Abstract
Aim
Identify and quantify factors describing variability of amikacin clearance in preterm neonates at birth.
Methods
Population pharmacokinetics of amikacin were estimated in a cohort of 205 extreme preterm neonates [post conception age (PCA) 27.8, SD 1.8, range 24–30 weeks; weight 1.07, SD 0.34, range 0.45–1.98 kg, postnatal age <72 h]. Covariate analysis included weight, PCA, Apgar score, prophylactic administration of a nonsteroidal anti-inflammatory drug (NSAID) to the neonate, maternal indomethacin and betamethasone administration, and chorioamnionitis.
Results
A one-compartment linear disposition model with zero order input (0.3 h i.v. infusion) and first-order elimination was used. The population parameter estimate for volume of distribution (V) was 40.2 l per 70 kg. Clearance (CL) increased from 0.486 l h−1 per 70 kg at 24 weeks PCA to 0.940 l h−1 per 70 kg by 30 weeks PCA. The population parameter variability (PPV) for CL and V was 0.336 and 0.451. The use of a NSAID (either aspirin or ibuprofen) in the first day of life reduced amikacin clearance by 22%. Overall 65% of the variability of CL was predictable. Weight explained 48%, PCA 15% and NSAIDs 2%.
Conclusions
Size and post-conception age are the major contributors to clearance variability in extreme premature neonates (<31 weeks PCA). The large (35% of total) unexplained variability in clearance reinforces the need for target concentration intervention to reduce variability in exposure to a safe and effective range.
Keywords: amikacin, extreme preterm infants, nonselective COX inhibitors, pharmacokinetics, target concentration intervention, variability
Introduction
Bactericidal effectiveness of amikacin, like any other aminoglycoside, mainly relates to intermittent, discontinuous peak concentrations due to the postantibiotic effect, while renal side-effects and ototoxicity relate to the average plasma concentration, based on saturation of renal and cochlear cell binding sites. The combination of effectiveness and safety has resulted in the concept of administration of larger doses with extended dosing intervals between consecutive administrations [1–6]. For example, using once daily dosing, the initial concentration of gentamicin is recommended to be 15–30 mg l−1 compared with 5–10 mg l−1 in a three times a day dosing [7].
It is assumed that an initial amikacin target concentration range of 15–30 mg l−1 and trough concentration of less than 5 mg l−1, corresponding to an average steady-state concentration of about 10 mg l−1, are adequate targets in a ‘once daily’ approach, but the between-subject variability in pharmacokinetics (PK) makes it difficult to achieve a safe and effective dose regimen in the individual premature neonate. Nothing appears to be known about within-patient variability in this age group (between occasion variability) which is the limiting factor for using target concentration intervention (TCI) to achieve safe and effective levels of variability [7].
The effect of ibuprofen on amikacin clearance in a cohort of 73 extremely premature neonates [post conception age (PCA) age below 31 weeks] inlcuded in the Multicentre Ibuprofen Prophylaxis Study (MIPS) trial was recently described [8]. Based on these observations, a more elaborated, PCA-based dosing chart was implemented in the Gasthuisberg Neonatal Intensive Care Unit (NICU) with an increase of the time interval between consecutive administrations of amikacin if ibuprofen was coadministered [3, 9].
We had the opportunity to examine the effect of these dosing adaptations in a cohort of 205 extreme premature neonates admitted in a time interval before (1999–2002) and following implementation (2002 to October 2004). In addition, a population-based approach was used to predict sources of the variability. This current study investigated pharmacokinetics only and did not consider pharmacodynamics except to acknowledge empirically derived target concentrations.
Methods
Patients
Perinatal data of infants admitted in the unit since 1 January 1996 were available in a prospectively collected database. These data were searched for all neonates admitted from January 1999 to September 2004 in the first day of life with a PCA below 31 weeks, postnatal age <3 days and on respiratory support. Neonates were included in this retrospective study if data on two (peak and trough) serum samples of amikacin were available. Maternal and neonatal characteristics (PCA at birth, birth weight, Apgar score at 1 and 10 min, antenatal betamethasone, antenatal indometacin, chorio-amnionitis) were extracted from this database. Based on the above-mentioned criteria, the study involved 205 preterms with a mean PCA of 27.8 (SD 1.8) weeks and a mean birth weight of 1.07 (SD 0.34) kg.
Before the Gasthuisberg NICU contributed to the MIPS trial (1999), acetylsalicylic acid (4 × 20 mg kg−1 day−1 of acetylsalicylic acid-lysine, i.e. 4 × 11 mg kg−1 day−1 of acetylsalicylic acid for 2 days) was routinely administered in premature neonates who developed respiratory distress syndrome necessitating respiratory support and surfactant administration.
During inclusion in the MIPS trial (2000–2001) premature neonates with a PCA <31 weeks at birth were randomized in a double-blind manner to receive either ibuprofen-lysine or placebo (normal saline) in the first 3 days of life. The first dose (10 mg kg−1, 1 ml kg−1) of ibuprofen-lysine was administered as an i.v. infusion of 15 min in the first 6 h of life. The two consecutive doses (5 mg kg−1, 0.5 ml kg−1) were administered 24 and 48 h later. Exclusion criteria for the MIPS trial were perinatal asphyxia (Apgar score at 5 min <5), serum creatinine >1.3 mg dl−1, clinical bleeding tendency or thrombocytopenia (platelet count <60 000 mm−3), life-threatening septicaemia or documented intraventricular haemorrhage before inclusion [8]. Both nonsteroidal anti-inflammatory drugs (NSAIDs) were administered to induce closure of an asymptomatic patent ductus arteriosus.
The MIPS trial was approved by the local ethics committee of the University Hospital, Gasthuisberg, Leuven and neonates were included following informed consent from parents [8]. In contrast to the MIPS trial, only data in premature neonates on respiratory support were evaluated in this current study. Shortly after finalization of the MIPS trial, we temporarily continued to administer ibuprofen in premature neonates who required respiratory support and surfactant administration. No additional ethical approval was sought for the collection and the retrospective analysis of amikacin and clinical data used in this present report.
Amikacin: drug administration and sampling
Amikacin (20 mg kg−1 per 36 h in neonates with a PCA below 30 weeks and 20 mg kg−1 per 24 h in neonates with a PCA of ≥30 weeks) and ampicillin (2 × 50 mg kg−1 day−1) was the standard empirical treatment for suspected early-onset bacterial infection in the Gasthuisberg NICU until shortly after the finalization of the MIPS trial. A more complex PCA-based dosing chart was implemented in 2002 based on the suggestions of Langhendries et al. (PCA <28 weeks, 20 mg kg−1 per 42 h; PCA 28–30 weeks, 20 mg kg−1 per 36 h; PCA 31–33 weeks, 18.5 mg kg−1 per 30 h; PCA 34–37 weeks, 17 mg kg−1 per 24 h; PCA >37 weeks, 15.5 mg kg−1 per 24 h) with an additional dosing interval increase of 6 h if ibuprofen was coadministered or if neonates had suffered asphyxia or hypoxia [3].
Amikacin (Amukin®; Bristol Myers Squibb, Braine-l’Alleud, Belgium) was given as an i.v. infusion over 20 min by syringe driver (SIMS Graseby®, Watford, UK). Administration was initiated shortly after admission. Blood samples for therapeutic drug monitoring were collected by arterial line or venous puncture just before (‘trough’) and 1 h after initiation of administration (‘peak’) of the second dose of amikacin.
Assay
Amikacin serum concentration measurements were performed using fluorescence polarization immunoassay (TDx; Abbott Laboratories, Diagnostics Division, Abbott Park, IL, USA) in the hours following sample collection and were reported in mg l−1. Drug recovery from extraction was 100% (SD 2.6%) over the tested concentration range 3–35 mg l−1 Precision was assessed at 5, 15 and 30 mg l−1; these yielded a within-run coefficient of variation (CV) 1.37–2.09%, between-day CV 0–1.74% and a total CV of 2.6–3.2%. The minimal quantifiable concentration was 0.8 mg l−1 defined by a CV of <20% (Abott information). The CV was typically <5% based on internal quality assessment covering the concentration range up to 50 mg l−1.
Pharmacokinetic analysis
Population parameter estimations
Population parameter estimates were obtained using a nonlinear mixed effects approach (NONMEM) [10]. Using this methodology, one can account for population parameter variability (between and within subjects) and residual variability (random effects) as well as parameter differences predicted by covariates (fixed effects). The between-subject variability in model parameters was modelled by exponentiating random effects (equivalent to assuming a log-normal distribution) Residual unexplained variability was modelled using an additive random effect (equivalent to assuming a normal distribution).
This error model assumes that the residual variability is the same order of magnitude over the whole range of measurements. The population parameter variability is modelled in terms of random effect (η) variables. Each of these variables is assumed to have mean 0 and a variance denoted by ω2, which is estimated. We report the estimate of ω for each variability component. The covariance between two elements of η (e.g. CL and V) is a measure of statistical association between these two variables. Their covariance is related to their correlation, i.e.
The covariance of clearance and distribution volume variability was estimated.
Covariate analysis
The parameter values were standardized for a body weight of 70 kg using an allometric model [11, 12].
where Pi is the parameter in the ith individual, Wi is the weight in the ith individual and Pstd is the parameter in an individual with a weight Wstd of 70 kg. This standardization has a strong theoretical and empirical basis and allows comparison of neonatal parameter estimates with those reported for adults. The PWR exponent was 0.75 for clearance and 1 for distribution volumes [13–15].
The quality of fit of the pharmacokinetic model to the data was judged by NONMEM's objective function and by visual examination of plots of observed vs. predicted concentrations. Models were nested and an improvement in the objective function was referred to the χ2 distribution to assess significance, e.g. an objective function change (OBJ) of 3.84 is significant at α = 0.05.
Covariate analysis included a model investigating age-related changes for clearance:
where CLstd are the population estimates for CL at 24 weeks, respectively, standardized to a 70-kg person using allometric models; PCA is the post-conception age in weeks; SLPCL is a parameter describing changes of clearance with PCA.
An indicator variable was applied to those subjects given a nonselective NSAID (ibuprofen or acetylsalicylic acid). The effect of coadministration of ibuprofen or acetylsalicylic acid on amikacin clearance was investigated by applying a scaling factor (FNSAID) to clearance in those neonates given a nonselective COX-inhibitor. The influence of prenatal betamethasone, prenatal indomethacin, perinatal chorioamnionitis, Apgar score at 1 and 10 min (continuous variable) were also investigated in a similar manner. A predictive check was performed by simulating 1000 patient profiles from the final model and its parameters. The 95% prediction interval was compared with the time course of observed concentrations.
Target concentration intervention
Population parameter estimates and their variability from the final model were used to estimate individual Bayesian parameter predictions of clearance (CL) and volume (V) in 1000 simulated neonates of similar PCA to the premature neonates in the present report. Target concentrations of 25 mg l−1 (peak) and 4 mg l−1 (trough) were assumed [16–18] Dose for each individual subject [(peak − trough) × (V)] and dosing interval {[Ln(peak) − Ln(trough)] × V/CL} were calculated by assuming bolus input and one compartment disposition. Calculated dosing intervals were categorized into four discrete time intervals for practical dosing recommendations, i.e. 24 h (= 30 h), 36 h (>30 to 42 h), 48 h (>42 to 60 h) and 72 h (>60 h), similar to those used in the Langhendries nomogram [3]. Because of the sparse design, our study was unable to estimate within-subject variability (WSV). However, the reliability of using TCI depends on the magnitude of WSV. Estimates of WSV were derived from adult aminoglycoside literature [7]. In the absence of any other information it was assumed that the WSV as a fraction of the unexplained population parameter variability (PPV) was the same in adults and neonates. Concentrations from these dosing regimens and parameter predictions were simulated to assess the predictive performance of target concentration intervention.
The population mean parameters, between-subject variance and residual variance were estimated using the first-order conditional estimate method using ADVAN 1 TRANS 2 of NONMEM V. The convergence criterion was three significant digits. A Compaq Digital Fortran Version 6.6A compiler with Intel Celeron 333-MHz CPU (Intel Corp., Santa Clara, CA, USA) under MS Windows XP (Microsoft Corp., Seattle, WA, USA) was used to compile and execute NONMEM.
Results
Clinical characteristics of neonates before (1999–2002) and following (2002 to October 2004) implementation of the more elaborated PCA-based scheme for amikacin administration are shown in Table 1. No significant differences in clinical characteristics between both groups were observed. Data from 410 drug assay samples in these 205 subjects were available to study the PK of amikacin. Although mean trough levels of amikacin were significantly lower in the second time interval (8.2 mg l−1vs. 4.8 mg l−1, P < 0.001), there were still 29 (38.2%) neonates with a trough amikacin concentration above 5 mg l−1.
Table 1.
Clinical characteristics of neonates before and after the implementation of a post conception age (PCA)-based scheme for amikacin administration
| 1999–2002 | 2002–04 | P-value | |
|---|---|---|---|
| Number of neonates | 129 | 76 | |
| Neonatal survival (day 28) | 96% | 92% | NS |
| Gestational age (weeks) | 28 (24–30) | 28 (24–30) | NS |
| Birth weight (g) | 1047 (346) | 1110 (341) | NS |
| Prenatal indomethacin | 10 | 2 | NS |
| Prenatal betamethasone | 103 | 58 | NS |
| NSAID administration | 116 | 41 | NS |
| Peak amikacin (mg l−1) | 45.7 (17.8) | 38.13 (12.4) | NS |
| range | 10.4–100 | 14.2–71.2 | |
| Trough amikacin (mg l−1) | 8.2 (4.4) | 4.8 (2.4) | <0.001 |
| range | 1.1–21.8 | 0.8–15 |
Data are reported by mean and standard deviation or by median and range. Clinical characteristics and drug assay samples in infants admitted before and since implementation of the more elaborated PCA-based scheme for amikacin were compared (Mann–Whitney U, χ2).
Covariate effects were observed for size, post-conception age and early neonatal coadministration of a NSAID (aspirin or ibuprofen). There was no effect attributable to prenatal betamethasone, prenatal indomethacin, perinatal chorioamnionitis, or Apgar score.
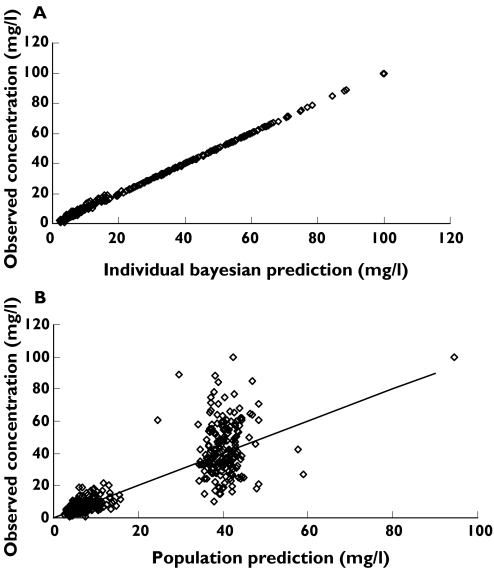
Individual concentration predictions are based on values of maximum a posteriori (MAP) Bayesian estimates of the parameters, while predicted population concentrations are based on population parameters and covariate information (Figure 1A,B).
Figure 1.
(A) Individual Bayesian concentration predictions based on values of the parameters for the specific individual are compared with those observed. (B) Population predictions are compared with those observed. The line x = y is the line of identity
Parameter estimates are shown in Table 2. Clearance (CL) increased from 0.486 l h−1 per 70 kg at 24 weeks PCA to 0.940 l h−1 per 70 kg by 30 weeks PCA. The PPV for clearance without covariates in the model was 56.5% and with covariates was 33.6%. This difference between PPV with and without covariates is a measure of the predictable decrease in PPV due to covariates. The ω2 estimates for the different components contributing to variability are shown in Table 3. The ratio of the population parameter variability predictable from covariates (PPVP2) to the total population parameter variance obtained without covariate analysis (PPV2) indicates the relative importance of covariate information. For example, the ratio of 0.65 achieved for clearance in this current study indicates that 65% of the overall variance in clearance is predictable from covariate information. Weight was used to predict size using allometric models and contributed 48% of variance. PCA at birth contributed 14% and the coadministration of a NSAID in the first day 2%. Weight and PCA independently contributed to prediction of variability (Table 3a).
Table 2.
Amikacin population pharmacokinetic parameter estimates
| Parameter | Estimate | PPV | %SE |
|---|---|---|---|
| CLstd (l h−1 per 70 kg) at 24 weeks PCA, without NSAID | 0.486 | 0.336 | 7.2 |
| Vstd (l per 70 kg) | 40.2 | 0.451 | 3.3 |
| SLPCL (l week−1) | 0.11 | – | 12.5 |
| FNSAID | 0.788 | – | 4.5 |
| Residual unidentified variability (mg l−1) | 1.59 | 31.7 |
PPV is the population parameter variability expressed as the square root of its variance, SE is the standard error of the estimate. V = [Vstd ×(Wt/70)] l per 70 kg; CL =[CLstd ×(Wt/70) 0.75] ×EXP[SLPCL ×(PCA-24) ×FNSAID] l h−1 per 70 kg where Vstd and CLstd are the population estimates for V and CL, respectively, standardized to a 70-kg person using allometric models; PCA is the post-conception age in weeks; SLPCL is the factor relating PCA to developmental changes in CL; FNSAID is a scaling factor for those premature neonates given a NSAID. Err is the residual additive error.
Table 3.
Effect of covariate analysis on variance of clearance (ω2)
| (a) Impact of each covariate alone | |||||
|---|---|---|---|---|---|
| Individual covariate alone | PPVt2 | PPVR2 | PPVP2 | PPVP2/PPVt2 | OBJ |
| No covariates | 0.319 | 0.319 | 0 | 0 | 2073.0 |
| Allometric scaling | 0.3191 | 0.165 | 0.154 | 48.3% | 1973.9 |
| Post-conception age | 0.3191 | 0.2 | 0.119 | 37.3% | 2012.8 |
| NSAID use | 0.3191 | 0.301 | 0.018 | 5.6% | 2047.6 |
| Sequential nested model | PPVt2 | PPVR2 | PPVP2 | PPVP2/PPVt2 | OBJ |
|---|---|---|---|---|---|
| No covariates | 0.319 | 0.319 | 0 | 0 | 2073.0 |
| Allometric scaling | 0.3191 | 0.165 | 0.154 | 48.3% | 1973.9 |
| Post-conception age | 0.3191 | 0.119 | 0.200 | 62.7% | 1919.0 |
| NSAID use | 0.3191 | 0.113 | 0.206 | 64.6% | 1893.2 |
(b) Impact of each covariate when added sequentially to the model
Assumed from no covariate model estimate.
Assumed from no covariate model estimate. PPVt2is the total population parameter variability estimated without covariate analysis, PPVP2is the population parameter variability predictable from covariates, PPVR is the random PPV estimated on a parameter when covariate analysis is included. The ratio of the population parameter variability predictable from covariates (PPVP) to the total population parameter variability obtained without covariate analysis (PPVt2)(i.e.PPVP2/PPVt2) indicates the fraction of the total variability in the parameter that is predictable from covariates. OBJ is the objective function value measuring the goodness of fit.
Mean age related PK predictions based on the covariate models are shown in Table 4. Parameters were estimated based on a standard adult weight of 70 kg to enable comparison with other studies and aminoglycosides. This table also expresses PK parameters per kg, based on the expected weight for each age group.
Table 4.
Weight- and age-related amikacin parameters and dosing recommendations predicted using the allometric ‘1/4 Power’ Model (per 70 kg)
| Age, weeks PCA | Weight, kg | CL, l h−1 per 70 kg | CL, l h−1 | V, l per 70 kg | V, l | Maintenance dose, mg | Dosing interval, h |
|---|---|---|---|---|---|---|---|
| 24 | 0.5 | 0.486 | 0.012 | 40.2 | 0.29 | 6.0 | 44 |
| 25 | 0.6 | 0.543 | 0.015 | 40.2 | 0.34 | 7.2 | 41 |
| 26 | 0.8 | 0.606 | 0.021 | 40.2 | 0.46 | 9.6 | 40 |
| 27 | 0.9 | 0.676 | 0.026 | 40.2 | 0.52 | 10.9 | 37 |
| 28 | 1 | 0.755 | 0.031 | 40.2 | 0.57 | 12.1 | 34 |
| 29 | 1.15 | 0.842 | 0.039 | 40.2 | 0.66 | 13.9 | 31 |
| 30 | 1.3 | 0.94 | 0.047 | 40.2 | 0.75 | 15.7 | 29 |
The predictive check on the final model demonstrated that the fraction of patients with concentrations <5 mg l−1 or >20 mg l−1 was very similar to similar fractions observed in the study population. Target concentration intervention leads to a substantial predicted improvement in concentrations in relation to the target of trough <5 mg l−1 and geometric mean of 10 mg l−1(Table 5).
Table 5.
Concentrations during the two study periods and for the simulation study. Target concentration intervention (TCI) resulted in improved concentration profiles
| Concentration | 1999–2002 n = 129 | 2002–2004 n = 76 | Total study period n = 205 | Predictive check n = 1000 | TCI n = 1000 |
|---|---|---|---|---|---|
| <5 mg l–1 | 27.9% | 61.8% | 40.5% | 36% | 87% |
| >20 mg l–1 | 94.5% | 93% | 94.2% | 94% | 77% |
| Average concentration1 | 18.8 mg l–1 | 12.9 mg l–1 | 16.5 mg l–1 | 16.6 mg l–1 | 8.8 mg l–1 |
Estimated from geometric mean of measured (or simulated) peak and trough concentrations.
Discussion
There is still uncertainty concerning the most safe and effective dosing regimen of aminoglycosides in neonates. Transient higher but less frequent maximum serum concentrations may allow a better peak concentration/minimal inhibitory concentration ratio, increasing bactericidal effectiveness and decreasing risk of bacterial resistance [1–3]. Due to a saturable process in binding of aminoglycosides to the renal proximal tubule brush border membranes, pulse administration probably diminishes the risk of nephrotoxicity. Although speculative, nephrotoxicity of amikacin may be lower in neonates due to reduced renal uptake capacity [4, 19, 20].
Target concentrations for amikacin have not been prospectively defined but clinical convention aims for a trough around 4 mg l−1 and a peak around 25 mg l−1 with an implicit average exposure of 10 mg l−1. Following implementation of a more complex PCA-based normogram for dose calculation of amikacin in premature neonates, a significant decrease in the mean trough concentration of amikacin was documented (Table 1). However, a majority (72%, Table 5) of neonates had a trough amikacin concentration above 5 mg l−1 in this study. Determination of dosing regimen by weight and PCA alone resulted in trough concentrations of >5 mg l−1 in 38% of neonates. This is compatible with studies in adults showing that covariate information (age, weight, sex, serum creatinine) is insufficient to achieve safe and effective concentrations [7].
A PK analysis seeking covariates to explain this observed variability in amikacin clearance was undertaken in a population of extreme preterm infants (24–30 weeks PCA) in their first days of life. We limited the cohort population to extreme preterm neonates in their first days of life since previous PK studies have focused on the relevance of PCA and postnatal age in more heterogeneous cohorts [3, 5, 6, 21, 22].
Langhendries et al. documented a mean amikacin clearance of 0.87 ml kg−1 min−1 in preterm [gestational age (GA) 28–30 weeks] and of 0.73 ml kg−1 min−1 in extreme preterm neonates (GA <28 weeks), while Kenyon observed a mean amikacin clearance of 0.75 ml kg−1 min−1 when only observations (n = 12) in preterm neonates with a GA <31 weeks were considered [3, 22]. Estimated amikacin clearance in our cohort (all <72 h, 76% cotreated with a NSAID) was 0.6 ml kg−1 min−1 in preterm (GA 28–30 weeks, mean weight 1.15 kg) and 0.44 ml kg−1 min−1 in extreme preterm neonates (GA <28 weeks, mean weight 0.8 kg).
In the present cohort, weight and PCA were the major contributors to clearance variability and coadministration of a NSAID also contributed to the variability observed. Neither prenatal drug exposure (maternal betamethasone, indomethacin) nor early neonatal characteristics (Apgar score) further contributed to the variability observed, leaving 35% of total variance in CL still unexplained.
Size has considerable impact on the variability of PK parameters in children and is often unaccounted for in neonatal PK studies [11, 12, 23]. Size was the primary covariate used in our analysis of the effects of age and weight. This deliberate choice was based on known biological principles. A great many physiological, structural and time-related variables scale predictably within and between species with weight exponents of 0.75, 1 and 0.25, respectively [15]. We have used these ‘1/4 power models’ in this current study rather than centred weight, or some other function of weight, because the ‘1/4 power models’ have a sound theoretical and observational basis in biology [13, 14]. The 3/4 power law for metabolic rates was derived from a general model that describes how essential materials are transported through space-filled fractal networks of branching tubes. By choosing weight as the primary covariate, the secondary effects of PCA could be investigated. We had no prior biological model for the change of clearance with time (PCA), but assumed a first-order process, which commonly underlies time varying processes in biology. With a wider range of PCA a different function may be found to be more useful. We have chosen to scale all parameter estimates to a standard weight of 70 kg to facilitate comparison with adult values.
Amikacin is almost exclusively cleared by renal elimination and amikacin CL reflects glomerular filtration rate (GFR) [3, 5, 6, 21, 24]. PCA is a predictor of amikacin clearance presumably because it predicts the time course of development of the GFR [24]. GFR changes are usually referenced to body surface area in children [25], a model that approximates the ‘3/4 power model’ and is equivalent to using 2/3 as the weight exponent. GFR matures during infancy and approaches an adult rate (6 l h−1 per 70 kg) by 6 months postnatal age [25–27]. The administration of either ibuprofen or aspirin to neonates in the first day of life to enhance closure of an asymptomatic patent ductus arteriosus was associated with a reduction of amikacin clearance by 21%. This observation correlates with experimental data in newborn rabbits and is not limited to extreme preterm neonates [9, 28–30].
We hypothesized that prenatal treatment with betamethasone might be associated with a more mature GFR at birth, based on experimental work in animals and earlier observations in human premature neonates [31–33]. There is conflicting evidence in the literature on the impact of prenatal betamethasone on renal clearance in human premature neonates, but we found no effect on amikacin clearance at birth attributable to maternal betamethasone.
Peak concentration reflects volume of distribution and dose given. Volume of distribution was associated with a PPV of 0.451. We were unable to show any effect attributable to PCA. This is in contrast to studies showing substantial changes in volume of distribution of several drugs during the first weeks of life in cohorts of more heterogeneous age [3, 5, 6, 21, 22, 34, 35]. Polar drugs such as aminoglycosides distribute rapidly into the extracellular fluid (ECF), but enter cells more slowly. The initial dose of such drug given is usually higher in preterms compared with full terms or children because of an increased ECF volume [36]. We were unable to detect a volume of distribution change with increasing PCA in this cohort of limited PCA range (24–30 weeks), but this does not eliminate potential PCA-independent differences in ECF [3, 5, 6]. Sepsis, for example, is associated with an increase in distribution volume in neonates [37]. In addition, preterms included in the present report are in their first day of life, precluding the ability to detect the effect of postnatal maturational changes in ECF [27, 36].
Finally, there are reports of substantial differences between prescribed and administered doses of drugs, including amikacin, in neonates, probably contributing to the estimated PK parameter variability and residual variance [38–40].
The more elaborated PCA-based dose procedure still was inadequate to reduce variability to a safe and effective range. The use of individual Bayesian estimates of clearance and volume to guide TCI improved future concentration predictions. Bayesian forecasting has proved useful for aminoglycoside dosing in children [41–43]. Bleyzac et al. have suggested that incorporation of renal maturation into the population model will further improve Bayesian adaptive control of therapeutics in neonates [6]. Such intervention is likely to prove more effective than traditional nomogram-based models, but unfortunately, is not yet feasible at birth since serum creatinine still reflects maternal renal function [44, 45]. Our study has shown that Bayesian forecasting of dose based on early blood concentration measurements can reduce the number of patients with trough concentrations >5 mg l−1 while still maintaining average concentrations close to 10 mg l−1.
The estimated unexplained variability (PPV = 0.336) in clearance of amikacin in a rather homogeneous cohort of extreme preterms is remarkably similar (PPV = 0.32) to that predicted in adults treated with aminoglycosides [7]. Variability could only in part be explained by weight, PCA and cotreatment with a NSAID in early neonatal life.
We recommend predicting initial doses based on weight and PCA with subsequent dose individualization based on Bayesian estimates of PK parameters from concentrations measured immediately before and 60 min after the second dose. With target concentrations of 25 mg l−1 peak and 4 mg l−1 trough (geometric mean 10 mg l−1), the maintenance dose is predicted from 21 × V mg−1, while the dosing interval is predicted from 1.8 × V/CL h−2 (Table 4). Practical dosing intervals can then be determined from their closeness to this prediction (e.g. 48 h at 1 kg, 24 weeks PCA; 12 h at 3 kg, 40 weeks PCA (Table 4).
Acknowledgments
The clinical research of K.A. is supported by the Fund for Scientific Research, Flanders (Belgium) by a Clinical Doctoral Grant (A 6/5 – KV – G 1). We gratefully acknowledge the assistance of Koen Desmet, Department of Laboratory Medicine, for analysing the amikacin samples.
References
- 1.Mattie H. The importance of pharmacokinetics and pharmacodynamics for effective treatment of infections. Clin Invest. 1993;71:480–2. doi: 10.1007/BF00180064. [DOI] [PubMed] [Google Scholar]
- 2.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–9. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Langhendries JP, Battisti O, Bertrand JM, Francois A, Kalenga M, Darimont J, Scalais E, Wallemacq P. Adaptation in neonatology of the once-daily concept of aminoglycoside administration: evaluation of a dosing chart for amikacin in an intensive care unit. Biol Neonate. 1998;74:351–62. doi: 10.1159/000014053. [DOI] [PubMed] [Google Scholar]
- 4.Langhendries JP, Battisti O, Bertrand JM, Francois A, Darimont J, Ibrahim S, Tulkens PM, Bernard A, Buchet JP, Scalais E. Once-a-day administration of amikacin in neonates: assessment of nephrotoxicity and ototoxicity. Dev Pharmacol Ther. 1993;20:220–30. doi: 10.1159/000457566. [DOI] [PubMed] [Google Scholar]
- 5.Treluyer JM, Merle Y, Tonnelier S, Rey E, Pons G. Nonparametric population pharmacokinetic analysis of amikacin in neonates, infants, and children. Antimicrob Agents Chemother. 2002;46:1381–7. doi: 10.1128/AAC.46.5.1381-1387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleyzac N, Varnier V, Labaune JM, Corvaisier S, Maire P, Jelliffe RW, Putet G, Aulanger G. Population pharmacokinetics of amikacin at birth and interindividual variability in renal maturation. Eur J Clin Pharmacol. 2001;57:499–504. doi: 10.1007/s002280100355. [DOI] [PubMed] [Google Scholar]
- 7.Matthews I, Kirkpatrick C, Holford N. Quantitative justification for target concentration intervention — parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol. 2004;58:8–19. doi: 10.1111/j.1365-2125.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwé W, Jespers A, Weyler J, Harrewijn I, Langhendries JP. Prohylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9449):1945–9. doi: 10.1016/S0140-6736(04)17477-1. [DOI] [PubMed] [Google Scholar]
- 9.Allegaert K, Cossey V, Langhendries JP, Naulaers G, Vanhole C, Devlieger H, Van Overmeire B. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol Neonate. 2004;86:207–11. doi: 10.1159/000079618. [DOI] [PubMed] [Google Scholar]
- 10.Sheiner LB, Beal SL. NONMEM Users Guide. San Francisco: Division of Pharmacology, University of California; 1979. [Google Scholar]
- 11.Anderson BJ, Meakin GH. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth. 2002;12:205–19. doi: 10.1046/j.1460-9592.2002.00616.x. [DOI] [PubMed] [Google Scholar]
- 12.Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–32. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 13.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276(5309):122–6. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 14.West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–9. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 15.Peters HP. Physiological correlates of size. In: Beck E, Birks HJB, Conner EF, editors. The Ecological Implications of Body Size. Cambridge: Cambridge University Press; 1983. pp. 48–53. Chapter 4. [Google Scholar]
- 16.Prins JM, Weverling GJ, de Blok K, van Ketel RJ, Speelman P. Validation and nephrotoxicity of a simplified once-daily aminoglycoside dosing schedule and guidelines for monitoring therapy. Antimicrob Agents Chemother. 1996;40:2494–9. doi: 10.1128/aac.40.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Hoog M, van den Anker JN. Aminoglycosides and glycopeptides. In: Yaffe S, Aranda JV, editors. Neonatal and Pediatric Pharmacology. 3. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 377–401. [Google Scholar]
- 18.Holford NHG. Target concentration intervention: beyond Y2K. Br J Clin Pharmacol. 1999;48:9–13. doi: 10.1046/j.1365-2125.1999.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PD, Bennett DB, Gleason CR, Hottendorf GH. Correlation between renal membrane binding and nephrotoxicity of aminoglycosides. Antimicrob Agents Chemother. 1987;31:570–4. doi: 10.1128/aac.31.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koren G, Klein J, MacLeod SM. The dissociation between aminoglycoside serum concentrations and nephrotoxicity. Life Sci. 1988;43:1817–23. doi: 10.1016/0024-3205(88)90281-0. [DOI] [PubMed] [Google Scholar]
- 21.Padovani EM, Pistolesi C, Fanos V, Messori A, Martini N. Pharmacokinetics of amikacin in neonates. Dev Pharmacol Ther. 1993;20:167–73. doi: 10.1159/000457558. [DOI] [PubMed] [Google Scholar]
- 22.Kenyon CF, Knoppert DC, Lee SK, Vandenberghe HM, Chance GW. Amikacin pharmacokinetics and suggested dosage modifications for the preterm infant. Antimicrob Agents Chemother. 1990;34:265–8. doi: 10.1128/aac.34.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson B. Disentangling PK-PD in neonates. Arch Dis Child Fetal Neonatal Ed. 2004;89:F3–4. doi: 10.1136/fn.89.1.F3-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koren G, James A, Perlman M. A simple method for the estimation of glomerular filtration rate by gentamicin pharmacokinetics during routine drug monitoring in the newborn. Clin Pharmacol Ther. 1985;38:680–5. doi: 10.1038/clpt.1985.245. [DOI] [PubMed] [Google Scholar]
- 25.Bergstein JM. Introduction to glomerular diseases. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 16. Philadelphia: W.B. Saunders Co; 2000. pp. 1574–5. [Google Scholar]
- 26.van den Anker JN, Schoemaker RC, Hop WC, van der Heijden BJ, Weber A, Sauer PJ, Neijens HJ, de Groot R. Ceftazidime pharmacokinetics in preterm infants: effects of renal function and gestational age. Clin Pharmacol Ther. 1995;58:650–9. doi: 10.1016/0009-9236(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 27.Arant BS., Jr Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978;92:705–12. doi: 10.1016/s0022-3476(78)80133-4. [DOI] [PubMed] [Google Scholar]
- 28.Guignard JP. The adverse renal effects of prostaglandin-synthesis inhibitors in the newborn rabbit. Semin Perinatol. 2002;26:398–405. doi: 10.1053/sper.2002.37310. [DOI] [PubMed] [Google Scholar]
- 29.Allegaert K, Cossey V, Debeer A, Langhendries JP, Van Overmeire B, de Hoon J, Devlieger H. The impact of ibuprofen on renal clearance in preterm infants is independent of the gestational age. Pediatr Nephrol. 2005;20:740–3. doi: 10.1007/s00467-005-1842-8. [DOI] [PubMed] [Google Scholar]
- 30.Allegaert K, Vanhole C, de Hoon J, Guignard JP, Tibboel D, Devlieger H, Van Overmeire B. Non-selective cyclo-oxygenase inhibitors and glomerular filtration rate in preterm neonates. Pediatr Nephrol. 2005 doi: 10.1007/s00467-005-1998-2. 10.1007/s00467-005-1998-2. [DOI] [PubMed] [Google Scholar]
- 31.van den Anker JN, Hop WC, de Groot R, van der Heijden BJ, Broerse HM, Lindemans J, Sauer PJ. Effects of prenatal exposure to betamethasone and indomethacin on the glomerular filtration rate in the preterm infant. Pediatr Res. 1994;36:578–81. doi: 10.1203/00006450-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Smith LM, Ervin MG, Wada N, Ikegami M, Jobe AH. Single and multiple prenatal glucocorticoid exposures improve preterm newborn lamb cardiovascular and renal function similarly. Am J Obstet Gynecol. 2003;188:444–53. doi: 10.1067/mob.2003.36. [DOI] [PubMed] [Google Scholar]
- 33.MacKintosh D, Baird-Lambert J, Drage D, Buchanan N. Effects of prenatal glucocorticoids on renal maturation in newborn infants. Dev Pharmacol Ther. 1985;8:107–14. doi: 10.1159/000457028. [DOI] [PubMed] [Google Scholar]
- 34.Anderson BJ, Woollard GA, Holford NHG. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol. 2000;50:125–34. doi: 10.1046/j.1365-2125.2000.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–17. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 36.Friis-Hansen B. Body water compartments in children: changes during growth and related changes in body composition. Pediatrics. 1961;28:169–81. [PubMed] [Google Scholar]
- 37.Lingvall M, Reith D, Broadbent R. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br J Clin Pharmacol. 2005;59:54–61. doi: 10.1111/j.1365-2125.2005.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philips JB, 3rd, Geerts M, Dew A, Cassady G. The accuracy of amikacin administration in neonates. Pediatr Pharmacol (New York) 1983;3:127–30. [PubMed] [Google Scholar]
- 39.Reilly KM. Problems in administration techniques and dose measurement that influence accuracy of i.v. drug delivery. Am J Hosp Pharm. 1987;44:2545–50. [PubMed] [Google Scholar]
- 40.Allen EM, Van Boerum DH, Olsen AF, Dean JM. Difference between the measured and ordered dose of catecholamine infusions. Ann Pharmacother. 1995;29:1095–100. doi: 10.1177/106002809502901104. [DOI] [PubMed] [Google Scholar]
- 41.Kraus DM, Dusik CM, Rodvold KA, Campbell MM, Kecskes SA. Bayesian forecasting of gentamicin pharmacokinetics in pediatric intensive care unit patients. Pediatr Infect Dis J. 1993;12:713–8. doi: 10.1097/00006454-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Carlstedt BC, Uaamnuichai M, Day RB, Bowman L, Brater DC. Aminoglycoside dosing in pediatric patients. Ther Drug Monit. 1989;11:38–43. doi: 10.1097/00007691-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Vinks AA. The application of population pharmacokinetic modeling to individualized antibiotic therapy. Int J Antimicrob Agents. 2002;19:313–22. doi: 10.1016/s0924-8579(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 44.Ensom MH, Davis GA, Cropp CD, Ensom RJ. Clinical pharmacokinetics in the 21st century. Does the evidence support definitive outcomes? Clin Pharmacokinet. 1998;34:265–79. doi: 10.2165/00003088-199834040-00001. [DOI] [PubMed] [Google Scholar]
- 45.van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21:63–73. doi: 10.1097/00007691-199902000-00010. [DOI] [PubMed] [Google Scholar]