Abstract
Aims
The strength of sedation due to antihistamines can be evaluated by using positron emission tomography (PET). The purpose of the present study is to measure histamine H1 receptor (H1R) occupancy due to olopatadine, a new second-generation antihistamine and to compare it with that of ketotifen.
Methods
Eight healthy males (mean age 23.5 years-old) were studied following single oral administration of olopatadine 5 mg or ketotifen 1 mg using PET with 11C-doxepin in a placebo-controlled crossover study design. Binding potential ratio and H1R occupancy were calculated and were compared between olopatadine and ketotifen in the medial prefrontal (MPFC), dorsolateral prefrontal (DLPFC), anterior cingulate (ACC), insular (IC), temporal (TC), parietal (PC), occipital cortices (OC). Plasma drug concentration was measured, and correlation of AUC to H1R occupancy was examined.
Results
H1R occupancy after olopatadine treatment was significantly lower than that after ketotifen treatment in the all cortical regions (P < 0.001). Mean H1R occupancies for olopatadine and ketotifen were, respectively: MPFC, 16.7 vs. 77.7; DLPFC, 14.1 vs. 85.9; ACC, 14.7 vs. 76.1; IC, 12.8 vs. 69.7; TC, 12.5 vs. 66.5; PC, 13.9 vs. 65.8; and OC, 19.5 vs. 60.6. Overall cortical mean H1R occupancy of olopatadine and ketotifen were 15% and 72%, respectively. H1R occupancy of both drugs correlated well with their respective drug plasma concentrations (P < 0.001).
Conclusion
It is suggested that 5 mg oral olopatadine, with its low H1R occupancy and thus minimal sedation, could safely be used an antiallergic treatment for various allergic disorders.
Abbreviations
histamine H1 receptor (H1R), histamine H1 receptor occupancy (H1RO), dopamine D2 receptor (D2R), positron emission tomography (PET), blood–brain barrier (BBB), binding potential ratio (BPR), distribution volume (DV)
Keywords: olopatadine, ketotifen, histamine H1-receptor (H1R), histamine H1-receptor occupancy, positron emission tomography (PET), second-generation antihistamine, first-generation antihistamine, placebo-controlled crossover study design
Introduction
Histamine H1 receptor (H1R) antagonists, or antihistamines, are often used for treatment of allergic disorders such as seasonal rhinitis and conjunctivitis. Antihistamines act mainly on the peripheral system but can induce sedation as a central side-effect. This undesirable side-effect is caused by the blockade of nerve transmission in the histaminergic neurone system, which projects from the nucleus in the posterior hypothalamus to almost all cortical areas [1–3]. First-generation antihistamines, such as ketotifen and d-chlorpheniramine, can easily penetrate the blood–brain barrier, and tend to occupy a large proportion of postsynaptic H1Rs (>50%) [4–8]. Second-generation antihistamines, such as fexofenadine and terfenadine, are significantly less effective at penetrating the blood–brain barrier and H1Rs are slightly occupied (<20%) as demonstrated using positron emission tomography (PET) [4, 9]. Variation in cerebral H1R occupancy (H1RO) of antihistamines may result from their different permeability through the blood–brain barrier. Thus, the sedative property of antihistamines can be evaluated by the permeability of the blood–brain barrier, measured with PET and [11C]doxepin that can easily penetrate the blood–brain barrier and bind to available H1Rs in the brain, following administration of the target drug.
Functional neuroimaging techniques such as PET are widely used to evaluate the action and determine minimal effective doses of psychoactive drugs. Indeed, there have been many studies to measure the receptor occupancy of dopaminergic [10–17] and serotonergic [18–22] in schizophrenic and depressive patients. For example, using PET with [11C]raclopride, Nyberg and colleagues demonstrated that the suitable daily dose of risperidone was 4 mg, which achieved sufficiently high dopamine D2 receptor (D2R) occupancy of 72%, and that the previous recommended standard dose of 6 mg daily, often accompanied by extrapyramidal side-effects, achieved unnecessarily high D2R occupancy of 82%[13].
In the case of antihistamines, lower H1RO in the brain is favoured since they act peripherally. All first-generation antihistamines have sedative properties because of their high blood–brain barrier permeability. However, in the case of second-generation antihistamines, users tend to underestimate their sedative profiles and be less cautious when driving or operating potentially dangerous machinery [1–3]. Moreover, people may take second-generation antihistamines at double or triple doses when recommended doses fail to achieve the desired effects.
Recently, second-generation antihistamines have been further divided into two subgroups. The first includes drugs that cause little sedation at low or recommended doses, but cause dose-related cognitive impairment at higher doses. The other category consists of drugs that do not cross the blood–brain barrier, and thus induce no sedation even at exceeded doses [2, 3]. Thus, it is important to define the sedative threshold for each newly developed drug before launch.
Olopatadine (KW-4679), a new second-generation antihistamine developed in Japan, is widely used as an eye solution for allergic conjunctivitis [23–31] and as an oral treatment for allergic rhinitis and skin diseases [32–35]. Previous studies have compared its efficacy with that of other antiallergic drugs [24, 35]. However, only few animal studies [36–38] and one human study [39] have investigated sedative profile of olopatadine. The primary aim of the present study is to measure H1RO of olopatadine using PET and to compare it with that of ketotifen, a typical sedative antihistamine [40–43], in a placebo-controlled crossover study design. This study design is different from that of our previous studies where control data were obtained from different subjects [4– 9].
Methods
The present study was approved by the Committees on Clinical Investigation at both Tohoku University Graduate School of Medicine and Tokyo Metropolitan Institute of Gerontology (TMIG), Japan, and was performed in accordance with the policy of the Declaration of Helsinki. All experiments were performed at the Positron Medical Centre of TMIG.
Subjects and study design
Eight male Japanese subjects (mean age ± SD: 23.9 ± 1.2 years), recruited by advertisement as study-subjects, were given a description of the study, and their written informed consents were obtained. All subjects were in good health with no clinical history of major physical or mental illnesses, showed no abnormality in brain MRI, and were not receiving any concomitant medication likely to interfere with the study results. Alcohol, nicotine, caffeine, grapefruit and grapefruit juice were forbidden during the study period, and food intake was controlled on the test day and the day before PET measurement. The subjects were requested to finish a light meal by at least 3 h before the study started.
The eight subjects underwent PET measurement after single oral administration of olopatadine 5 mg, ketotifen 1 mg, or a lactobacteria preparation 6 mg used as placebo in a three-way crossover study, with minimum washout intervals of 7 days between treatments. The lactobacteria preparation has been widely used as placebo in Japan, and its administration has resulted in no statistical difference between pre- and postadministration in previous cognitive studies at our department [7, 9, 44]. The present study was single-blinded as the study investigators had to report on each medication, and tmax of olopatadine (1.0 ± 0.3 h) was significantly smaller than that of ketotifen (2.8 ± 0.2 h). After drug administration, each subject was asked to lie down in a comfortable position. Blood samples were collected from the subjects before drug administration and at 30, 60, 90, 120 and 150 min postadministration of olopatadine, or at 60, 120, 180, 210 and 240 min postadministration of ketotifen.
Measurement of drug concentrations
Plasma olopatadine and ketotifen concentrations were measured using liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS) with an electric spray ionization method. The MS/MS system was an API 4000 (MDS Sciex, Ontario, Canada) in the case of olopatadine or an API 3000 in the case of ketotifen.
For measurement of plasma olopatadine concentration, the Solid Phase Extraction (SPE) cartridge (OASIS HLB, 30 mg/mL, Waters Corporation, Milford, MA, USA) was pretreated with 1 mL of methanol and 1 mL of water. An internal standard solution (150 µL, 250 ng mL−1) and water (150 µL) were added to each plasma sample and the mixture was applied onto the SPE cartridge. LC was performed on a Shimadzu 10 A Vp HPLC instrument (Shimadzu Co., Kyoto, Japan) equipped with an analytical column. Separations were carried out on a C30 reversed-phase HPLC column (Develosil C30-UG-5, Nomura Chemical, Seto, Japan) at a flow rate of 0.2 mL min−1. Detection of olopatadine was based on fragmentation of the precursor ion of m/z = 338 to product ion m/z = 165; the internal standard was based on fragmentation of the precursor ion of m/z = 353 to product ion m/z = 248 under multiple reaction monitoring mode. The lowest detectable concentration was around 0.4 ng mL−1 and a coefficient of variation (CV) of olopatadine plasma concentrations measured for quality control ranged from 5.3% to 9.2%. Values below the detectable threshold were extrapolated from the data.
Conditions for measurement of plasma ketotifen concentration were as follows: LC separation was performed on an Agilent 1100 system (Agilent Technologies, Waldbronn, Germany) with a SYNERGI MAX 2.0 × 50 mm (Phenomenex, Torrance, CA, USA) column at a flow rate of 0.25 mL min−1. The reconstituted extract (15 µL) was injected onto an HPLC system with an isocratic mobile phase of 65: 35 v/v 10 mmol L−1 ammonium acetate-acetonitrile and a 5.0-min run-time. Positive ions were detected on an API3000 system at a 500 °C nebulizer gas temperature, 3500 V IonSpray voltage, 7 L min−1 (air) turbo gas, Concentration 8 (air) nebulizer gas, Concentration 8 (nitrogen) curtain gas and Concentration 10 (nitrogen) collision gas. Ion detection was based on monitoring [M + H]+ ions in the analyte and internal standard in the first quadruple and their corresponding product ions in the third quadruple with a dwell time of 500 ms. Chromatographic data for multiple reaction monitoring (MRM) were collected using Analyst software (version 1.1, AB/MDS SCIEX). The lowest detectable concentration was around 0.1 ng mL−1 and a CV of ketotifen concentrations measured for quality control ranged from 0.2% to 5.3%. Values below the detectable threshold were extrapolated from the data.
To examine the relationship between estimated binding potential ratio of [11C]doxepin and plasma concentration of each drug, the area under the curve (AUC) of olopatadine was calculated for 0–150 min (AUC0−2.5 h) postadministration and that of ketotifen for 0–240 min (AUC0−4 h) postadministration.
PET tracer and Image acquisition
[11C]doxepin was prepared by [11C]methylation of desmethyl doxepin with [11C]methyl triflate as described previously [45, 46]. [11C]doxepin radiochemical purity was over 99%, and its specific radioactivity at the time of injection was 58.9 ± 30.1 GBq µmol−1 (2719 ± 1113 mCi µmol−1). Saline solution containing [11C]doxepin was intravenously injected into each subject at a time corresponding to tmax of each drug (60 min postadministration of olopatadine or 160 min postadministration of ketotifen). The injected dose and cold mass of [11C]doxepin were 259.1 ± 29.5 MBq (7.00 ± 0.80 mCi), and 6.29 ± 5.32 nmol, respectively, and the radiological dose was calculated based on a previous paper on radiological exposure [47]. Blood samples were taken 10 min postinjection of the tracer to measure radioactivity in the plasma. Labelled metabolites in the plasma were analysed by HPLC as described previously [48]. The percentage of unchanged doxepin was 93.9 ± 3.2 at 10 min postinjection.
Approximately 60 min after [11C]doxepin injection, the subjects were positioned on the coach of the PET scanner (Headtome-V: Shimadzu Co., Kyoto, Japan) so that the transaxial slices were parallel to the orbitomeatal line and a 7-min-long transmission scan was started using 68Ge/68Ga line source for tissue attenuation correction. The subjects were then scanned in order to detect high-energy photon emissions (511 keV) from the [11C]doxepin injected into them. The emission scan was conducted in a three-dimensional (3D) mode, lasting for 15 min (70–85 min postinjection of [11C]doxepin), which acquired 30 slices with 128-by-128 voxels, and at spatial resolutions of 4.5 mm full-width-half-maximum (FWHM) in the transaxial plane and 5.8 mm FWHM in the z-axis [46]. Sensitivity for a 20-cm-long cylindrical phantom was 48.6 kcps kBq−1 mL−1 (1.8 Mcps µCi−1 mL−1) in the 3D mode [49].
PET brain images, after being corrected for tissue attenuation, were reconstructed with a filtered back projection algorithm. The brain images were then normalized by plasma radioactivity at 10 min postinjection to yield static distribution volume images according to our static scan protocol [46, 48]. Our previous investigations confirmed that [11C]doxepin-H1Rs binding was better described with a two-compartment rather than a three-compartment model, proposing the use of distribution volume as an index of [11C]doxepin binding [46]. We also confirmed that our static scan protocol produced reliable distribution volume values with high correlation efficient (r = 0.94) [48].
Three brain images obtained from each subject on different days, following oral administration of olopatadine, ketotifen or placebo, were slightly shifted in x, y and z directions. Using the subject's own MRI-T1 image as a reference, the images were coregistered to the identical stereotaxic brain coordinate system using Statistical Parametric Mapping (SPM99: Welcome Department, UK) software package [50] (Figure 1A). The MRI images were obtained with a SIGNA 1.5 Tesla machine (General Electric Inc., WI, US), TMIG.
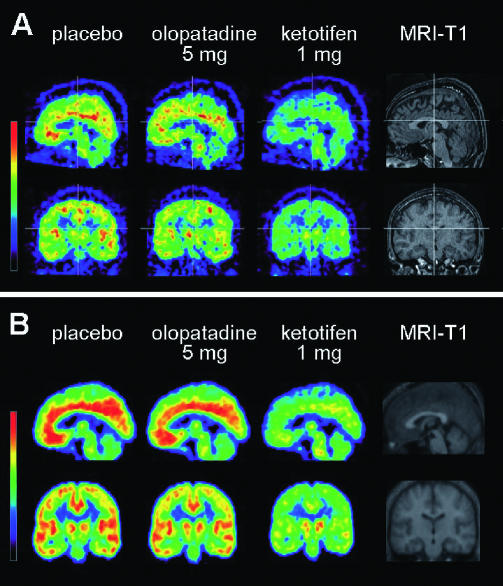
Figure 1.
Distribution pattern of 11C-doxepin in the brain of human subjects. Brain distribution volume (DV) of [11C]doxepin was examined in healthy male subjects with PET in three drug conditions, such as placebo (left), olopatadine 5 mg (centre) and ketotifen 1 mg (right) for each subject, as well as their own MRI-T1 image (far right), demonstrated in the sagittal (top) and coronal (bottom) sections for each treatment. (A) Brain DV images of an individual human subject, where the three PET images were coregistered to their own MRI-T1 image as a reference. (B) Mean brain DV images (n = 8) averaged from the eight individual brain images following transformation to the standard brain space (spatial normalization). Both demonstrate that ketotifen treatment results in significantly lower DV than the other drug conditions
Regions of interest (ROIs) were first placed on various brain regions in the MRI-T1 images with precise anatomical information, in the following brain regions: the medial prefrontal (MPFC), dorsolateral prefrontal (DLPFC), anterior cingulate (AC), insular (IC), temporal (TC), parietal (PC), occipital (OC) cortices and on the cerebellum. The ROI information was automatically copied onto the three coregistered distribution volume brain images, and regional distribution volume values were measured in the identical locations in the three drug conditions. Mean voxel values were calculated for the above cortical regions, and binding potential ratio were calculated for each cortical region using the following equation: BPR = [(DV of each region – DV of cerebellum)/DV of cerebellum][8, 9]. Finally, H1RO of olopatadine and that of ketotifen were calculated for each cortical region based on the following equation: H1RO = [(BPR of placebo – BPR with a given antihistamine)/BPR of placebo] × 100 [8, 9, 18, 20].
For visualization at a whole-brain level, distribution volume brain images were statistically analysed on a voxel-by-voxel basis by SPM99, following spatial normalization and smoothing. In spatial normalization, original distribution volume brain images were transformed to the standard anatomical space to minimize intersubject variation in brain structure [50]. Following the spatial normalization, the images were smoothed by an isotropic Gaussian kernel with FWHM of 12 mm to raise signal : noise (S/N) ratios. Differences in parameter values between olopatadine or ketotifen and placebo (control) were statistically analysed by paired t-test (under multisubjects and different conditions), and regional maxima of statistical significance (P < 0.001) were projected onto the surface-rendered MRI-T1 standard brain images (Figure 2). Precise locations of the statistically significant regions were identified using Co-Planar Stereotaxic Atlas [51].
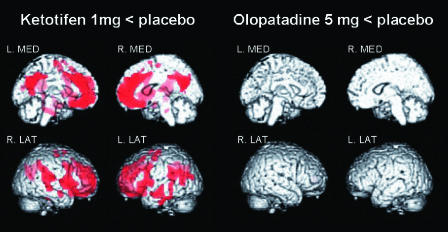
Figure 2.
Results of voxel-by-voxel comparison of brain distribution volume (DV) images. The red colour shows areas of significantly lower DV after ketotifen treatment vs. after placebo treatment (‘Ketotifen 1 mg < placebo’ in the left columns). In contrast, there are no areas of significantly lower DV after olopatadine treatment than after placebo treatment (‘Olopatadine 5 mg < placebo’ in the right columns). In both columns, significant areas are demonstrated in four aspects such as left and right medial (L. MED and R. MED) and right and left lateral (R. LAT and L. LAT) aspects (P < 0.001, uncorrected, using SPM99)
Statistical analysis
Differences in binding potential ratio between olopatadine, ketotifen and placebo were examined using anova multiple comparison with Bonferroni correction. The difference in H1RO between olopatadine and ketotifen was examined using paired Student t-test. The relationship between plasma drug concentration and H1RO value was examined using Pearson's correlation test. A probability of P < 0.05 was considered to be statistically significant. All statistical examinations were performed using SPSS for Windows 11.0.1 (Japanese version).
Results
Mean plasma concentrations and AUCs of olopatadine and ketotifen are as shown in Table 1. Mean plasma concentrations of olopatadine and ketotifen reached peak values at 60 min (45.3 ng mL−1) and at 180 min (0.25 ng mL−1) postadministration, respectively, indicating significantly different tmax for the two drugs (at 60 min for olopatadine and at 180 min for ketotifen). Large coefficients of variation (CVs) for the plasma drug concentrations at five measurement points indicated the presence of large intersubject variations in pattern of time-concentration curves, as indicated in Table 1.
Table 1.
Plasma concentrations of olopatadine and ketotifen (n = 8)
| Olopatadine (ng/ml) | Ketotifen (ng/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Time (min) | Mean | S.D. | C.V. | Time (min) | Mean | S.D. | C.V. |
| 0 | 0.0 | 0.0 | 0 | 0.00 | 0.00 | ||
| 30 | 34.0 | 22.9 | 67.4% | 60 | 0.10 | 0.05 | 50.0% |
| 60 | 45.3 | 13.4 | 29.6% | 120 | 0.23 | 0.19 | 82.6% |
| 90 | 39.4 | 10.7 | 27.2% | 180 | 0.25 | 0.12 | 48.0% |
| 120 | 29.4 | 6.9 | 23.5% | 210 | 0.20 | 0.13 | 65.0% |
| 150 | 24.6 | 6.0 | 24.4% | 240 | 0.17 | 0.11 | 64.7% |
| AUC0−2.5 h[ng/mL*h] | 80.2 | 4.4 | 5.5% | AUC0−4 h[ng/mL*h] | 0.64 | 0.31 | 48.4% |
AUC0−2.5 h for olopatadine was 80.2 ng mL−1 h−1 and its CV was small (5.5%), indicating that there was no large intersubject variation in olopatadine AUC (Table 1). AUC0−4 h for ketotifen was 0.64 ng mL−1 h−1 with a large CV (48.4%), indicating that the intersubject variability for ketotifen AUC was large, possibly due to the low plasma ketotifen concentrations near to the detectable threshold (Table 1).
The radioactivity distribution pattern of [11C]doxepin is shown in Figure 1. The distribution volume brain image following treatment with olopatadine was similar to that following treatment with placebo in an individual subject (Figure 1A). The same trend was consistently observed in the averaged distribution volume brain image, based on the spatially normalized brain images of the eight subjects (Figure 1B), representing the mean radioactivity distribution pattern. High radioactivity was observed in the MPFC, DLPFC, ACC, IC, TC, PC, OC, and thalamus following treatment with olopatadine and placebo (Figure 1). In contrast, the radioactivity distribution pattern following treatment with ketotifen was much lower than that following the treatment with olopatadine or placebo.
Using SPM99 on a voxel-by-voxel basis, parametric brain distribution volume images following treatment with olopatadine or ketotifen were statistically compared with those following treatment with placebo. In Figure 2, the red areas show brain regions where distribution volumes were significantly lower (P < 0.001) following treatment with ketotifen than following treatment with placebo (Figure 2, left). Areas such as ACC, MPFC, DLPFC, and TC demonstrated significantly low distribution volumes after treatment with ketotifen compared with placebo (Table 2). Conversely, SPM analysis did not reveal any brain area where distribution volumes were significantly lower following treatment with olopatadine compared with placebo (Figure 2, right).
Table 2.
Precise information of regions with significant dicrease in specific binding
| Regions | Brodmann's area | x, y, z {mm} | Voxel number | T values | Z values | P values |
|---|---|---|---|---|---|---|
| Anterior cingulate gyrus | 32 | 0, 34, 26 | 393 | 9.46 | 5.85 | <0.001 |
| Fusiform gyrus | 20 | −42, −20, −38 | 26 | 8.76 | 5.63 | <0.001 |
| Medial frontal gyrus | 10 | −2, 46, −8 | 189 | 7.99 | 5.36 | <0.001 |
| Precuneus | 31 | −2, −62, 28 | 145 | 7.99 | 5.36 | <0.001 |
| Posterior cingulate gyrus | 31 | 6, −66, 22 | 7.12 | 5.02 | <0.001 | |
| Middle temporal gyrus | 21 | −58, −10, −18 | 17 | 7.26 | 5.08 | <0.001 |
| Superior temporal gyrus | 22 | 56, 8, 0 | 28 | 7.17 | 5.04 | <0.001 |
| Superior temporal gyrus | 42 | 58, −26, 18 | 37 | 6.99 | 4.97 | <0.001 |
| Middle frontal gyrus | 10 | 32, 58, 8 | 23 | 6.83 | 4.90 | <0.001 |
| Superior frontal gyrus | 9 | −24, 54, 30 | 8 | 6.74 | 4.87 | <0.001 |
| Medial frontal gyrus | 10 | 6, 62, −4 | 13 | 6.71 | 4.85 | <0.001 |
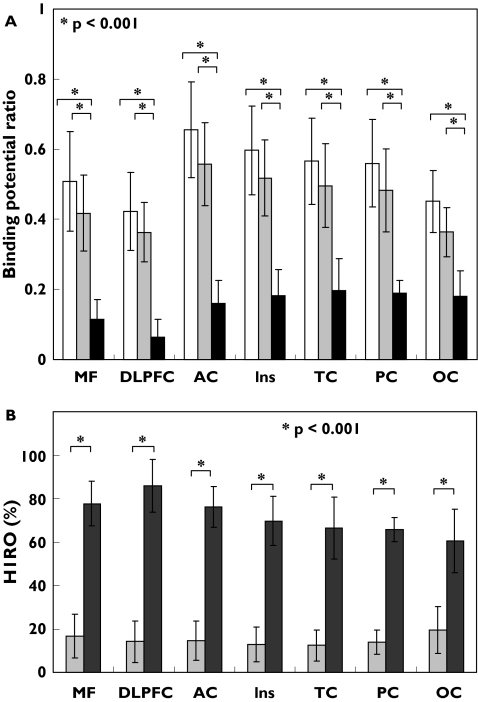
Binding potential ratio values in H1R-rich regions such as MPFC, DLPFC, ACC, IC, TC, PC and OC were evaluated based on ROI analysis (Figure 3A). Binding potential ratio values following treatment with olopatadine were only slightly different from those following treatment with placebo. However, binding potential ratio values following treatment with ketotifen were significantly lower than those following treatment with placebo or olopatadine (P < 0.001 for all regions studied) with the following 95% CI values for mean binding potential ratio differences from placebo: MPFC, 0.25, 0.54; DLPFC, 0.25, 0.47; ACC, 0.35, 0.64; IC, 0.28, 0.55; TC, 0.22, 0.52; PC, 0.24, 0.50; and OC, 0.17, 0.37; and from olopatadine: MPFC, 0.16, 0.45; DLPFC, 0.19, 0.41; ACC, 0.25, 0.54; IC, 0.20, 0.47; TC, 0.15, 0.45; PC, 0.16, 0.43; and OC, 0.08, 0.28.
Figure 3.
Region of interest (ROI)-based analyses of (A)binding potential ratios (BPR) and (B) histamine H1 receptor occupancy (H1RO) in the cortex. ROI measurements were taken in the medial prefrontal (MPFC), dorsolateral prefrontal (DLPFC), anterior cingulate (AC), insular (IC), temporal (TC), parietal (PC), occipital cortex (OC) after treatment with antihistamines. Comparison of BPRs shows differences in the sedative properties of the three drugs such as placebo (PLA), olopatadine (OLO) and ketotifen (KET) (A). H1RO by the two antihistamines are shown taking H1RO by the placebo as 0% (B). P < 0.001, statistically examined by anova followed by multiple comparison by Bonferroni test. The error bars represent interindividual variability (SD). PLA (□); OLO (▪); KET (▪)
H1RO values following treatment with olopatadine or ketotifen were also calculated using the value of H1RO following treatment with placebo as baseline (0%) (Figure 3B). Mean H1RO following treatment with olopatadine was approximately 15% (mean H1RO ± SD MPFC, 16.7 ± 10.0; DLPFC, 14.1 ± 9.6; ACC, 14.7 ± 9.1; IC, 12.8 ± 7.9; TC, 12.5 ± 7.1; PC, 13.9 ± 5.6; and OC, 19.5 ± 10.6) and that following treatment with ketotifen was approximately 72% (mean H1RO ± SD MPFC, 77.7 ± 10.3; DLPFC, 85.9 ± 12.2; ACC, 76.1 ± 9.5; IC, 69.7 ± 11.3; TC, 66.5 ± 14.2; PC, 65.8 ± 5.5; and OC, 60.6 ± 14.6). H1RO values following treatment with olopatadine were significantly lower than those following treatment with ketotifen (P < 0.001) for all cortical regions studied with the following mean binding potential ratio differences (95% CI) between olopatadine and ketotifen: MPFC, 51.8, 70.2; DLPFC, 59.8, 83.7; TC, 40.1, 58.5; PC, 38.5, 54.7; OC, 26.6, 48.2; ACC, 50.7, 72.2; IC, 42.3, 60.8. These data demonstrate that binding potential ratio following treatment with olopatadine is substantially higher than that following treatment with ketotifen in all cortical regions studied.
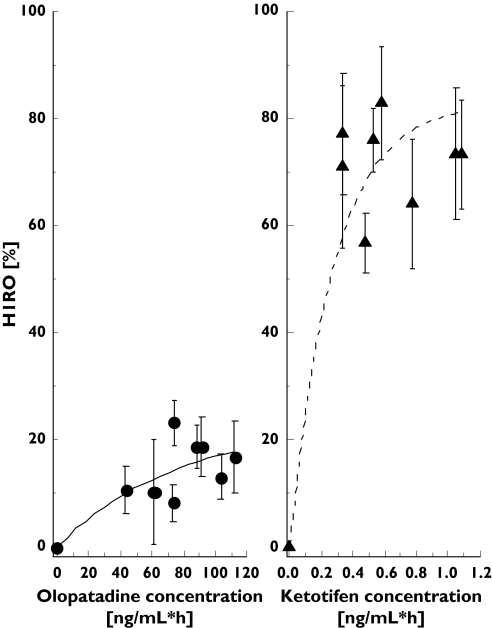
Correlations between H1ROs and AUCs of olopatadine and ketotifen concentrations were examined across the subjects. The H1ROs correlated well to the AUCs with the correlation coefficients of 0.83 for both drug conditions (Pearson's test, P < 0.001). H1RO of olopatadine rose slowly with increment in olopatadine AUC0−2.5 h. H1RO of ketotifen tended to rise rapidly with increment in ketotifen AUC0−4 h(Figure 4). Although the CV of ketotifen AUC0−4 h was high, possibly because ketotifen concentrations took very low values near to the detectable threshold, the result of significant correlation between ketotifen AUC0−4 h and H1RO was in accordance with the results of previous studies on other sedative antihistamines such as d-chlorpheniramine [7, 8].
Figure 4.
Relationship between mean H1RO and olopatadine (left) and ketotifen plasma concentrations (right). Plasma concentrations of the two antihistamines are presented as area under the curve (AUC). Correlation efficients examined by Pearson's correlation test were 0.76 for olopatadine (P < 0.001) and 0.86 for ketotifen (P < 0.001). H1RO of ketotifen rises rapidly with increments in plasma concentration whereas H1RO of olopatadine rises slowly with increments in plasma concentration. The error bars represent intraindividual variability (SEM)
Discussion
Recently, noninvasive in vivo measurement of neuroreceptor occupancy has been conducted in humans for the development of various psychoactive drugs [10–22]. In the present study, H1RO of olopatadine, a second-generation antihistamine, was compared with that of ketotifen, a typical sedative antihistamine, in a single-blinded placebo-controlled crossover study design.
H1RO after a single oral administration of olopatadine 5 mg or ketotifen 1 mg was calculated as approximately 15% and 72%, respectively. The high value for ketotifen corresponds with the H1RO value reported in a previous clinical trial (76.8%, n = 3) where the averaged baseline value (n = 6) was obtained from different subjects [6]. It has also been reported that, single-oral administration of d-chlorpheniramine (2 mg) achieved approximately 50–77% of H1RO [4, 8]. In addition, brain H1RO due to d-chlorpheniramine seems to increase rapidly in a concentration-dependent manner [7, 8] in a similar fashion to that due to ketotifen in the present study, although the large CV value of ketotifen AUC0−4 h would limit the reliability of the correlation analysis (Figure 4). Previous PET studies demonstrated that first-generation antihistamines occupied more than 50% of available H1Rs. This high H1RO is associated with high prevalence of sleepiness and cognitive decline [5, 8].
Conversely, H1RO after single-oral administration of olopatadine (5 mg) was much lower than that of first-generation antihistamines (15%vs. >50%). This result corresponds with the categorization of olopatadine as a second-generation antihistamine. Previous studies have demonstrated H1RO values due to other second-generation antihistamines: epinastine 20 mg (8.2–13.2%) [5, 6], terfenadine 60 mg (12.1–17.1%) [4, 6], astemizole 10 mg (28.7%), azelastine 1 mg (20.3%), mequitazine 3 mg (22.2%) [6] and ebastine 10 mg (9.9%) [8]. Second-generation antihistamines occupy around 0–20% of brain H1Rs [6]. Single-oral doses of cetirizine 20 mg and fexofenadine 120 mg, both double oral doses in Japan, have been reported to achieve 26% and 0%, respectively [9]. Based on such findings, second-generation antihistamines can be further separated into two subgroups according to their blood–brain barrier permeabilities [2, 3]; one category that causes little sedation at low doses, but causes dose-related cognitive impairment at higher doses, as seen with cetirizine; the other category that does not cross the blood–brain barrier and therefore induces no sedation even at exceeded doses, as seen with fexofenadine [9].
Such variation in blood–brain barrier permeability among antihistamines has been explained by various factors such as different lipophilicity, molecular size and different actions of drug transporters. Lipophilic antihistamines, as seen with many first-generation antihistamines, can be absorbed in a full amount in the gut, and can freely penetrate the blood–brain barrier. In the case of second-generation antihistamines, with decreased lipophilicity, absorption in the gut would be limited. P-glycoprotein, an efflux pump expressed in the blood–brain barrier, gut barrier and in other organs, may be playing the most important role in blood–brain barrier permeability [52]. In the case of fexofenadine, a known substrate of P-gp, both gut absorption and blood–brain barrier permeability would decrease further. In the blood–brain barrier particularly, there is a strict barrier with tight junctions between capillary endothelial cells and with astroglial processes, where few fexofenadine molecules can penetrate and enter the brain. Whether olopatadine is a substrate of P-gp is currently under investigation.
The socially important detail is that although some second-generation antihistamines appear to be nonsedative, they are mildly sedative with increased doses. Based on these findings, a recent expert meeting (the Consensus Group on New Generation Antihistamines: CONGA) states that H1RO measured by PET should be under 20% at the highest recommended dose [2]. From this standpoint, olopatadine seems to belong to the same category as cetirizine, as its brain H1RO seems to increase in a concentration-dependent manner as demonstrated in Figure 4. This assumption corresponds with a recent human study demonstrating that olopatadine 10 mg (a double oral dose in Japan) induced mild psychomotor impairment among healthy subjects [39]. In addition, animal studies may provide further suggestions [33, 36–38]. No EEG changes were observed after oral administration of olopatadine in rabbits [33] and in rats [36], whereas oral ketotifen induced significant sedation in both animal studies [33, 36]. Another rat study demonstrated that 10 mg kg−1 oral administration of olopatadine did not affect behaviour in rats whereas 50 mg kg−1 oral administration of olopatadine induced significant sedation [37]. It is assumed that the therapeutic dose of olopatadine (single oral dose at 5 mg) is reasonably safe and suitable in terms of avoiding sedative side-effects. The final conclusions regarding the sedative effects of single oral administration of olopatadine 5 mg should be drawn combined with the results of a planned double-blinded placebo-controlled study on psychomotor performance and subjective sleepiness.
The present study succeeded in demonstrating sedation due to antihistamines in healthy subjects, but it does not describe subjective sedation owing to other origins, as seen in allergic patients even at preadministration of antihistamines and relieved postadministration [53]. Other causes for such subjective sedation would be the contribution of other chemical mediators (prostaglandins, etc) and mental and physical factors due to irritating symptoms such as nasal plugging and sand eyes. The authors do not yet know the extent to which such factors would affect PET results of a similar study conducted in patients with active allergy.
This is the first PET study on antihistamines, following a placebo-controlled crossover study design where we were able to minimize potential errors due to intersubject variability. This is probably the most important advantage of our study design, which makes interpretation of results easier and clearer. To our knowledge, placebo-controlled crossover study design was first used with PET to investigate dopamine D1 or D2 receptor occupancies by new antagonists such as NNC 756 [10], sertindole [12] and risperidone [13]. Only two complete placebo-controlled crossover studies [18, 21] and a few partially crossover studies regarding serotonin receptors (5HT1A receptors) were available [19, 22]. Compared with PET studies in a clinical trial design, the number of complete placebo-controlled PET studies is generally limited [11, 14–17,54].
Placebo-controlled crossover studies are disadvantaged by the increased radiological exposure, as each subject is scanned more than twice. Investigators are therefore advised to minimize total radiation exposure to subjects by choosing a minimum radiological dose and by using 3D data acquisition mode with high sensitivity. In addition, mental and physical stress of the subjects should be decreased by simplifying measurement protocol, as in the present study where complete datasets were obtained for all of the eight subjects. In a study by Martinez et al.[18] only 6 of the 11 subjects completed all four 100-min-long PET scans planned, which reflects the difficulty of conducting crossover PET studies.
In summary, we examined H1RO of olopatadine at its highest recommended single oral dose (5 mg) and compared it with that of single oral administration of ketotifen (1 mg) using PET measurement in a placebo-controlled crossover study. Olopatadine occupied approximately 15% of available H1Rs in the human brain whereas approximately 72%of H1Rs were occupied by 1 mg of ketotifen. It is therefore suggested that oral administration of olopatadine (5 mg), with its low H1RO and thus minimal sedation, could safely be used an antiallergic treatment for various allergic disorders.
It would be of a great aid in estimating the appropriate therapeutic doses of new antihistamines and other drugs using PET measurement and the minimum number of subjects (6 to 10 subjects). Collection of more H1RO data is encouraged for establishment of a reliable international database for evaluation of the sedative profile of antihistamines.
Acknowledgments
This work was in part supported by Grants-in-Aid for scientific research (No. 12557007, 14370027, 14770489) from the Japan Society of Promotion of Science (JSPS) and a 21st Century COE program (Bio-nano-technology) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by Sagawa Traffic Safety Foundation (to Tashiro M). The authors thank the subjects in the PET measurement, Ms Miyoko Ando for care of subjects and Dr Kazunori Kawamura for the preparation of [11C]doxepin.
References
- 1.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–30. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST, Canonica GW, Simons FE, Taglialatela M, Tharp M, Timmerman H, Yanai K. Consensus Group on New-Generation Antihistamines (CONGA): present status and recommendations. Clin Exp Allergy. 2003;33(9):1305–24. doi: 10.1046/j.1365-2222.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 3.Casale TB, Blaiss MS, Gelfand E, Gilmore T, Harvey PD, Hindmarch I, Simons FE, Spangler DL, Szefler SJ, Terndrup TE, Waldman SA, Weiler J, Wong DF. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111(5):S835–42. doi: 10.1067/mai.2003.1550. [DOI] [PubMed] [Google Scholar]
- 4.Yanai K, Ryu JH, Watanabe T, Iwata R, Ido T, Sawai Y, Ito K, Itoh M. Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br J Pharmacol. 1995;116(1):1649–55. doi: 10.1111/j.1476-5381.1995.tb16386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanai K, Ryu JH, Watanabe T, Iwata R, Ido T, Asakura M, Matsumura R, Itoh M. Positron emission tomographic study of central histamine H1-receptor occupancy in human subjects treated with epinastine, a second-generation antihistamine. Meth Find Exp Clin Pharmacol. 1995;17(Suppl C):64–9. [PubMed] [Google Scholar]
- 6.Yanai K, Okamura N, Tagawa M, Itoh M, Watanabe T. New findings in pharmacological effects induced by antihistamines: from PET studies to knock-out mice. Clin Exp Allergy. 1999;29(Suppl 3):29–36. discussion 37-8. [PubMed] [Google Scholar]
- 7.Okamura N, Yanai K, Higuchi M, Sakai J, Iwata R, Ido T, Sasaki H, Watanabe T, Itoh M. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. Br J Pharmacol. 2000;129(1):115–23. doi: 10.1038/sj.bjp.0702994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagawa M, Kano M, Okamura N, Higuchi M, Matsuda M, Mizuki Y, Arai H, Iwata R, Fujii T, Komemushi S, Ido T, Itoh M, Sasaki H, Watanabe T, Yanai K. Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)- chlorpheniramine, a classical antihistamine. Br J Clin Pharmacol. 2001;52(5):501–9. doi: 10.1046/j.1365-2125.2001.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tashiro M, Sakurada Y, Iwabuchi K, Mochizuki H, Kato M, Aoki M, Funaki Y, Itoh M, Iwata R, Wong DF, Yanai K. Central effects of fexofenadine and cetirizine. Measurement of psychomotor performance, subjective sleepiness, and brain histamine H1-receptor occupancy using 11C-doxepin positron emission tomography. J Clin Pharmacol. 2004;44(8):890–900. doi: 10.1177/0091270004267590. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson P, Farde L, Halldin C, Sedvall G, Ynddal L, Sloth-Nielsen M. Oral administration of NNC 756 – a placebo controlled PET study of D1-dopamine receptor occupancy and pharmacodynamics in man. Psychopharmacology (Berl) 1995;119(1):1–8. doi: 10.1007/BF02246046. [DOI] [PubMed] [Google Scholar]
- 11.de Haan L, van Bruggen M, Lavalaye J, Booij J, Dingemans PM, Linszen D. Subjective experience and D2 receptor occupancy in patients with recent-onset schizophrenia treated with low-dose olanzapine or haloperidol: a randomized, double-blind study. Am J Psychiatry. 2003;160(2):303–9. doi: 10.1176/appi.ajp.160.2.303. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg S, Olsson H, Nilsson U, Maehlum E, Halldin C, Farde L. Low striatal and extra-striatal D2 receptor occupancy during treatment with the atypical antipsychotic sertindole. Psychopharmacology (Berl) 2002;162(1):37–41. doi: 10.1007/s00213-002-1083-5. [DOI] [PubMed] [Google Scholar]
- 13.Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156(6):869–75. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 14.Bernardo M, Parellada E, Lomena F, Catafau AM, Font M, Gomez JC, Lopez-Carrero C, Gutierrez F, Pavia J, Salamero M. Double-blind olanzapine vs. haloperidol D2 dopamine receptor blockade in schizophrenic patients: a baseline-endpoint. Psychiatry Res. 2001;107(2):87–97. doi: 10.1016/s0925-4927(01)00085-3. [DOI] [PubMed] [Google Scholar]
- 15.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 16.Martinot JL, Paillere-Martinot ML, Poirier MF, Dao-Castellana MH, Loc’h C, Maziere B. In vivo characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology (Berl) 1996;124(1–2):154–8. doi: 10.1007/BF02245616. [DOI] [PubMed] [Google Scholar]
- 17.Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33(4):227–35. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 18.Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, Parsey RV, Hashimoto T, Huang Y, Shinn A, Van Heertum R, Abi-Dargham A, Caltabiano S, Malizia A, Cowley H, Mann JJ, Laruelle M. Differential occupancy of somatodendritic and postsynaptic 5HT (1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24(3):209–29. doi: 10.1016/S0893-133X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 19.Rabiner EA, Bhagwagar Z, Gunn RN, Sargent PA, Bench CJ, Cowen PJ, Grasby PM. Pindolol augmentation of selective serotonin reuptake inhibitors: PET evidence that the dose used in clinical trials is too low. Am J Psychiatry. 2001;158(12):2080–2. doi: 10.1176/appi.ajp.158.12.2080. [DOI] [PubMed] [Google Scholar]
- 20.Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, de Haes JU, de Vries M, Grasby PM. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl) -1-piperazinyl]butyl]-1, 2-benzisothiazol-3-(2H) -one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl) -1-piperazinyl) ethyl) -N-(2-py ridinyl) cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301(3):1144–50. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama T, Suhara T, Okubo Y, Ichimiya T, Yasuno F, Maeda J, Takano A, Saijo T, Suzuki K. In vivo drug action of tandospirone at 5-HT1A receptor examined using positron emission tomography and neuroendocrine response. Psychopharmacology (Berl) 2002;165(1):37–42. doi: 10.1007/s00213-002-1234-8. [DOI] [PubMed] [Google Scholar]
- 22.Andree B, Nyberg S, Ito H, Ginovart N, Brunner F, Jaquet F, Halldin C, Farde L. Positron emission tomographic analysis of dose-dependent MDL 100, 907 binding to 5–hydroxytryptamine−2A receptors in the human brain. J Clin Psychopharmacol. 1998;18(4):317–23. doi: 10.1097/00004714-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Abelson MB, Spitalny L. Combined analysis of two studies using the conjunctival allergen challenge model to evaluate olopatadine hydrochloride, a new ophthalmic antiallergic agent with dual activity. Am J Ophthalmol. 1998;125(6):797–804. doi: 10.1016/s0002-9394(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 24.Katelaris CH, Ciprandi G, Missotten L, Turner FD, Bertin D, Berdeaux G. A comparison of the efficacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and cromolyn sodium 2% ophthalmic solution in seasonal allergic conjunctivitis. Clin Ther. 2002;24(10):1561–75. doi: 10.1016/s0149-2918(02)80060-1. [DOI] [PubMed] [Google Scholar]
- 25.Spangler DL, Bensch G, Berdy GJ. Evaluation of the efficacy of olopatadine hydrochloride 0.1%ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. Clin Ther. 2001;23(8):1272–80. doi: 10.1016/s0149-2918(01)80106-5. [DOI] [PubMed] [Google Scholar]
- 26.Berdy GJ, Spangler DL, Bensch G, Berdy SS, Brusatti RC. A comparison of the relative efficacy and clinical performance of olopatadine hydrochloride 0.1% ophthalmic solution and ketotifen fumarate 0.025% ophthalmic solution in the conjunctival antigen challenge model. Clin Ther. 2000;22(7):826–33. doi: 10.1016/S0149-2918(00)80055-7. [DOI] [PubMed] [Google Scholar]
- 27.Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05% Acta Ophthalmol Scand Suppl. 2000;230:64–5. doi: 10.1034/j.1600-0420.2000.078s230064.x. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl. 2000;230:52–5. doi: 10.1034/j.1600-0420.2000.078s230052.x. [DOI] [PubMed] [Google Scholar]
- 29.Butrus S, Greiner JV, Discepola M, Finegold I. Comparison of the clinical efficacy and comfort of olopatadine hydrochloride 0.1% ophthalmic solution and nedocromil sodium 2% ophthalmic solution in the human conjunctival allergen challenge model. Clin Ther. 2000;22(12):1462–72. doi: 10.1016/s0149-2918(00)83044-1. [DOI] [PubMed] [Google Scholar]
- 30.Abelson MB, Welch DL. An evaluation of onset and duration of action of patanol (olopatadine hydrochloride ophthalmic solution 0.1%) compared to Claritin (loratadine 10 mg) tablets in acute allergic conjunctivitis in the conjunctival allergen challenge model. Acta Ophthalmol Scand Suppl. 2000;230:60–3. doi: 10.1034/j.1600-0420.2000.078s230060.x. [DOI] [PubMed] [Google Scholar]
- 31.Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999;117(5):643–7. doi: 10.1001/archopht.117.5.643. [DOI] [PubMed] [Google Scholar]
- 32.Ohmori K, Hayashi K, Kaise T, Ohshima E, Kobayashi S, Yamazaki T, Mukouyama A. Pharmacological pharmacokinetic and clinical properties of olopatadine hydrochloride, a new antiallergic drug. Jpn J Pharmacol. 2002;88(4):379–97. doi: 10.1254/jjp.88.379. [DOI] [PubMed] [Google Scholar]
- 33.Ishii H, Sasaki Y, Manabe H, Ikemura T, Sato H, Ichikawa S, Shiozaki S, Kitamura S, Ohmori K. [General pharmacology of KW-4679, an antiallergic drug (1st report): effects on central nervous system, autonomic nervous system and peripheral nervous system] Clin Pharmacol Ther. 1995;5(8):1421–40. in Japanese. [Google Scholar]
- 34.Hamilton SA, Duddle J, Herdman MJ, Trigg CJ, Davies RJ. Comparison of a new antihistaminic and antiallergic compound KW 4679 with terfenadine and placebo on skin and nasal provocation in atopic individuals. Clin Exp Allergy. 1994;24(10):955–9. doi: 10.1111/j.1365-2222.1994.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 35.Morita K, Koga T, Moroi Y, Urabe K, Furue M. Rapid effects of olopatadine hydrochloride on the histamine-induced skin responses. J Dermatol. 2002;29(11):709–12. doi: 10.1111/j.1346-8138.2002.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamei C, Ichiki C, Izumo T, Ohishi H, Yoshida T, Tsujimoto S. Effect of the new antiallergic agent olopatadine on EEG spectral powers in conscious rats. Arzneimittelforschung. 1996;46(8):789–93. [PubMed] [Google Scholar]
- 37.Kamei C, Nishiga M, Shigemoto Y, Konishi M, Shinomiya K. [Effect of epinastine hydrochloride (Alesion) on the central nervous system] Jpn Pharmacol Ther. 2002;30(2):91–5. [Google Scholar]
- 38.Nishiga M, Fujii Y, Konishi M, Hossen MA. Effects of second-generation histamine H1 receptor antagonists on the active avoidance response in rats. Clin Exp Pharmacol Physiol. 2003;30(1–2):60–3. doi: 10.1046/j.1440-1681.2003.03791.x. [DOI] [PubMed] [Google Scholar]
- 39.Kamei H, Noda Y, Ishikawa K, Senzaki K, Muraoka I, Hasegawa Y, Hindmarch I, Nabeshima T. Comparative study of acute effects of single doses of fexofenadine, olopatadine, d-chlorpheniramine and placebo on psychomotor function in healthy volunteers. Hum Psychopharmacol. 2003;18(8):611–8. doi: 10.1002/hup.538. [DOI] [PubMed] [Google Scholar]
- 40.Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40(3):412–48. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- 41.Simons FE, Fraser TG, Reggin JD, Simons KJ. Comparison of the central nervous system effects produced by six H1-receptor antagonists. Clin Exp Allergy. 1996;26(9):1092–7. [PubMed] [Google Scholar]
- 42.Matejcek M, Irwin P, Neff G, Abt K, Wehrli W. Determination of the central effects of the asthma prophylactic ketotifen, the bronchodilator theophylline, and both in combination: an application of quantitative electroencephalography to the study of drug interactions. Int J Clin Pharmacol Ther Toxicol. 1985;23(5):258–66. [PubMed] [Google Scholar]
- 43.Vollmer R, Matejcek M, Greenwood C, Grisold W, Jellinger K. Correlation between EEG changes indicative of sedation and subjective responses. Neuropsychobiology. 1983;10(4):249–53. doi: 10.1159/000118019. [DOI] [PubMed] [Google Scholar]
- 44.Tagawa M, Kano M, Okamura N, Higuchi M, Matsuda M, Mizuki Y, Arai H, Fujii T, Komemushi S, Itoh M, Sasaki H, Watanabe T, Yanai K. Differential cognitive effects of ebastine and (+) -chlorpheniramine in healthy subjects: correlation between cognitive impairment and plasma drug concentration. Br J Clin Pharmacol. 2002;53(3):296–304. doi: 10.1046/j.0306-5251.2001.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwata R, Pascali C, Bogni A, Miyake Y, Yanai K, Ido T. A simple loop method for the automated preparation of (11C) raclopride from (11C) methyl triflate. Appl Radiat Isot. 2001;55(1):17–22. doi: 10.1016/s0969-8043(00)00368-7. [DOI] [PubMed] [Google Scholar]
- 46.Mochizuki H, Kimura Y, Ishii K, Oda K, Sasaki T, Tashiro M, Yanai K, Ishiwata K. Quantitative measurement of histamine H (1) receptors in human brains by PET and [11C]doxepin. Nucl Med Biol. 2004;31(2):165–71. doi: 10.1016/j.nucmedbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Hayashi Y, Watabe H, Matsumoto M, Horikawa T, Fujiwara T, Ito M, Yanai K. Estimation of organ cumulated activities and absorbed doses on intakes of several 11C labelled radiopharmaceuticals from external measurement with thermoluminescent dosimeters. Phys Med Biol. 1998;43(2):389–405. doi: 10.1088/0031-9155/43/2/013. [DOI] [PubMed] [Google Scholar]
- 48.Mochizuki H, Kimura Y, Ishii K, Oda K, Sasaki T, Tashiro M, Yanai K, Ishiwata K. Simplified PET measurement for evaluating histamine H1 receptors in human brains using [11C]doxepin. Nucl Med Biol. 2004. pp. 1005–11. [DOI] [PubMed]
- 49.Fujiwara T, Watanuki S, Yamamoto S, Miyake M, Seo S, Itoh M, Ishii K, Orihara H, Fukuda H, Satoh T, Kitamura K, Tanaka K, Takahashi S. Performance evaluation of a large axial field-of-view PET scanner SET-2400w. Ann Nucl Med. 1997;11(4):307–13. doi: 10.1007/BF03165298. [DOI] [PubMed] [Google Scholar]
- 50.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 51.Talairach J, Tournoux P. Stuttgart. Germany: Georg Thieme Verlag; 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 52.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53(5):526–34. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaeth J, Klimek L, Mosges R. Sedation in allergic rhinitis is caused by the condition and not by antihistamine treatment. Allergy. 1996;51(12):893–906. doi: 10.1111/j.1398-9995.1996.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 54.Bench CJ, Lammertsma AA, Dolan RJ, Grasby PM, Warrington SJ, Gunn K, Cuddigan M, Turton DJ, Osman S, Frackowiak RS. Dose dependent occupancy of central dopamine D2 receptors by the novel neuroleptic CP-88,059–01: a study using positron emission tomography and 11C-raclopride. Psychopharmacology (Berl) 1993;112(2–3):308–14. doi: 10.1007/BF02244926. [DOI] [PubMed] [Google Scholar]