Abstract
Aims
The effect of enzyme induction on the pharmacokinetics of pioglitazone, a thiazolidinedione antidiabetic drug that is metabolized primarily by CYP2C8, is not known. Rifampicin is a potent inducer of several CYP enzymes and our objective was to study its effects on the pharmacokinetics of pioglitazone in humans.
Methods
In a randomized, two-phase crossover study, ten healthy subjects ingested either 600 mg rifampicin or placebo once daily for 6 days. On the last day, they received a single oral dose of 30 mg pioglitazone. The plasma concentrations and cumulative excretion of pioglitazone and its active metabolites M-IV and M-III into urine were measured up to 48 h.
Results
Rifampicin decreased the mean total area under the plasma concentration-time curve (AUC0−∞) of pioglitazone by 54% (range 20–66%; P = 0.0007; 95% confidence interval −78 to −30%) and shortened its dominant elimination half-life (t1/2) from 4.9 to 2.3 h (P = 0.0002). No significant effect on peak concentration (Cmax) or time to peak (tmax) was observed. Rifampicin increased the apparent formation rate of M-IV and shortened its tmax (P < 0.01). It also decreased the AUC0−∞ of M-IV (by 34%; P = 0.0055) and M-III (by 39%; P = 0.0026), shortened their t1/2 (M-IV by 50%; P = 0.0008, and M-III by 55%; P = 0.0016) and increased the AUC0−∞ ratios of M-IV and M-III to pioglitazone by 44% (P = 0.0011) and 32% (P = 0.0027), respectively. Rifampicin increased the M-IV/pioglitazone and M-III/pioglitazone ratios in urine by 98% (P = 0.0015) and 95% (P = 0.0024). A previously unrecognized metabolite M-XI, tentatively identified as a dihydroxy metabolite, was detected in urine during both phases, and rifampicin increased the ratio of M-XI to pioglitazone by 240% (P = 0.0020).
Conclusions
Rifampicin caused a substantial decrease in the plasma concentration of pioglitazone, probably by induction of CYP2C8. Concomitant use of rifampicin with pioglitazone may decrease the efficacy of the latter drug.
Keywords: pioglitazone, rifampicin, drug interaction, CYP2C8, pharmacokinetics, metabolism
Introduction
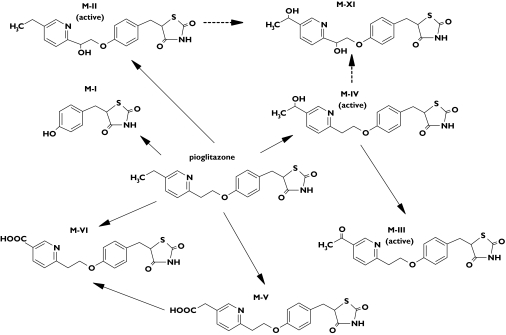
Pioglitazone is a thiazolidinedione compound used in the treatment of type 2 diabetes. It is an insulin sensitizer that acts as agonist of the peroxisome proliferator-activated receptor subtype gamma (PPAR-γ) [1]. Pio-glitazone is rapidly absorbed, its oral bioavailability exceeds 80%, and it is extensively metabolized by hydroxylation and oxidation to active and inactive metabolites in the liver [2, 3]. The main active metabolites are M-IV (a hydroxy derivative) and M-III (a keto derivative); the latter being formed from M-IV (Figure 1) [3]. Another metabolite M-II also has pharmacological activity, but its concentrations are low and it does not significantly contribute to the total amount of active species [2]. The circulating concentrations of the metabolites M-IV and M-III are equal to or greater than those of the parent pioglitazone and they have considerably longer half-lives than pioglitazone [3]. In vitro studies suggest the drug is metabolized by several cytochrome P450 (CYP) enzymes, but mainly by CYP2C8 and CYP3A4 [2–4]. The lipid-lowering fibrate gemfibrozil, an inhibitor of CYP2C8 [5], increases the mean area under the plasma concentration-time curve (AUC) of pioglitazone by 3.2-fold, whereas the antimycotic itraconazole, a potent inhibitor of CYP3A4, has no effect on the pharmacokinetics of pioglitazone [6]. These findings suggest that pioglitazone is metabolized mainly by CYP2C8 in vivo.
Figure 1.
The metabolism of pioglitazone in humans [2]. M-XI is a previously unrecognized metabolite
Rifampicin, an antibiotic used mainly in the treatment of tuberculosis, is a potent inducer of several drug-metabolizing enzymes, and it markedly decreases the plasma concentrations of many drugs [7]. Rifampicin has its greatest effect on the expression of CYP3A4 [7], but it also induces CYP2C8, for example in human hepatocytes [8–10]. Rifampicin has recently been found to decrease the AUC of the thiazolidinedione rosiglitazone, which is primarily metabolized by CYP2C8, by over 50%[11, 12], as well as increasing the expression of CYP2C8 protein in small bowel enterocytes [13]. The aim of this study was to investigate the effects of rifampicin on the pharmacokinetics of pioglitazone.
Methods
Subjects
Ten healthy subjects (seven men, three women; age range 21–24 years; weight range 57–79 kg; height range 168–182 cm) participated in the study after each gave a written informed consent and were ascertained to be healthy by medical history, clinical examination, and routine laboratory tests. None received continuous medication or oral contraceptives or was a smoker. The study protocol was approved by the Ethics Committee for Studies In Healthy Subjects and Primary Care of the Helsinki and Uusimaa Hospital District, and by the Finnish National Agency for Medicines.
Study design
A randomized, two-phase crossover study with a washout period of 4 weeks was carried out. Each subject took 600 mg rifampicin (one tablet of Rimapen, Orion Pharma, Espoo, Finland) or placebo orally once daily at 20.00 h for 6 days. On day 6 at 09.00 h, a single dose of 30 mg pioglitazone (one tablet of Actos, Takeda Europe, London, UK) was administered orally with 150 mL water. The subjects fasted for 9 h before pioglitazone intake, and received a standard meal 3 h and light standard meals 7 h and 11 h after dosing.
Blood and urine sampling
Venous blood samples (10 mL) were drawn from a cannulated forearm vein before administration of pioglitazone and 1, 2, 3, 4, 5, 7, 9, 12, 24 and 48 h later. Plasma was separated immediately and stored at −70 °C until analysis. Urine was collected cumulatively in fractions of 0–12, 12–24 and 24–48 h after the administration of pioglitazone. After each collection period, the volume of urine was measured and an aliquot was stored at −70 °C.
Analysis of drug and metabolites
Samples (0.5 mL) were spiked with 50 µL of internal standard (rosiglitazone 3 µg mL−1 in 20% methanol) followed by extraction into 5 mL of ethyl acetate. After evaporation of the organic phase under nitrogen (45 °C), the residues were dissolved in 100 µL of mobile phase (acetonitrile-water 45 : 55 v/v) and transferred into autosampler vials.
The concentrations of pioglitazone and its metabolites in plasma were measured by use of SCIEX API 3000 and the concentrations in urine by use of SCIEX API 2000 tandem mass spectrometry (Sciex Division of MDS Inc, Toronto, Ontario, Canada) coupled to an Agilent 1100 series liquid chromatography system (Agilent Technologies, Waldbronn, Germany). Chromatography was performed on an XTerra RP C18 column (3.9 × 100 mm; Waters Corp., Milford, Massachusetts, USA) using a mobile phase consisting of 10 mm ammonium acetate (A) (pH 9.0, adjusted with 25% ammonia solution) and acetonitrile (B). An aliquot (5 µL) of sample was injected at a mobile phase flow rate of 400 µL min−1. The mobile phase gradient comprised of 0 min at 5% B, 1 min to 20% B, 12.4 min to 38% B, 2 min at 100% B and 5.6 min at 5% B, giving a total chromatographic run time of 21 min. The mass spectrometers were operated in positive atmospheric pressure chemical ionization (APCI) mode with selected reaction monitoring (SRM). The ion transitions monitored were m/z 357 to m/z 134 for pioglitazone, m/z 371 to m/z 148 for M-III, m/z 373 to m/z 150 for M-IV, m/z 387 to m/z 164 for M-V, m/z 389 to m/z 166 for a previously unrecognized metabolite (M-XI, dihydroxypioglitazone, Figure 1) and m/z 358 to m/z 135 for rosiglitazone. These transitions represent the product ions of the [M + H]+ ions. A weighted (1/x) linear regression was used to generate calibration curve from standards (0–2000 ng mL−1; r2 > 0.999) and to calculate the concentrations of samples. The limit of quantification for pioglitazone in plasma was 0.1 ng mL−1 and the day-to-day coefficient of variation (CV) was 3.6% at 20 ng mL−1, 3.3% at 200 ng mL−1, and 6.3% at 2000 ng mL−1 (n = 5). The limit of quantification for pioglitazone in urine was 0.1 ng mL−1 and the CV was below 8% at relevant concentrations. Pioglitazone was obtained from Sigma-Aldrich Chemie (Steinheim, Germany) and rosiglitazone from SmithKline Beecham plc (Middlesex, UK). Because the reference compounds for the metabolites were not available, their concentrations are given in arbitrary units (U mL−1) relative to the ratio of the peak height of the metabolite to the peak height of the internal standard. The detector response for each metabolite was confirmed to be linear over the relevant concentration range by means of sample dilution (5, 25, 125 and 625-fold dilutions; r2 > 0.998). A signal to noise ratio of 10 : 1 was used as the limit of determination. The CV for the determination of the metabolites in plasma and urine was below 8% at relevant concentrations (n = 8). Rifampicin did not interfere with the assay.
Pharmacokinetic analysis
The pharmacokinetics of pioglitazone were characterized by the peak concentration in plasma (Cmax), the time to Cmax (tmax), the dominant half-life (t1/2, the half-life of the phase contributing most to the area under the curve), the terminal half-life (t1/2,terminal) and the area under the plasma concentration-time curve from 0 to 48 h (AUC0−48) or to infinity (AUC0−∞). The pharmacokinetics of the metabolites M-IV and M-III were characterized by Cmax, tmax, t1/2, AUC0−48 and AUC0−∞. An apparent formation rate constant (kf) was calculated for M-IV. Values for Cmax and tmax were taken directly from the original data. For each subject, the log-linear phases of the concentration-time curve for pioglitazone and the metabolites M-IV and M-III were identified visually. The plasma concentrations of pioglitazone declined biphasically, particularly during rifampicin treatment. Therefore, for the dominant elimination phase, the elimination rate constant (ke) was determined by linear regression analysis using the first log-linear phase of the descending plasma concentration curve. For the slow elimination phase, starting between 9 and 12 h, a terminal elimination rate constant (ke,terminal) was determined by using the log-linear terminal part of the plasma pioglitazone concentration-time curve. The dominant and terminal half-lives for parent pioglitazone were calculated from the equations t1/2 = ln2/ke and t1/2,terminal= ln2/ke,terminal. The AUC for pioglitazone was calculated by the linear trapezoidal rule for the ascending phase and the log-linear trapezoidal rule for the descending phase, with extrapolation to infinity when appropriate, by division of the last measured concentration by ke,terminal. The plasma concentrations of M-IV and M-III declined monophasically and the elimination rate constants (ke) were determined by linear regression analysis of the log-linear part of the concentration-time curve. The t1/2 was calculated from the equation t1/2 = ln2/ke and the AUC by the linear and log-linear trapezoidal rules with extrapolation to infinity by dividing the last measured concentration by ke. The kf for the metabolite M-IV was calculated by the method of residuals from the ascending part of the metabolite concentration-time curve [14]. All calculations were performed using MK-model, version 5.0 (Biosoft, Cambridge, UK).
The cumulative 48 h excretion [Ae(0–48)] of pioglitazone (ng) and its metabolites in arbitrary units (U) into urine were determined, and the renal clearance (Clrenal) of pioglitazone was calculated from the expression Ae(0–48)/AUC0−48.
Statistical analysis
Results are expressed as mean values ± SD in the text and tables, and, for clarity, as mean values ± SEM in the figures. Ninety-five per cent confidence intervals were calculated on the mean differences between the placebo and rifampicin phases for all pharmacokinetic parameters except for tmax. The pharmacokinetic variables between the two phases were compared by use of a paired t-test, or in the case of tmax, by the Wilcoxon signed-rank test. The level of statistical significance was P < 0.05. The analyses were performed with Systat for Windows version 6.0.1 (SPSS Inc., Chigago, IL, USA).
Results
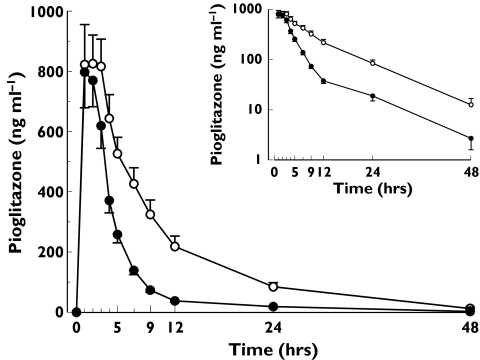
There was no apparent difference in the plasma concentrations of pioglitazone between the study phases up to 2 h after the ingestion of pioglitazone. From 3 h onwards plasma pioglitazone concentrations were significantly lower during the rifampicin phase than during placebo administration (Figure 2). Rifampicin decreased the mean AUC0−∞ of pioglitazone by 54% (range 20–66%; P = 0.0007; 95% confidence interval −78 to −30%) and shortened its dominant elimination t1/2 from 4.9 h to 2.3 h (P = 0.0002), compared with placebo (Table 1 and Figure 2). Rifampicin had no significant effect on the Cmax, tmax or t1/2,terminal of pioglitazone.
Figure 2.
Mean ± SEM plasma concentration-time curves for pioglitazone in ten healthy subjects after a single oral dose of 30 mg pioglitazone on the last day of a 6-day treatment with placebo (○) or 600 mg rifampicin (•) once daily. The inset depicts the same data on a semilogarithmic scale
Table 1.
Pharmacokinetic data for pioglitazone in ten healthy subjects after a single oral dose of 30 mg pioglitazone on the last day of a 6-day treatment with 600 mg rifampicin or placebo given once daily
| Variable | Placebo phase(control) | Rifampicinphase | Rifampicin phasepercentage ofcontrol (range) | Mean differencebetween phases(95% CI) | P-value |
|---|---|---|---|---|---|
| Pioglitazone | |||||
| Cmax (ng mL−1) | 932 ± 335 | 888 ± 335 | 95 (52–167) | −44 (−339, 251) | 0.7446 |
| tmax (h) | 1 (1–3) | 1.5 (1–2) | 1.0000 | ||
| t1/2 (h) | 4.9 ± 1.0 | 2.3 ± 0.6 | 47 (29–110) | −2.6 (−3.6, −1.7) | 0.0002 |
| t1/2, terminal (h) | 8.1 ± 1.8 | 8.1 ± 4.0 | 99 (58–289) | −0.1 (−3.4, 3.3) | 0.9672 |
| AUC0−48 (mg L−1 h) | 8.44 ± 3.50 | 3.93 ± 1.19 | 47 (35–81) | −4.51 (−6.51, −2.51) | 0.0006 |
| AUC0−∞ (mg L−1 h) | 8.61 ± 3.66 | 3.98 ± 1.20 | 46 (34–80) | −4.63 (−6.72, −2.55) | 0.0007 |
| Clrenal (ml h−1) | 0.12 ± 0.05 | 0.12 ± 0.06 | 99 (49–143) | −0.001 (−0.03, 0.03) | 0.9264 |
| M-IV | |||||
| kf (h−1) | 0.19 ± 0.05 | 0.44 ± 0.19 | 231 (114–475) | 0.25 (0.10, 0.40) | 0.0041 |
| Cmax (U mL−1) | 0.87 ± 0.28 | 1.02 ± 0.19 | 116 (63–219) | 0.14 ± (−0.12, 0.41) | 0.2479 |
| tmax (h) | 12 (9–24) | 8 (3–12) | 0.0072 | ||
| t1/2 (h) | 32 ± 10 | 16 ± 3 | 50 (32–95) | −16 (−23, −9) | 0.0008 |
| AUC0−48 (U mL−1 h) | 29.2 ± 8.3 | 27.2 ± 3.7 | 93 (66–166) | −1.9 (−7.4, 3.5) | 0.4431 |
| AUC0−∞ (U mL−1 h) | 48.2 ± 17.5 | 32.0 ± 5.1 | 66 (49–155) | −16.2 (−26.3, −6.1) | 0.0055 |
| M-III | |||||
| Cmax (U mL−1) | 0.63 ± 0.22 | 0.81 ± 0.32 | 129 (51–244) | 0.18 (−0.06, 0.42) | 0.1220 |
| tmax (h) | 12 (9–24) | 6 (3–9) | 0.0076 | ||
| t1/2 (h) | 39 ± 16 | 18 ± 4 | 45 (30–120) | −22 (−33, −11) | 0.0016 |
| AUC0−48 (U mL−1 h) | 21.1 ± 6.5 | 20.4 ± 7.3 | 97 (55–165) | −0.7 (−5.0, 3.5) | 0.7067 |
| AUC0−∞ (U mL−1 h) | 39.8 ± 17.0 | 24.3 ± 8.5 | 61 (45–99) | −15.5 (−24.0, −7.0) | 0.0026 |
Values shown as mean ± SD unless otherwise indicated, tmax data as median (range). CI, confidence interval; Cmax, observed peak plasma concentration; tmax, time to reach Cmax; t1/2, dominant elimination half-life; t1/2,terminal, terminal elimination half-life; AUC0−48, area under the concentration vs. time curve to 48 h; AUC0−∞, area under the concentration versus time curve to infinity; kf, apparent formation rate constant; U, arbitrary units (relative to the ratio of the peak height of the metabolite to the peak height of the internal standard).
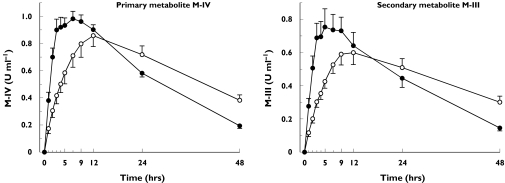
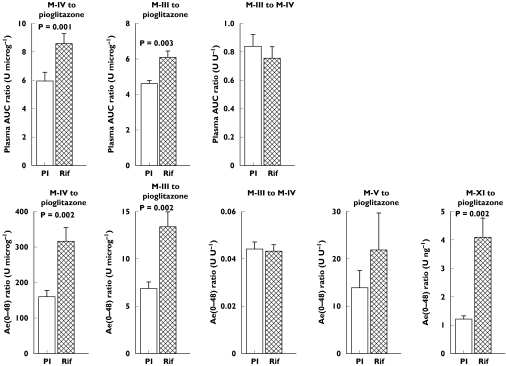
Compared with placebo, rifampicin increased the apparent formation rate (kf) of M-IV by 131% (P = 0.0041) (Table 1, Figure 3). The tmax of M-IV and M-III were also reached significantly earlier during the rifampicin phase than during placebo administration. In addition, rifampicin (a) shortened the t1/2 of M-IV and M-III by 50% (P = 0.0008) and 55% (P = 0.0016), respectively, and (b) decreased the AUC0−∞ of M-IV and M-III by 34% (P = 0.0055) and 39% (P = 0.0026), respectively, whereas their AUC0−48 values remained unaffected. Rifampicin increased the M-IV/pioglitazone AUC0−∞ ratio by 44% (P = 0.0011) and the M-III/pioglitazone AUC0−∞ ratio by 32% (P = 0.0027) (Figure 4).
Figure 3.
Mean ±SEM plasma concentration-time curves for metabolites M-IV and M-III in ten healthy volunteers after a single oral dose of 30 mg pioglitazone on the last day of a 6-day treatment with placebo (○) or 600 mg rifampicin (•) once daily
Figure 4.
Mean ±SEM AUC ratios and Ae ratios for pioglitazone and its metabolites in 10 healthy subjects after a single oral dose of 30 mg pioglitazone on the last day of a 6-day treatment with placebo (Pl) or 600 mg rifampicin (Rif) given once daily
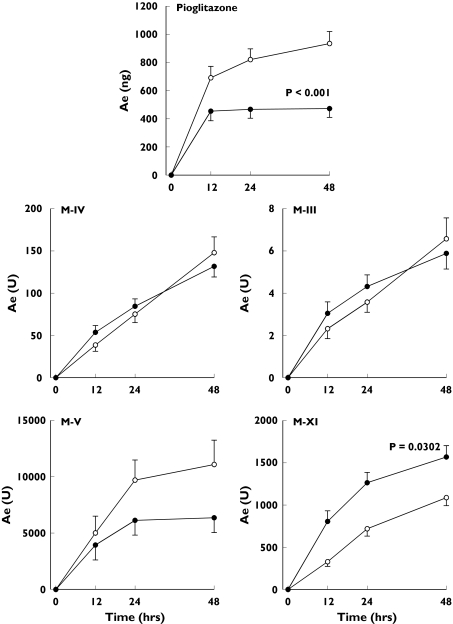
Rifampicin decreased the Ae(0–48) of pioglitazone by 49% (P < 0.0001), and that of M-V by 43% (P = 0.0694), whereas the Ae(0–48) of M-IV and M-III remained unchanged (Figure 5). The Ae(0–48) of M-XI was increased by 44% (P = 0.0302). Rifampicin raised the M-IV/pioglitazone Ae(0–48) ratio by 98% (P = 0.0015), the M-III/pioglitazone ratio by 95% (P = 0.0024), and the M-XI/pioglitazone ratio by 240% (P = 0.0020) (Figure 4). The renal clearance of pioglitazone was unaffected by rifampicin.
Figure 5.
Mean ±SEM amounts of pioglitazone, M-IV, M-III, M-V and M-XI excreted in urine within 48 h [Ae(0–48)] in 10 healthy subjects after a single oral dose of 30 mg pioglitazone on the last day of a 6-day treatment with 600 mg rifampicin (•) or placebo (○) once daily
Discussion
Rifampicin was found to decrease substantially the plasma concentration of pioglitazone from 3 h onwards, shorten its dominant elimination t1/2, enhance the formation of its metabolites and increase the metabolite/pioglitazone ratio in plasma and urine. These findings indicate that rifampicin induces the metabolism of pioglitazone during its elimination phase. The shortening of the t1/2 of the active metabolites M-IV and M-III by rifampicin is consistent with the induction of the further metabolism of these compounds. Some interindividual variation in the extent of the interaction was evident, with the decrease in the AUC of pioglitazone ranging from 20% to 66%.
CYP2C8, and to a lesser extent CYP3A4 and CYP2C9, catalyse the in vitro metabolism of pioglitazone [2–4]. In a previous study, the CYP2C8 inhibitor gemfibrozil increased the mean AUC of pioglitazone by 3.2-fold, whereas the CYP3A4-inhibitor itraconazole had no effect on pioglitazone pharmacokinetics [6]. These findings suggest that pioglitazone is metabolized mainly by CYP2C8 in vivo. Rifampicin induces a range of proteins involved in drug metabolism and transport, including several CYP enzymes, some conjugating (phase II) enzymes, and drug transporters such as P-glycoprotein [7]. Although rifampicin has its greatest effect on drugs that are CYP3A4 substrates [15–19], it can it also induce the metabolism of drugs that are substrates of CYP2C8 [11, 12, 20]. Because itraconazole has no statistically significant effect on the pharmacokinetics of pioglitazone, induction of CYP3A4 probably does not explain the effect of rifampicin on pioglitazone pharmacokinetics. Taken together, these findings indicate that rifampicin decreases plasma concentrations and urinary excretion of pioglitazone mainly by inducing its CYP2C8-catalysed biotransformation in the liver. The Cmax of pioglitazone remained unaffected, which is typical for drugs with low hepatic extraction ratio and negligible first-pass metabolism, such as this drug has [2].
After its peak, the plasma pioglitazone concentrations declined biphasically, as has been observed in previous pharmacokinetic studies [21]. The majority of the elimination of pioglitazone and its metabolite formation takes place during the first phase, and rifampicin considerably shortened the ‘dominant elimination half-life’ of pioglitazone associated with this phase. However, the long-terminal half-life of pioglitazone observed in the later phase remained unaffected by rifampicin. The parameter could reflect, for example, the release of pioglitazone from peripheral tissues, which are not changed by induction.
The urinary excretion of pioglitazone was decreased during rifampicin treatment, although the renal clearance of the drug was unchanged. During the rifampicin phase the elimination of pioglitazone into urine was nearly complete by 12 h, which is in agreement with the time-course of the plasma concentration data and with the inducibility of pioglitazone metabolism by rifampicin. Rifampicin increased the apparent formation rate (kf) of the metabolite M-IV, shortened its tmax and increased the M-IV/pioglitazone ratio in plasma and urine, indicating that rifampicin increased the activity of this metabolic step. The formation of the secondary metabolite M-III is dependent on that of its precursor M-IV. Rifampicin did not affect the M-III/M-IV AUC and Ae ratios, which suggests that it does not induce this secondary metabolic step. The relative contribution of the active metabolites M-IV and M-III to the biological activity of pioglitazone is probably increased by rifampicin. M-V is an inactive polar metabolite, which was found in detectable amounts only in urine. Rifampicin showed a tendency to decrease the excretion of M-V into urine, which could be explained by the lower plasma concentrations of pioglitazone.
In addition to the six major metabolites of pioglitazone, four other metabolites (M-VII to M-X) have been identified in vitro and in animals [22]. In the present study, a previously unrecognized metabolite M-XI was found in urine. Its mass to charge ratio 389 suggests that the compound is a dihydroxy metabolite of pioglitazone, formed by further hydroxylation of M-II or M-IV. The neutral loss scan of m/z 389 resulted in the same neutral 223 amu fragment as did parent pioglitazone and its other metabolites. During the placebo phase, the Ae(0–48) of M-XI was approximately one-tenth of that of M-V, which is the main compound in urine. M-XI was not detected in plasma. The rapid excretion of M-XI into urine is consistent with a polar nature. The increased excretion of M-XI during rifampicin treatment strongly suggests that its formation is inducible.
The absolute concentrations of the metabolites could not be quantified in this study, because authentic standards were not available. However, we were still able to study the effect of rifampicin on the relative changes in the metabolite concentrations.
In the present study, rifampicin was given for 6 days. Maximal induction of intestinal CYP enzymes and transporters is achieved in about 1 week, whereas that for hepatic induction can take longer [7, 18, 23]. Thus it is possible that a more prolonged treatment with rifampicin could result in an increase in the magnitude of the rifampicin–pioglitazone interaction. Recent studies also suggest that rifampicin may act as both an inducer and an inhibitor of CYP2C8 and CYP3A4. The decrease in plasma drug concentration was found to be smaller, when rifampicin and the target drug (e.g. repaglinide) are administrated at the same time [24, 25]. Thus, the time interval between administration of rifampicin and pioglitazone may also affect the magnitude of the interaction.
The blood glucose-lowering effect of pioglitazone develops gradually over a period of weeks and is dose-dependent [26]. The decrease in the plasma concentrations of pioglitazone and its active metabolites by rifampicin may decrease the blood glucose-lowering efficacy of pioglitazone, although this was not investigated in the present single dose study. Therefore, it is advisable to monitor blood glucose concentrations when starting treatment with rifampicin and to adjust pioglitazone dosage as necessary. Furthermore, it may be important to decrease the dosage of pioglitazone when rifampicin treatment is discontinued.
In previous studies in healthy subjects treatment with rifampicin (600 mg daily for 5 or 6 days) was found to decrease the AUC of rosiglitazone, which is mainly metabolized by CYP2C8, by 54–65%[11, 12]. In addition, rifampicin decreases the AUC of the meglitinide analogues repaglinide (a substrate of CYP2C8 and CYP3A4) [20] and nateglinide (a substrate of CYP2C9 and CYP3A4) [27], and that of the sulphonylurea drugs glyburide, glimepiride, glipizide and gliclazide (all substrates of CYP2C9) by 22–70%[28–30]. In these studies, rifampicin has also decreased the glucose-lowering effects of repaglinide, glyburide and gliclazide. It is also possible that other CYP2C8 enzyme inducers, such as phenobarbital [9], can decrease the plasma pioglitazone concentrations.
In conclusion, pretreatment with rifampicin causes a substantial decrease in the plasma concentrations of pioglitazone and its active metabolites, probably by induction CYP2C8. Concomitant use of rifampicin or other potent enzyme inducer may decrease the clinical efficacy of pioglitazone, although this could not be established in the present single dose study in healthy subjects.
Acknowledgments
We would like to thank Mrs Kerttu Mårtensson, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance. This study was supported by grants from the Helsinki University Central Hospital Research Fund, the National Technology Agency and the Sigrid Jusélius Foundation, Finland.
References
- 1.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 2.Eckland DA, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes. 2000;108(Suppl 2):234–42. [Google Scholar]
- 3.Product information for Actos. [20 Dec, 2004]; Available from: http://www.actos.com/pi.pdf.
- 4.Hanefeld M. Pharmacokinetics and clinical efficacy of pioglitazone. Int J Clin Pract. 2001;121(Suppl):19–25. [PubMed] [Google Scholar]
- 5.Wang J-S, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola T, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther. 2005;77:404–14. doi: 10.1016/j.clpt.2004.12.266. [DOI] [PubMed] [Google Scholar]
- 7.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 8.Gerbal-Chaloin S, Pascussi JM, Pichard-Garcia L, Daujat M, Waechter F, Fabre JM, Carrere N, Maurel P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29:242–51. [PubMed] [Google Scholar]
- 9.Raucy JL, Mueller L, Duan K, Allen SW, Strom S, Lasker JM. Expression and induction of CYP2C P450 enzymes in primary cultures of human hepatocytes. J Pharmacol Exp Ther. 2002;302:475–82. doi: 10.1124/jpet.102.033837. [DOI] [PubMed] [Google Scholar]
- 10.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan LS, LeCluyse EL, Zech K, Robertson P, Jr, Koch P, Antonian L, Wagner G, Yu L, Parkinson A. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31:421–31. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Kim K, Jung W, Kim S, Shin J. Effect of rifampin on the pharmacokinetics of rosiglitazone in Korean healthy subjects. Clin Pharmacol Ther. 2004;75:157–62. doi: 10.1016/j.clpt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Niemi M, Backman JT, Neuvonen PJ. Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone. Clin Pharmacol Ther. 2004;76:239–49. doi: 10.1016/j.clpt.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Glaeser H, Drescher S, Eichelbaum M, Fromm MF. Influence of rifampicin on the expression and function of human intestinal cytochrome P450 enzymes. Br J Clin Pharmacol. 2005;59:199–206. doi: 10.1111/j.1365-2125.2004.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 3. Baltimore: Williams & Wilkins; 1995. Appendix I–C. Estimation of absorption kinetics from plasma concentration data; pp. 478–84. [Google Scholar]
- 15.Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59:7–13. doi: 10.1016/S0009-9236(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 16.Villikka K, Kivistö KT, Backman JT, Olkkola KT, Neuvonen PJ. Triazolam is ineffective in patients taking rifampin. Clin Pharmacol Ther. 1997;61:8–14. doi: 10.1016/S0009-9236(97)90176-4. [DOI] [PubMed] [Google Scholar]
- 17.Kyrklund C, Backman JT, Kivistö KT, Neuvonen M, Laitila J, Neuvonen PJ. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 2000;68:592–7. doi: 10.1067/mcp.2000.111414. [DOI] [PubMed] [Google Scholar]
- 18.Fromm MF, Busse D, Kroemer HK, Eichelbaum M. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology. 1996;24:796–801. doi: 10.1002/hep.510240407. [DOI] [PubMed] [Google Scholar]
- 19.Holtbecker N, Fromm MF, Kroemer HK, Ohnhaus EE, Heidemann H. The nifedipine–rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos. 1996;24:1121–3. [PubMed] [Google Scholar]
- 20.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- 21.Baba S. Pioglitazone a review of Japanese clinical studies. Curr Med Res Opin. 2001;17:166–89. doi: 10.1185/0300799039117059. [DOI] [PubMed] [Google Scholar]
- 22.Shen Z, Reed JR, Creighton M, Liu DQ, Tang YS, Hora DF, Feeney W, Szewczyk J, Bakhtiar R, Franklin RB, Vincent SH. Identification of novel metabolites of pioglitazone in rat and dog. Xenobiotica. 2003;33:499–509. doi: 10.1080/0049825031000085951. [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Shin JG, Chong WS, Kim S, Lee JS, Jang IJ, Shin SG. Time course of the changes in prednisolone pharmacokinetics after co-administration or discontinuation of rifampin. Eur J Clin Pharmacol. 1993;45:287–9. doi: 10.1007/BF00315399. [DOI] [PubMed] [Google Scholar]
- 24.Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brøsen K. Rifampicin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur J Clin Pharmacol. 2004;60:109–14. doi: 10.1007/s00228-004-0746-z. [DOI] [PubMed] [Google Scholar]
- 25.Kajosaari LI, Laitila J, Neuvonen PJ, Backman JT. Metabolism of repaglinide by CYP2C8 and CYP3A4 in vitro: effect of fibrates and rifampicin. Basic Clin Pharmacol Toxicol. 2005. pp. 249–56. [DOI] [PubMed]
- 26.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose–response study. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 27.Niemi M, Neuvonen M, Juntti-Patinen L, Backman JT, Neuvonen PJ. Effect of fluconazole on the pharmacokinetics and pharmacodynamics of nateglinide. Clin Pharmacol Ther. 2003;74:25–31. doi: 10.1016/S0009-9236(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 28.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69:400–6. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 29.Niemi M, Kivistö KT, Backman JT, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of glimepiride. Br J Clin Pharmacol. 2000;50:591–5. doi: 10.1046/j.1365-2125.2000.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JY, Kim KA, Park PW, Park CW, Shin JG. Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther. 2003;74:334–40. doi: 10.1016/S0009-9236(03)00221-2. [DOI] [PubMed] [Google Scholar]