Abstract
Aims
To study regional differences and identify determinants of antibiotic consumption in ambulatory care in Hungary.
Methods
Regional distribution-based antibiotic sales data were converted into a number of defined daily doses (DDD) per 1000 inhabitant-days. Correlations were assessed with the Spearman rank test.
Results
There were large and stable interregional differences in antibiotic consumption. They were associated with socio-economic determinants, e.g. the population receiving free access to medicines and receiving regular social assistance.
Conclusions
More detailed studies are needed to better understand the determinants of antibiotic use in these specific patient populations and to identify additional determinants at regional level.
Keywords: antibacterials, community consumption, determinants of antibiotic use, drug utilization, regional differences
Introduction
There is growing concern about increasing antibiotic use and the consequent emergence of antibiotic-resistant microorganisms, now reaching alarming levels in some European countries [1, 2]. Antibiotic overuse and misuse are major factors contributing to the development of resistance. Therefore, information on the trends, patterns and determinants of antibiotic consumption is essential to determine areas where improvement is needed. Following the recognition of a collective struggle to fight increasing antimicrobial resistance in European countries [3], comprehensive systems for the surveillance of antibiotic consumption and of bacterial resistance have recently been set up in Europe, and Hungary has joined these projects. Our study aimed at complementing information from these multinational surveillance projects by specifically focusing on regional variations in antibiotic consumption in Hungarian ambulatory care.
Methods
For each Hungarian region (county), yearly wholesale data on the community pharmacy sales of antibacterials for systemic use in humans were kindly provided by IMS PharmMIS Consulting Company. In Hungary, drug utilization in nursing homes, social homes, foster homes, prisons and dentists is allocated to community care. Products were classified at the substance level according to the 2003 version of the World Health Organization ATC (Anatomical–Therapeutic–Chemical) classification [4]. When not provided by the 2003 version of the ATC classification, e.g. generations of cephalosporins, further grouping of substances was made according to a more recent version of the ATC classification [5]. Demographic data on each region (county) were obtained from the 2003 yearbook of the Hungarian National Statistics Institute [6]. Consumption in each region was expressed as a number of defined daily doses (DDD) per 1000 inhabitant-days [4].
To explore the reasons for regional differences in total antibiotic consumption, we tested associations between total antibiotic consumption and possible determinants of use [7, 8].
Regional level data on the following possible determinants were extracted from the 2003 yearbooks of the Hungarian National Statistics Institute [6]: population density, prevalence of chronic obstructive pulmonary disease (COPD), of diabetes, and of malignant neoplasms, proportion of population aged 0–5 years, < 14 years and > 60 years, proportion of infants breastfed exclusively at 6 months of age, proportion of infants breastfed at least partially at 6 months of age, prevalence of vaccination against influenza, average monthly net income, gross domestic product (GDP) per inhabitant, number of persons receiving free access to selected medicines from the public health system without quantity limit (known in Hungary as ‘public medicine service’) per 10 000 inhabitants, number of persons regularly receiving social assistance per 10 000 inhabitants, number of persons per 100 rooms, percent of homes with premises for bathing and washing, number of enrolled patients per general practioner (GP), yearly number of consultations and home visits per GP, and density of pharmacies.
Statistical analyses were performed with SPSS (version 12.0.1; SPSS Inc., Chicago, IL, USA). Associations between possible determinants and total antibiotic consumption were tested by the two-tailed Spearman coefficient (r) for nonparametric correlations. Because multiple hypotheses were tested, the Bonferroni correction was used and only associations with a P < 0.003 (0.05/19) were considered statistically significant.
Results
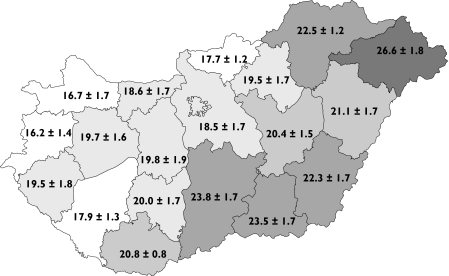
Nationwide total antibiotic consumption in the Hungarian community remained relatively stable during the period 1996–2003 (mean ± SD 20.2 ± 1.4 DDD per 1000 inhabitant-days). However, there were large variations depending on the region (Figure 1). For each year during 1996–2003, there was a more than 1.5-fold difference (mean ± SD 1.7 ± 0.1) between the regions with the lowest and and the highest total antibiotic consumption.
Figure 1.
Average ± standard deviation of regional antibiotic consumption (defined daily doses per 1000 inhabitant-days) in community care, Hungary, 1996–2003
The ranking of the individual regions according to their total antibiotic consumption was basically the same during the whole 8-year study period (Spearman, 0.83 < r < 0.97, P < 0.00001 for all combinations of year tested), hence certain regions remained high consumers of antibiotics, whereas others remained low consumers.
At both endpoints, i.e. 1996 and 2003, the largest interregional variations (ratio max./min. > 2) in the consumption of antibiotic groups were recorded for penicillins with extended spectrum, first- and third-generation cephalosporins, sulphonamides and trimethoprim, lincosamides and quinolones (Table 1).
Table 1.
Mean, minimum and maximum regional antibiotic consumption, Hungary, 1996 and 2003
| 1996 | 2003 | |||||||
|---|---|---|---|---|---|---|---|---|
| DDD per 1000 inhabitant-days | Ratio max./min. | DDD per 1000 inhabitant-days | Ratio max./min. | |||||
| Antibiotic class | Mean ± SD1 | Min. | Max. | max./min | Mean ± SD1 | Min. | Max. | max./min |
| Antibacterials for systemic use (J01) | 19.7 ± 2.2 | 15.5 | 24.1 | 1.6 | 19.6 ± 2.8 | 15.5 | 26.7 | 1.7 |
| Tetracyclines (J01AA) | 3.3 ± 0.6 | 2.4 | 4.4 | 1.8 | 1.9 ± 0.3 | 1.5 | 2.5 | 1.7 |
| Beta-lactam antibiotics, penicillins (J01C) | 7.4 ± 1.3 | 4.7 | 10.8 | 2.3 | 8.4 ± 1.6 | 5.7 | 12.7 | 2.2 |
| Penicillins with extended spectrum (J01CA) | 3.5 ± 0.9 | 1.4 | 5.2 | 3.8 | 3.0 ± 1.0 | 1.1 | 5.3 | 5.0 |
| Beta-lactamase-sensitive penicillins (J01CE) | 1.4 ± 0.2 | 1.1 | 1.8 | 1.7 | 0.9 ± 0.2 | 0.6 | 1.3 | 2.2 |
| Penicillin combinations incl. beta-lactamase inhibitors (J01CR) | 2.6 ± 0.5 | 1.8 | 4.0 | 2.3 | 4.5 ± 0.7 | 3.4 | 6.3 | 1.9 |
| Cephalosporins and related substances (J01DA) | 2.2 ± 0.4 | 1.6 | 2.9 | 1.8 | 2.3 ± 0.4 | 1.8 | 3.3 | 1.8 |
| First-generation cephalosporins (J01DB2) | 0.4 ± 0.1 | 0.3 | 0.6 | 2.4 | 0.1 ± 0.1 | 0.1 | 0.3 | 4.6 |
| Second-generation cephalosporins (J01DC2) | 1.7 ± 0.3 | 1.2 | 2.2 | 1.8 | 1.8 ± 0.3 | 1.3 | 2.4 | 1.9 |
| Third-generation cephalosporins (J01DD2) | 0.2 ± 0.1 | 0.1 | 0.3 | 5.5 | 0.4 ± 0.1 | 0.3 | 0.7 | 2.3 |
| Sulphonamides and trimethoprim (J01EE) | 4.2 ± 0.8 | 2.7 | 6.2 | 2.3 | 2.5 ± 0.9 | 1.8 | 5.0 | 2.8 |
| Macrolides and lincosamides (J01F) | 2.0 ± 0.3 | 1.4 | 2.3 | 1.6 | 3.0 ± 0.4 | 2.1 | 3.6 | 1.7 |
| Macrolides (J01FA) | 1.7 ± 0.2 | 1.2 | 2.0 | 1.7 | 2.4 ± 0.3 | 1.7 | 2.9 | 1.7 |
| Lincosamides (J01FF) | 0.3 ± 0.1 | 0.2 | 0.5 | 2.8 | 0.6 ± 0.1 | 0.4 | 0.8 | 2.0 |
| Quinolones (J01M) | 0.6 ± 0.2 | 0.4 | 1.1 | 2.9 | 1.5 ± 0.3 | 1.1 | 2.1 | 2.0 |
SD, Standard deviation.
According to the ATC/DDD Index 2005.
The only two determinants which showed a significant association with total antibiotic consumption were the number of persons receiving free access to selected medicines from the public health system without quantity limit per 10 000 inhabitants (r = 0.84, P < 0.00001) and the number of persons regularly receiving social assistance per 10 000 inhabitants (r = 0.64, P < 0.001). No significant correlation was found for the other tested determinants, although there was a trend towards a positive association between antibiotic use and the prevalence of COPD (r = 0.54, P = 0.013), the number of yearly consultations and home visits per GP (r = 0.46, P = 0.041), and towards a negative association between antibiotic use and the percent of homes with premises for bathing and washing (r = –0.59, P = 0.006) and the GDP per inhabitant (r = –0.59, P = 0.006).
Discussion
After the political change in 1990, total antibiotic consumption in the Hungarian community decreased with minimal fluctuations and mild, slow realignment in the pattern of use thereafter [9, 10]. Antibiotic consumption in Hungary was, with 21.1 DDD per 1000 inhabitant-days, in the middle-range of European countries in 1998 [11, 12] and then decreased according to the ESAC (European Surveillance of Antibiotic Consumption) survey from 2002 [9]. However, Hungary should not be regarded as a homogeneous territory and the present study showed large and stable interregional variations in antibiotic consumption in the Hungarian community during 1996–2003.
Such regional differences have been reported by other European countries such as Denmark, Germany, Italy, Spain and Sweden [8, 13–16] and have been mentioned in an older publication in Hungarian [10]. In Hungary, the interregional variations of antibiotic consumption showed a West–East gradient in contrast to the pan-European remarkable North-South gradient [9]. When examining interregional variations, Germany is shown to have an opposite East–West gradient, whereas other countries have shown a North–South gradient (e.g. Italy, Spain and Sweden), or no clear pattern (e.g. Denmark) [8, 13–16]. With a ratio of 1.7 between the highest and the lowest antibiotic consumption, the extent of the regional variation was above that found in Denmark (1.4) and Sweden (1.5), but below that found in Germany (1.8), Spain (1.9) and Italy (2.2) [8, 13–16].
Hungarian citizens that benefit from the ‘public medicine service’ can receive certain medicines free of charge without limitation in quantity. This includes some antibiotics from each ATC group. The rate of persons having access to this service was positively associated with antibiotic consumption. This association, however, does not tell us whether citizens that benefit from this service have a poorer health status and more frequently suffer from community-acquired infections, or if doctors simply tend to prescribe medicines more frequently, including antibiotics, to such citizens that have free access to medicines. The other significant and positive association was between antibiotic consumption and the rate of regular recipients of social assistance – an indicator of poor social and economic conditions. It may be that recipients of social assistance more often suffer from community-acquired infections and therefore receive antibiotics more often. There was no relationship between antibiotic consumption and the average monthly net income and only a trend towards a negative association with the GDP per inhabitant. This could be explained by the fact that many population groups with low income, such as the unemployed and the retired, are excluded from the net income statistics in Hungary. There was also a trend towards a negative association between regional antibiotic use and the percent of homes with premises for bathing and washing. Again, this suggests that poor socio-economic status is a determinant of antibiotic consumption in Hungary, at least at regional level.
In Spain, regional variations in the proportion of population aged < 14 years were associated with antibiotic use while the proportion of elderly population was not [8]. In Hungary, we found no association between the proportions of various age classes and regional antibiotic use. Patients diagnosed with chronic diseases such as COPD, diabetes and malignant neoplasm are more susceptible to infections. There was no association between the prevalence of diabetes or malignant neoplasm and regional antibiotic use and only a trend towards association with the prevalence of COPD. These factors therefore cannot explain the large regional variation in antibiotic use in Hungarian community care. Additionally, there was little regional variation in the percentage of citizens vaccinated against influenza, which could not be an explanation for the large differences in antibiotic use.
Unfortunately, other determinants of antibiotic consumption, such as the incidence of community-acquired respiratory tract infections, prescriber- and patient-related factors or promotional activity, could not be studied because of the lack of data on these determinants. Data were available for some diseases such as AIDS or microbiological foodborne diseases; however, all AIDS cases are treated in the capital, Budapest, and the reported incidence of foodborne diseases was considered too low to be of relevance to this study.
In Hungary, each patient is enrolled with one GP. The average number of enrolled patients per GP does not vary much among regions and there was no relationship between antibiotic use and the number of enrolled patients per GP. Additionally, there was only a trend towards association between antibiotic use and GP activity measured by the yearly number of consultations and home visits per GP. There was no relationship between regional antibiotic use and the density of pharmacies. In Hungary, the number of pharmacies per population is controlled by law at 1 per 5000 and ends up being rather even over the country. Although antibiotics are only available from the pharmacy with a doctor's prescription, one cannot exclude regional differences in illegal, over-the-counter purchases without a prescription. This phenomenon, however, is believed to be rare in Hungary based on a comparison with reimbursement-based data for Hungary from the ESAC project [9]. Finally, since our data are based on sales, regional differences could in principle be related to differences in sales to foreign visitors from the seven countries that have common borders with Hungary. Such sales are known to happen, but their extent, as well as possible regional differences, are unknown. It is unlikely, however, that sales to foreign visitors explain the large differences in consumption observed between regions.
In conclusion, we showed that constant and large interregional differences in antibiotic consumption exist in Hungarian ambulatory care and that these are associated with socio-economic determinants such as the fraction of the population having access to free medicines or receiving regular social assistance. More detailed studies, e.g. patient interviews, are now needed to understand better the determinants of antibiotic use in these specific patient populations and design adequate interventions, including regulatory changes, to prevent possible abuse of antibiotics by such patients. Further specific studies should be implemented to identify additional determinants of antibiotic consumption at regional level.
Acknowledgments
Competing interests: None to declare.
References
- 1.Goossens H, Sprenger M. Community acquired infection and bacterial resistance. BMJ. 1998;7159:654–6. doi: 10.1136/bmj.317.7159.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronzwaer SL, Cars O, Büchholz U, Molstad S, Goettsch W, Veldhuijzen IK, Kool JL, Sprenger MJ, Degener JE. European Antimicrobial Resistance Surveillance System. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;3:278–82. doi: 10.3201/eid0803.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnet DL. Toward multinational antimicrobial resistance surveillance systems in Europe. Int J Antimicrob Agents. 2000;15:91–101. doi: 10.1016/s0924-8579(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 4.ATC Classification Index with DDDs. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2003. [Google Scholar]
- 5.ATC/DDD Index 2005. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2005. [30 July 2005]. Available at: http://www.whocc.no/atcddd/indexdatabase/ [Google Scholar]
- 6.Statistical Yearbook of Hungary, Yearbook of Health Statistics, Yearbook of Welfare Statistics, Yearbook of Housing Statistics 2003. Budapest: Hungarian Central Statistical Office; 2004. [Google Scholar]
- 7.Workshop 2: List of Possible Determinants of Antibiotic Use and Sources of Data. [30 July 2005]; European Conference on Antibiotic Use in Europe, Brussels, Belgium 15–17 November 2001. Available at: http://www.ua.ac.be/main.aspx?=*ESAC2&n=21673.
- 8.García-Rey C, Fenoll A, Aguilar L, Casal J. Effect of social and climatological factors on antimicrobial use and Streptococcus pneumoniae resistance in different provinces in Spain. J Antimicrob Chemother. 2004;54:465–71. doi: 10.1093/jac/dkh375. [DOI] [PubMed] [Google Scholar]
- 9.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 10.Graber H. [Utilisation of antibiotics in Hungary] Lege Artis Med. 1997;7:552–6. [Google Scholar]
- 11.Stratchounski L, Bedenkov A, Hryniewicz W, Krcmery LE, Semenov V. The usage of antibiotics in Russia and some countries in Eastern Europe. Int J Antimicrob Agents. 2001;18:283–6. doi: 10.1016/s0924-8579(01)00381-8. [DOI] [PubMed] [Google Scholar]
- 12.Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297–307. doi: 10.1016/s0924-8579(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 13.Boccia D, Alegiani SS, Pantosti A, Moro ML, Traversa G. The geographic relationship between the use of antimicrobial drugs and the pattern of resistance for Streptococcus pneumoniae in Italy. Eur J Clin Pharmacol. 2004;60:115–9. doi: 10.1007/s00228-003-0724-x. [DOI] [PubMed] [Google Scholar]
- 14.DANMAP 99—Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Copenhagen: Danish Veterinary Laboratory; 2000. [30 July 2005]. Available at http://www.dfvf.dk/Files/Filer/Zoonosecentret/Publikationer/Danmap/Danmap_1999.pdf. [Google Scholar]
- 15.De With K, Schröder H, Meyer E, Nink K, Hoffmann S, Steib-Bauert M, Kämmerer R, Ruess S, Daschner FD, Kern WV. [Antibiotic use in Germany and European comparison] Dtsch Med Wochenschr. 2004;129:1987–92. doi: 10.1055/s-2004-831838. [DOI] [PubMed] [Google Scholar]
- 16.SWEDRES 2002. A report on Swedish Antibiotic Utilisation and Resistance in Human Medicine. Solna, Sweden: the Swedish Strategic Programme for the Rational Use of Antimicrobial Agents (STRAMA), and the Swedish Institute for Infectious Disease Control; 2003. [30 July 2005]. Available at: http://www.smittskyddsinstitutet.se/upload/Publikationer/Swedres2002._pdf.pdf. [Google Scholar]