;86: 125-39. [DOI] [PubMed] [Google Scholar]
- 5.Torlesse H, Hodges M. Anthelminthic treatment and haemoglobin concentrations during pregnancy. Lancet 2000;356: 1083. [DOI] [PubMed] [Google Scholar]
- 6.Bundy DAP, Chan MS, Savioli L. Hookworm infection in pregnancy. Trans R Soc Trop Med Hyg 1995;89: 521-2. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RMA, Medley GF. Community control of helminth infections of man by mass and selective chemotherapy. Parasitology 1985;90: 629-60. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson LS. Impact of helminth infections on human nutrition: schistosomes and soil transmitted helminths. London: Taylor and Francis, 1987.
- 9.Cooper ES, Bundy DAP. Trichuris is not trivial. Parasitol Today 1988;4: 301-6. [DOI] [PubMed] [Google Scholar]
- 10.World Bank: World development report: investing in health. Oxford: Oxford University Press, 1993.
- 11.Roche M, Layrisse M. Hookworm anaemia. Am J Trop Med Hyg 1966;15: 1029-102. [PubMed] [Google Scholar]
- 12.Bundy DAP, Cooper ES. Trichuris and trichuriasis in humans. Adv Parasitol 1989;28: 107-73. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson LS, Latham M, Adams E, Kinoti SN, Pertiet A. Physical fitness, growth and appetite of Kenyan schoolboys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutr 1993;123: 1036-46. [DOI] [PubMed] [Google Scholar]
- 14.Partnership for Child Development. The health and nutritional status of schoolchildren in Africa: evidence from school-based programmes in Ghana and Tanzania. Trans R Soc Trop Med Hyg 1998;92: 254-61. [DOI] [PubMed] [Google Scholar]
- 15.Albonico M, Crompton DWT, Savioli L. Control strategies for human intestinal nematode infections. Adv Parasitol 1999;42: 277-341. [DOI] [PubMed] [Google Scholar]
- 16.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. BMJ 2000;320: 1697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael E. Treatment for intestinal helminth infection [letters]. BMJ 2000;321: 1224-5. [PMC free article] [PubMed] [Google Scholar]
- 18.Savioli L, Neira M, Albonico M, Beach MJ, Chwaya HM, Crompton DW, et al. Treatment for intestinal helminth infection [letters]. BMJ 2000;321: 1226-7. [PubMed] [Google Scholar]
- 19.Watkin NE, Pollitt E. “Stupidity or worms”: do intestinal worms impair mental performance? Psycol Bull 1997;121: 171-91. [DOI] [PubMed] [Google Scholar]
- 20.Pollitt E, Hathirat P, Kotchabhakdi N, Missell L, Valyasevi A. Iron deficiency and educational achievement in Thailand. Am J Clin Nutr 1989;50: 687-97. [DOI] [PubMed] [Google Scholar]
- 21.Kvalsvig JD, Cooppan RM, Connolly KJ. The effects of parasite infections on cognitive processes in children. Ann Trop Med Parasitol 1992;85: 551-68. [DOI] [PubMed] [Google Scholar]
- 22.Partnership for Child Development. Heavy schistosomiasis associated with poor short-term memory and slower reaction time in Tanzanian school children. Trop Med Int Health 2002;7: 104-17. [DOI] [PubMed] [Google Scholar]
- 23.Hadidjaja P, Bonanag E, Suyardi MA, Abidin SA, Ismid IS, Margono SS. The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides. Am J Trop Med Hyg 1998;59: 791-5. [DOI] [PubMed] [Google Scholar]
- 24.Miguel E, Kremer M. Worms: education and health externalities in Kenya (NBER Working Paper No w8481). Cambridge, MA: National Bureau of Economic Research, 2001. (www.nber.org/papers/W8481)
- 25.Montresor A, Crompton DWT, Gyorkos TW, Savioli L. Helminth control in school-age children. A guide for managers of control programmes. Geneva: World Health Organization, 2002. (www.who.int/entity/wormcontrol/documents/en/itoviii.pdf)
- 26.Bundy DAP, De Silva NR. Can we deworm this wormy world? Br Med Bull 1998;54: 421-32. [DOI] [PubMed] [Google Scholar]
- 27.Crompton DWT, Engels D, Montresor A, Neira MP, Savioli L. Action starts now to control disease due to schistosomiasis and soil-transmitted helminthiasis. Acta Trop 2003;86: 121-4. [DOI] [PubMed] [Google Scholar]
- 28.Hall A, Nokes C, Wen ST, Adjei S, Kihamia C, Mwanre L, et al. Alternatives to body weight for estimating the dose of praziquantel needed to treat schistosomiasis. Trans R Soc Trop Med Hyg 1999;93: 653-8. [DOI] [PubMed] [Google Scholar]
- 29.Montresor A, Engels D, Chitsulo L, Bundy DAP, Brooker S, Savioli L. Development and validation of a 'tablet pole' for the administration of praziquantel in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2001;95: 542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montresor A, Ramsan M, Engels D, Foum A, Savioli L. Field test of the 'dose pole' for praziquantel in Zanzibar. Trans R Soc Trop Med Hyg 2002;96: 323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montressor A, Awasthi S, Crompton DWT. Use of benzimidazoles in children younger than 24 months for treatment of soil transmitted helminthiasis. Acta Trop 2003;86: 223 -32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Silva NR, Sirisena JL, Gunasekera DP, Ismail MM, de Silva HJ. Effect of mebendazole therapy during pregnancy on birth outcome. Lancet 1999;353: 1145-9. [DOI] [PubMed] [Google Scholar]
- 33.Bradley M, Horton J. Assessing the risk of benzimidazole therapy during pregnancy. Trans R Soc Trop Med Hyg 2001;95: 72-3. [DOI] [PubMed] [Google Scholar]
- 34.FRESH. Focusing resources on effective school health: ensuring the quality and equity of basic education. Washington, DC: Unesco, Unicef, WHO, World Bank, 2000.
- 35.World Health Organization. Report of the WHO informal consultation on monitoring of drug efficacy in the control of schistosomiasis and intestinal nematodes. Geneva: WHO, 1996. (WHO/CDS/CPC/SIP/99.1.)


