Abstract
Aims
The primary aim of the study was to investigate the possible effect of the CYP2C8 3 allele and of grapefruit juice on the pharmacokinetics of repaglinide. Furthermore, the impact of a single dose of grapefruit juice on the pharmacokinetics of repaglinide in relation to dose.
Methods
Thirty-six healthy male subjects, genotyped for CYP2C8 3 (11 genotyped as CYP2C8 1/ 3, one as CYP2C8 3/ 3 and 24 as CYP2C8 1/ 1), participated in a randomized, cross-over trial. In the two phases, the subjects drank 300 mL water or 300 mL grapefruit juice, in randomized order, 2 h before administration of a single dose of either 0.25 mg or 2 mg repaglinide.
Results
Neither the mean AUC0−∞ (geometric mean ratio: 1.01; 95% CI: 0.93–1.1, P = 0.88) nor the mean Cmax (geometric mean ratio: 1.05; 95% CI: 0.94–1.2, P = 0.35) of repaglinide were statistically significantly different in the group carrying the CYP2C8 3 mutant allele compared with wild-types. Grapefruit juice caused a 19% decrease in the geometric mean ratio of the 3-hydroxyquinidine to quinidine ratio (difference: 0.81; 95% CI: 0.75–0.87, P < 0.0001), which was used as an index of CYP3A4 activity, and an increase in the mean AUC0−∞ of repaglinide (geometric mean ratio: 1.13; 95% CI: 1.04–1.2, P = 0.0048), but had no statistically significant effect on the t1/2. There was no statistically significant difference in blood glucose concentration in subjects who had or had not ingested grapefruit juice. The effect was more pronounced at the low dose of repaglinide (0.25 mg) than at the therapeutic dose of 2 mg.
Conclusions
The pharmacokinetics of repaglinide in subjects carrying the CYP2C8*3 mutant allele did not differ significantly from those in the wild-types. Grapefruit juice increased the bioavailability of repaglinide, suggesting significant intestinal elimination of the drug which was assumed to be primarily mediated by CYP3A4 in the gut.
Keywords: repaglinide, CYP2C8 polymorphism, grapefruit juice
Introduction
Repaglinide is a short-acting, oral, insulin secretagogue that is used in the treatment of type 2 diabetes mellitus. It is rapidly absorbed (tmax of 0.5–1.0 h), has an absolute bioavailability after oral administration of about 60%[1] and its t1/2 is approximately 1 h [2]. Repaglinide is almost completely metabolized by cytochrome P450 enzymes and less than 2% of an oral dose is excreted unchanged in humans [3].
CYP2C8 and CYP3A4 have been found to contribute to the in vitro metabolism of repaglinide [4]. The CYP3A4 inhibitors ketoconazole and clarithromycin have shown small but statistically significant effects on repaglinide AUC (15% and 40% increase, respectively), with a more pronounced effect on Cmax, whereas t1/2 was relatively unaffected [5, 6]. The CYP3A4 and CYP2C inducer rifampicin has been shown to increase the human metabolism of repaglinide [7, 8]. Gemfibrozil and trimethoprim, both inhibitors of CYP2C8 [9, 10] decrease the metabolism of repaglinide (increasing its AUC by 8-fold and 61%, respectively), and prolong its t1/2 by 2.4 h and 0.2 h, respectively [11, 12]. Furthermore, gemfibrozil in combination with the CYP3A4 inhibitor itraconazole increased the AUC of repaglinide by 19.4-fold and prolonged the blood glucose-lowering effect of the latter in healthy subjects [11]. Itraconazole alone had no significant effect on the t1/2 and only a minor effect on the AUC of repaglinide (causing a 1.4-fold increase) [11]. Thus, these in vivo data suggest a relatively high contribution of CYP2C8 to the hepatic clearance of the drug, whereas the contribution of CYP3A4 seems to be most pronounced during first pass in the gut and liver, or possibly if CYP2C8 activity is decreased.
Several mutations of the CYP2C8 gene have been characterized, namely CYP2C8 2 (Ile296Phe, with a frequency of 0.18 in African-Americans [13]), CYP2C8 3 (Arg139Lys, Lys399Arg, with a frequency of approximately 0.13 in Caucasians [13]), CYP2C8 4 (Ile264Met, with frequency of 0.075 in a Caucasian population [14]) and CYP2C8 5 (471delA, frameshift early stop codon, identified in one Japanese individual [15]). CYP2C8 3 has showed a decreased in vitro biotransformation of paclitaxel and arachidonic acid. However, a previous in vivo study has shown that subjects receiving a subtherapeutic (0.25 mg) dose of repaglinide and who carry the CYP2C8 3 mutant allele, had a 45% lower mean AUC and a 39% lower Cmax, compared with subjects carrying two CYP2C8 1 alleles [16].
Ingestion of grapefruit juice causes inhibition of intestinal CYP3A4 activity [17]. Different furanocoumarin derivatives in grapefruit juice are probably responsible for this effect [17–19], because they cause a mechanism-based inactivation of CYP3A4 [17] mainly in the gut wall [20]. Grapefruit juice inhibits the activities of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2E1, CYP2D6 in vitro[17]. However, its effect in vivo is limited [21, 22], probably due to the lower abundance of these CYPs in the gut wall [23].
The primary aim of the present study was to investigate the effect of the CYP2C8 3 single nucleotide polymorphism on the pharmacokinetics of repaglinide given at a therapeutic dose. A secondary aim was to characterize the effect of a single dose of grapefruit juice on the pharmacokinetics of repaglinide given at different doses.
Methods
This was an open-labelled, randomized, cross-over trial and subjects were divided into two groups, based on CYP2C8 genotype. Group 1 comprised 12 subjects carrying the CYP2C8 3 mutant allele (11 with the CYP2C8 1/ 3 genotype and one with the CYP2C8 3/ 3 genotype). Group 2 comprised 24 subjects with the CYP2C8 1/ 1 wild-type genotype. The grapefruit juice used in the study was from the same production batch (Rynkeby Foods A/S, Denmark, L4823BD2/19NOV04).
The subjects were to drink water or grapefruit juice 2 h before administration of a single dose of either 0.25 mg or 2 mg repaglinide (see Table 1).
Table 1.
Summary of trial design
| Visit | Group 1: Mutant allele (12 subjects) | Group 2: Wild-type (24 subjects) | ||||
|---|---|---|---|---|---|---|
| 1 | Screening | Screening | ||||
| 2 | Baseline quinidine 200 mg | Baseline quinidine 200 mg | ||||
| 3 | Grapefruit juice 300 mL/quinidine 200 mg | Grapefruit juice 300 mL/quinidine 200 mg | ||||
| Repaglinide 2 mg | Repaglinide 2 mg | Repaglinide 0.25 mg | ||||
| Sch. 1 (6 subj.) | Sch. 2 (6 subj.) | Sch. 1 (6 subj.) | Sch. 2 (6 subj.) | Sch. 3 (6 subj.) | Sch. 4 (6 subj.) | |
| 4 | Water/repaglinide 2 mg | Grapefruit juice/ repaglinide 2 mg | Water/repaglinide 2 mg | Grapefruit juice/ repaglinide 2 mg | Water/repaglinide 0.25 mg | Grapefruit juice/ repaglinide 0.25 mg |
| 5 | Grapefruit juice/ repaglinide 2 mg | Water/repaglinide 2 mg | Grapefruit juice/ repaglinide 2 mg | Water/repaglinide 2 mg | Grapefruit juice/ repaglinide 0.25 mg | Water/repaglinide 0.25 mg |
| 6 | Follow-up visit | Follow-up visit | ||||
Sch. = schedule, subj. = subject.
Subjects
Thirty-six nonsmoking, healthy male subjects aged 22–30 years were studied. All subjects gave written informed consent and the Regional Ethical Committee of Vejle and Funen Counties and the Danish National Board of Health approved the study, which was performed according to the Declaration of Helsinki.
Before inclusion, physical examination, laboratory testing and electrocardiography were performed (visit 1). Consumption of caffeine-containing food/beverages or alcohol was not allowed on study days. Subjects were asked not to consume grapefruit or grapefruit juice (except for the purpose of the study) during the whole experimental period.
Study procedure and sample collection
At visit 2, a 200-mg single oral dose of quinidine sulphate was administered with 150 mL water following an overnight fast. A single spot blood sample (10 mL) was collected after 4 h to obtain baseline measurements of the 3-hydroxyquinidine/quinidine ratio, used as a measure of CYP3A4 activity [24]. At visit 3, the subjects (following an overnight fast) drank 300 mL grapefruit juice, 2 h before taking the quinidine capsule with 150 mL water, and again a single blood sample (10 mL) was collected after 4 h. Plasma was separated by centrifugation (10 min at 7000 g) and frozen at −20 °C until analysis.
At visit 2, the 12 subjects in group 1 were randomized to follow either schedule 1 or 2 and the 24 subjects in group 2 were randomized to follow either schedule 1, 2, 3 or 4.
In schedule 1, the subjects drank 300 mL water at visit 4, after an overnight fast. Thirty minutes later, they were given an individualized standard breakfast. This was chosen by the subject, but was identical to that eaten at visit 4 and 5 and did not contain grapefruit/grapefruit juice or caffeine-containing food/beverages or alcohol. After a further 1.5 h, 2 mg of repaglinide was administered with 150 mL of water. Blood samples (4 mL) were collected from venous catheter at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6 and 7 h. Samples were cooled to 0 °C and after 20–30 min, serum was obtained by centrifugation (10 min at 7000 g, 4 °C) and stored at −20 °C until analysis. After a washout period of a minimum of 7 days, the subjects attended visit 5. Following an overnight fast, they drank 300 mL grapefruit juice, and 30 min later, they ate their standard breakfast (containing the same as at visit 4). After a further 1.5 h, 2 mg repaglinide was administered with 150 mL of water. Blood samples were taken as described above.
Schedules 2, 3, and 4 were performed in exactly the same way as schedule 1, with the following exceptions (Table 1).
In schedule 2, subjects drank 300 mL grapefruit juice at visit 4 and 300 mL water at visit 5. In schedule 3 the repaglinide dose was 0.25 mg. In schedule 4 subjects drank 300 mL grapefruit juice at visit 4 and 300 mL water at visit 5 and the repaglinide doses were 0.25 mg.
Blood glucose measurements were performed 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6 and 7 h after repaglinide administration, and glucose containing fluids and fruit (not grapefruit) was given when glucose concentrations fell below 3.6 mmol L−1. Adverse events were recorded on the days of medication. At the follow-up visit control, physical examination and laboratory testing were performed.
CYP2C8 genotyping
Genotyping for the CYP2C8 3 variant was performed according to a previously described procedure [25].
Drug and metabolite analysis
Serum repaglinide concentrations were measured by a previously described LC-MS/MS assay [26]. The limit of quantification was 0.55 nm (0.25 ng mL−1). The assay was validated in the concentration range 0.55–552.25 nm (0.250–250 ng mL−1) and the interassay coefficients of variation were <10%.
Quinidine and 3-hydroxyquinidine in plasma were analysed by a previously described HPLC method [27]. The limit of quantification was 5 nm (1.62 ng mL−1) for quinidine and 3 nm (1.02 ng mL−1) for 3-hydroxyquinidine, and the coefficient of variation was <5% determined at concentrations between 0.25 and 10.0 µM for both compounds. The metabolic ratio was defined as the 3-hydroxyquinidine/quinidine ratio in plasma.
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated by noncompartmental methods using the software package WinNonLin Pro Node PAL (Pharsight, Mountain View, CA, USA). The area under the serum concentration-time curve (AUC) of repaglinide was calculated using the linear trapezoidal method with extrapolation to infinity. Values for Cmax (maximum serum concentration) and tmax (time to maximum serum concentration) were obtained directly from the data. The terminal elimination half-life (t1/2) of repaglinide was calculated from the expression: t1/2 = ln2/λ, where λ is the terminal slope of the log serum concentration-time profile.
Statistical analyses
Sample size calculations were based on the primary outcome, represented by differences in repaglinide AUC0−∞ in carriers of CYP2C8 3 and wild-types. It was estimated that a true difference of at least 40% in AUC could be detected, given a two-sided α of 0.05 and a β of 80%, using an unbalanced design with 12 subjects in the mutant group and 24 in the wild-type group.
An unbalanced design was used as twice as many wild-types as carriers of CYP2C8 3 would enter the study. The pharmacokinetics of repaglinide in the wild-type group was studied at different doses in a randomized, parallel design. Proportionality with regard to repaglinide AUC has been established within the dose-range (0.25–2 mg) studied [28], and all 24 subjects were included in the mutant/wild-type analysis using a dose-corrected AUC.
The data are presented as median values with 90% interpercentile range. Before statistical analysis, all data except tmax were transformed to their natural logarithms to create a Gaussian distribution.
Analysis of the effects of CYP2C8 3 were performed using unpaired t-tests. Geometric ratios of mean data (baseline wild-types/baseline mutant allele) with 95% confidence intervals (CI) and P-values are presented.
Analysis of the effects of grapefruit juice was performed using paired t-tests. Geometric ratio of mean data (grapefruit juice/baseline) with 95% confidence intervals and P-values are presented. The effects on tmax are Hodges-Lehmann estimates of median differences with exact 95% confidence intervals. Statistical analyses were performed using GraphPad PrismTM v 3.03 and GraphPad QuickCalcs (http://graphpad.com/quickcalcs/index.cfm; GraphPad Software, Inc., San Diego, California USA), StatXact-3 (Cytel Software Corporation, Cambridge, Mass., USA) and Microsoft Excel.
Results
All subjects completed the study. No side-effects were noted except for one case of headache probably due to caffeine abstinence, and one of dysuria, probably not connected to the intake of study drugs. Blood glucose concentrations ranged from 2.2 to 7.7 mmol L−1 during days with repaglinide administration without symptoms of hypoglycaemia, because glucose was given when measurements fell below 3.6 mmol L−1. All control laboratory tests were within normal values.
The results of the effects of the CYP2C8 3 polymorphisms on repaglinide pharmacokinetics are summarized in Table 2 and Figure 1. Neither the mean AUC0−∞ (geometric mean ratio: 1.01; 95% CI: 0.93–1.1, P = 0.88) nor the mean Cmax (geometric mean ratio: 1.05; 95% CI: 0.94–1.2, P = 0.35) of repaglinide were significantly different in the group carrying the CYP2C8 3 mutant allele (group A, Table 2) compared with wild-type subjects who received 2 mg repaglinide (group B, Table 2). Repaglinide AUC0−∞ was also not significantly different in group A compared with the dose-corrected AUC0−∞ in all 24 wild-type subjects (group B + C, Table 2) (geometric mean ratio: 1.04; 95% CI: 0.95–1.1, P = 0.37).
Table 2.
The effect of of CYP2C8*3 mutation on repaglinide pharmacokinetics in 36 healthy male subjects divided into group (A) (B) and (C) after a single dose of either 2 mg or 0.25 mg repaglinide. (A) Carriers of CYP2C8*3 (2 mg repaglinide) (n = 12); (B) Wild-type (2 mg repaglinide) (n = 12); (C) Wild-type (0.25 mg repaglinide) (n = 12)
| Group | Median of Baseline results and 90% interpercentile range | Geometric mean ratio, 95% confidence interval and P value | |||
|---|---|---|---|---|---|
| Parameter | A | B | B + C | (Baseline B/Baseline A) | (Baseline B + C/Baseline A) |
| Cmax (ng/mL) | 19 | 19 | – | 1.05 | – |
| (9.3–31) | (15–34) | 0.94; 1.2] | |||
| P = 0.35 | |||||
| AUC(0→∞) | 26 | 30 | 33** | 1.01 | 1.04** |
| (ng*h/mL) | (22–66) | (25–52) | (24–83) | [0.93; 1.1]P = 0.88 | 0.95; 1.1]P = 0.37 |
Dose-corrected AUC(0–∞).
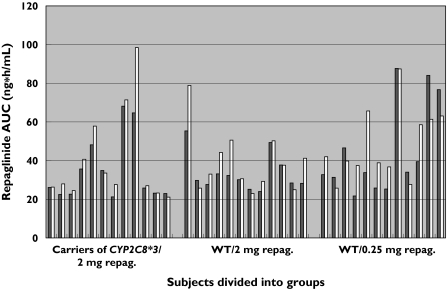
Figure 1.
Repaglinide (repag.) AUC0−∞ (area under the serum concentration-time curve) following a 2-mg oral dose of the drug and dose-corrected AUC0−∞ following a 0.25-mg oral dose in 36 healthy male subjects before (baseline) and after a single dose of 300 mL grapefruit juice (GFJ). WT = CYP2C8 wild-types. Baseline (▪); after GFJ (□)
The results of the effects of grapefruit juice are summarized in Tables 3 and 4 and illustrated in Figure 1. Grapefruit juice decreased the geometric mean of 3-hydroxyquinidine to quinidine ratio in the whole group of 36 subjects by approximately 19% (geometric mean ratio: 0.81; 95% CI: 0.75–0.87, P < 0.0001).
Table 3.
The effect of a single dose of 300 mL grapefruit juice (GFJ) on repaglinide pharmacokinetics and the ratio of 3-OH-quinidine to quinidine in 36 healthy male subjects as a whole (ALL) and in group (A) (B) and (C) after a single dose of either 2 mg or 0.25 mg repaglinide. (ALL) All 36 subjects; (A) Carriers of CYP2C8*3 (2 mg repaglinide) (n = 12); (B) Wild-type (2 mg repaglinide) (n = 12); (C) Wild-type (0.25 mg repaglinide) (n = 12)
| Median and 90% Interpercentile range | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | ALL | A | B | C | ||||
| Parameter | Baseline | After GFJ | Baseline | After GFJ | Baseline | After GFJ | Baseline | After GFJ |
| Age (years) | – | – | 25.5 | – | 25 | – | 26 | – |
| (24–27.9) | (22.6–26) | (24–29.5) | ||||||
| Weight (kg) | – | – | 78.5 | – | 82.5 | – | 79.5 | – |
| (68.7–89.5) | (68.1–98) | (73.7–103.8) | ||||||
| Cmax (ng/mL) | – | – | 19 | 18 | 19 | 22 | 2.3 | 3.0 |
| (9.3–31) | (14–41) | (15–34) | (17–33) | (1.2–3.9) | (2.0–4.0) | |||
| tmax (h) | – | – | 1.5 | 1.3 | 1.0 | 1.0 | 1.5 | 1.0 |
| (0.75–2.5) | (0.62–1.7) | (0.5–2.0) | (0.64–2.5) | (0.5–2.7) | (0.63–1.5) | |||
| t1/2 (h) | – | – | 1.1 | 1.1 | 1.3 | 1.5 | 1.0 | 0.8 |
| (0.72–1.5) | (0.95–1.9) | (1.0–1.8) | (0.91–2.9) | (0.64–1.4) | (0.56–2.5) | |||
| AUC(0→∞) | 32** | 38** | 26 | 28 | 30 | 35 | 34** | 41** |
| (ng h/mL) | (22–79) | (23–81) | (22–66) | (22–84) | (25–52) | (24–63) | (24–86) | (27–75) |
| 3-OH- | 0.11 | 0.085 | 0.13 | 0.085 | 0.11 | 0.089 | 0.11 | 0.080 |
| quinidine/ | (0.067–0.17) | (0.056–0.15) | (0.059–0.17) | (0.047–0.13) | (0.092–0.18) | (0.072–0.15) | (0.067–0.13) | (0.066–0.14) |
| quinidine | ||||||||
Dose-corrected AUC(0–∞).
Table 4.
Statistical analysis of 300 mL grapefruit juice (GFJ) on repaglinide pharmacokinetics and the ratio of 3-OH-quinidine to quinidine in 36 healthy male subjects as a whole (ALL) and divided into group (A) (B) and (C) after a single dose of either 2 mg or 0.25 mg repaglinide. (ALL) All 36 subjects; (A) Carriers of CYP2C8*3 (2 mg repaglinide) (n = 12); (B) Wild-type (2 mg repaglinide) (n = 12); (C) Wild-type (0.25 mg repaglinide) (n = 12)
| Group | Statistical Inference | |||
|---|---|---|---|---|
| Parameter | ALL | A | B | C |
| Cmax (ng/mL)† | – | 1.2 | 1.1 | 1.3 |
| [0.94; 1.5] | [0.94; 1.3] | [1.04; 1.7]* | ||
| P = 0.13 | P = 0.22 | P = 0.028 | ||
| tmax (h)‡ | – | −0.48 | 0.25 | −0.50 |
| [−0.76; 0.09] | [−0.53; 1.0] | [−1.4; −0.38]* | ||
| t1/2 (h)† | – | 1.2 | 1.1 | 0.95 |
| [0.95; 1.5] | [0.9; 1.4] | [0.69; 1.32] | ||
| P = 0.12 | P = 0.27 | P = 0.75 | ||
| AUC(0→∞) (ng*h/mL)† | 1.13** | 1.11 * | 1.13 | 1.14** |
| [1.04; 1.2] | [1.01; 1.2] | [0.99; 1.3] | [0.92; 1.4] | |
| P = 0.0048 | P = 0.028 | P = 0.061 | P = 0.22 | |
| 3-OH-quinidine/quinidine† | 0.81 | 0.74 | 0.79 | 0.89 |
| [0.75; 0.87]* | [0.65; 0.85]* | [0.7; 0.89]* | [0.78; 1.02] | |
| P < 0.0001 | P = 0.0005 | P = 0.0013 | P = 0.092 | |
Geometric mean ratio (After GFJ/Baseline), 95% confidence interval and P value.
Hodges-Lehmann estimates of median difference and exact 95% confidence interval.
95% CI does not include 1 for Geometric Mean ratio or 0 for Hodges-Lehmann estimates.
Dose-corrected AUC(0-∞).
Grapefruit juice caused a statistically significant increase in the mean AUC0−∞ and dose-corrected mean value in the entire group of 36 subjects (geometric mean ratio: 1.13; 95% CI: 1.04–1.2, P = 0.0048). There was no statistical significant difference in blood glucose concentrations (lowest value recorded between 0 and 2 h following repaglinide administration) between days with and without grapefruit juice (mean difference (grapefruit juice minus baseline): −0.17; 95% CI: −0.39–0.055, P = 0.14, paired t-test).
Stratified by genotype and repaglinide dose as described above (Table 4), only a significant increase in mean repaglinide AUC0−∞ in group A (geometric mean ratio: 1.11; 95% CI: 1.01–1.2, P = 0.028) and a significant increase in mean Cmax in group C (geometric mean ratio: 1.3; 95% CI: 1.04–1.7, P = 0.028) were found after grapefruit juice intake. A statistically significant decrease in median tmax was observed in group C (median difference: −0.50; 95% CI: −1.4 to −0.38). The half-life was not significantly affected by grapefruit juice in any of the groups.
Discussion
In this study, the pharmacokinetics of repaglinide was compared between subjects carrying CYP2C8 3 and wild-type subjects. A statistically significant difference between the two groups was not found. CYP2C8 3 is the most common CYP2C8 mutant allele present in Caucasian populations, where it has been found at frequencies of 0.13–0.17 [13, 14, 29].
CYP2C8*3 has been reported to exhibit decreased activity, in vitro toward CYP2C8 substrates paclitaxel and arachidonic acid [13, 14, 30], but not toward the N-deethylation of the antiarrhythmic drug amiodarone [31]. There are limited data on the in vivo effect of the CYP2C8 3 variant on drug metabolism. One study showed that heterozygotes of CYP2C8 3 had a significantly increased metabolism of repaglinide compared with homozygotes of CYP2C8 1[16]. In another report significantly decreased metabolism of (R)-ibuprofen in carriers of the CYP2C8 3 allele compared with homozygotes of CYP2C8 1 was observed [29].
One explanation for the results in the present study, where no effect of CYP2C8 genotype on repaglinide metabolism was observed, could be that the wild-type group also contained carriers of other CYP2C8 mutant alleles such as CYP2C8 4[13]. However, as the frequency of these alleles in Caucasian populations has been reported to be quite low, other mechanisms may be more important. Another possibility is that CYP3A4 may make a greater contribution to the in vivo biotransformation of repaglinide in subjects with a low level of CYP2C8 activity. However, baseline indices of CYP3A4 activity did not differ between groups (Table 3). Alternatively, the CYP2C8 3 allele may not affect the kinetic parameters for all substrates to an equal extent. Whereas the pharmacokinetics of paclitaxel and arachidonic acid are altered [13, 14, 30], those of repaglinide (present work) and amiodarone [31] are not.
In the previous study showing that the presence of CYP2C8 3 was associated with increased repaglinide metabolism, a low dose of repaglinide (0.25 mg) was used [16]. In the present work, subjects received a clinically relevant dose of 2 mg repaglinide, and no effect of the CYP2C8 3 was found. It could be speculated that at low doses levels of repaglinide, the relative contribution of CYP2C8 and CYP3A4 might be different than at clinically relevant doses.
The decrease in the quinidine metabolic ratio following ingestion of grapefruit juice is consistent with inhibition of CYP3A4. The relatively small magnitude of this decrease (19%) supports the hypothesis that only the intestinal fraction of CYP3A4 is affected.
Repaglinide AUC and dose-corrected AUC were increased overall by 13% after grapefruit juice intake in a statistically significant fashion. This observation together with a lack of influence on t1/2 suggests that the presystemic metabolism of repaglinide is most likely to be mediated by intestinal CYP3A4.
The extent of the increase in mean repaglinide AUC after grapefruit juice observed in this study was relatively small compared with that caused by CYP2C8 inhibitors gemfibrozil (8.1-fold) and trimethoprim (61%), and CYP3A4 inhibitor clarithromycin (40%). This is a likely to be a consequence of grapefruit juice only inhibiting the intestinal fraction of CYP3A4, and the fact that the oral bioavailability of repaglinide is about 60%[1]. These previous studies used a much lower dose of repaglinide (0.25 mg), which also confounds a direct comparison with our data. Studies using a clinically relevant dose of 2 mg have shown an increase in repaglinide AUC of only approximately 15% by ketoconazole, a potent CYP3A4 inhibitor, and 8% by simvastatin, a CYP3A4 substrate [5] and an inhibitor of CYP2C8 in vitro[32]. It is possible that other enzymes could be involved to a greater extent in the metabolism of repaglinide at this higher dose. Stratified analysis of the influence of grapefruit juice revealed that statistically significant effects were only observed for Cmax and tmax in subjects carrying the wild-type allele, and who received the low dose of the drug. All these data indicate a larger potential for drug interactions with repaglinide following low dose (0.25 mg) administration. As inhibitors of CYP2C8 [11, 12] generally seem to have a larger effect on the t1/2 of repaglinide compared with inhibitors of CYP3A4 [5, 6], CYP2C8 might contribute mainly to the hepatic clearance of the drug, whereas the contribution of CYP3A4 might be more pronounced during the first-pass metabolism in the gut and liver or if CYP2C8 activity is decreased.
As grapefruit juice has been found to inhibit other CYP enzymes including CYP2C9 and CYP2C19 [17], it is possible that CYP2C8-mediated repaglinide biotransformation could be inhibited by grapefruit juice as well. However, as only very limited amounts of CYP2C8 mRNA and no expression of the protein have been detected in human duodenum and small intestine [33], the presence of CYP2C8 in the intestine is probably very limited, and significant in vivo effects of grapefruit juice on this enzyme seems unlikely. The in vivo effects of grapefruit juice on the efflux transporter P-glycoprotein, which is also present in the gut wall, have been somewhat contradictory [22, 34, 35, 36]. However, repaglinide is not a substrate of P-glycoprotein (personal communication, September 2003, data on file at Novo Nordisk A/S), any inhibition of P-glycoprotein would have no influence on the pharmacokinetics of repaglinide.
The family of human organic anion transporting polypeptides (OATPs) contains members that are exclusively located in the basolateral membrane of hepatocytes, for example OATP1B1 (previously OATP-C) and OATP8, as well as members that are expressed in other tissues including placenta, intestine, kidney and lungs, for example OATP-A and OATP-B [37, 38]. These transporters actively mediate the cellular uptake of several endogenous and exogenous compounds. Grapefruit juice has been shown to decrease the activity of OATPs in vitro[39] and several fruit juices including grapefruit decrease the in vivo uptake of the antihistamine fexofenadine, which is mediated by OATPs in humans [39]. If the uptake of repaglinide into enterocytes is also mediated by OATPs, inhibition of these transporters by grapefruit juice could counteract the increase in bioavailability caused by inhibition of CYP3A4. This could represent another explanation of the relatively small effect of grapefruit juice on repaglinide bioavailability observed in the present study. A recent study strongly indicates that the cellular uptake of repaglinide is mediated by OATPs [37].
We conclude that the CYP2C8 3 mutant allele does not influence the pharmacokinetics of repaglinide to a clinically relevant degree. Grapefruit juice increased the bioavailability of repaglinide, suggesting intestinal elimination of repaglinide by CYP3A4 in the gut. Concomitant administration of repaglinide and grapefruit juice has no effect on blood glucose concentration, and thus the interaction is unlikely to be of clinical relevance.
Acknowledgments
Novo Nordisk A/S, Denmark, financed this study. Declaration of compliance: this experiment complies with the current law of the country in which it was performed (Denmark).
Rynkeby Foods A/S, Denmark, is thanked for donating the grapefruit juice used in the study.
The work was carried out at Odense University Hospital and Clinical Pharmacology, University of Southern Denmark, Winsløwparken 192, 5000 Odense C, Denmark.
References
- 1.Hatorp V, Oliver S, Su C-APF. Bioavailability of repaglinide, a novel antidiabetic agent, administered orally in tablet or solution form or intravenously in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:636–41. [PubMed] [Google Scholar]
- 2.Hatorp V, Huang W, Strange P. Repaglinide pharmacokinetics in healthy young adult and elderly subjects. Clin Ther. 1999;21:702–10. doi: 10.1016/S0149-2918(00)88321-6. [DOI] [PubMed] [Google Scholar]
- 3.van Heiningen PN, Hatorp V, Kramer Nielsen K, Hansen KT, van Lier JJ, van De Merbel NC, Oosterhuis B, Jonkman JHG. Absorption, metabolism and excretion of a single oral dose of 14C-repaglinide during repaglinide multiple dosing. Eur J Clin Pharmacol. 1999;55:521–5. doi: 10.1007/s002280050667. [DOI] [PubMed] [Google Scholar]
- 4.Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305–14. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatorp V, Hansen KT, Thomsen MK. Influence of drugs interacting with CYP3A4 on the pharmacokinetics, pharmacodynamics, and safety of the prandial glucose regulator repaglinide. J Clin Pharmacol. 2003;43:649–60. [PubMed] [Google Scholar]
- 6.Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58–65. doi: 10.1067/mcp.2001.116511. [DOI] [PubMed] [Google Scholar]
- 7.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- 8.Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brøsen K. Rifampicin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur J Clin Pharmacol. 2004;60:109–14. doi: 10.1007/s00228-004-0746-z. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 10.Wen X, Wang J, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and Sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Metab Dispos. 2002;30:631–5. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 11.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 12.Niemi M, Kajosaari LI, Neuvonen M, Backman JT, Neuvonen PJ. The CYP2C8 inhibitor trimethoprim increases the plasma concentration of repaglinide in healthy subjects. Br J Clin Pharmacol. 2003;57:441–7. doi: 10.1046/j.1365-2125.2003.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, Goldstein JA. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics. 2001;11:597–607. doi: 10.1097/00008571-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, Van Houdt J, Hendricks J, Mannens G, Bohets H, Williams FM, Armstrong M, Crespi CL, Daly AK. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6α-hydroxylase activity in human liver microsomes. Biochem Pharmacol. 2002;64:1579–89. doi: 10.1016/s0006-2952(02)01354-0. [DOI] [PubMed] [Google Scholar]
- 15.Soyama A, Saito Y, Komamura K, Ueno K, Kamakura S, Ozawa S, Sawada J. Five novel single nucleotide polymorphisms in the CYP2C8 gene, one of which induces a frame-shift. Drug Metabol Pharmacokin. 2002;17:SNP7 (374)–SNP10 (377). doi: 10.2133/dmpk.17.374. [DOI] [PubMed] [Google Scholar]
- 16.Niemi M, Leathart JB, Neuvonen M, Backman JT, Daly AK, Neuvonen PJ. Polymorphism in CYP2C8 is associated with reduced plasma concentrations of repaglinide. Clin Pharmacol Ther. 2003;74:380–7. doi: 10.1016/S0009-9236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 17.He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF, Hollenberg PF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–9. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 18.Edwards DJ, Bellevue FH, Woster PM. Identification of 6′,7′-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–90. [PubMed] [Google Scholar]
- 19.Fukuda K, Ohta T, Oshima Y, Ohashi N, Yoshikawa M, Yamazoe Y. Specific CYP3A4 inhibitors in grapefruit juice: furocoumarin dimers as components of drug interaction. Pharmacogenetics. 1997;7:391–6. doi: 10.1097/00008571-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Veronese ML, Gillen LP, Burke JP, Dorval EP, Hauck WW, Pequignot E, Waldman SA, Greenberg HE. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003;43:831–9. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]
- 21.Tassaneeyakul W, Tassaneeyakul W, Vannaprasaht S, Yamazoe Y. Formation of omeprazole sulphone but not 5-hydroxyomeprazole is inhibited by grapefruit juice. Br J Clin Pharmacol. 2000;49:139–44. doi: 10.1046/j.1365-2125.2000.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Marco MP, Edwards DJ, Wainer IW, Ducharme MP. The effect of grapefruit juice and seville orange juice on the pharmacokinetics of dextromethorphan: the role of gut CYP3A and P-glycoprotein. Life Sci. 2002;71:1149–60. doi: 10.1016/s0024-3205(02)01799-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang QY, Dunbar D, Ostrowska A, Zeisloft S, Yang J, Kaminsky LS. Characterisation of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–9. [PubMed] [Google Scholar]
- 24.Damkier P, Brosen K. Quinidine as a probe for CYP3A4 activity: Intrasubject variability and lack of correlation with probe-based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin Pharmacol Ther. 2000;68:199–209. doi: 10.1067/mcp.2000.108532. [DOI] [PubMed] [Google Scholar]
- 25.Yasar U, Lundgren S, Eliasson E, Bennet A, Wiman B, de Faire U, Rane A. Linkage between the CYP2C8 and CYP2C9 genetic polymorphisms. Biochem Biophys Res Communic. 2002;299:25–8. doi: 10.1016/s0006-291x(02)02592-5. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen MS, Chassard D, Evène E, Nielsen KK, Jørgensen M. Pharmacokinetics of repaglinide in healthy Caucasian and Japanese Subjects. J Clin Pharmacol. 2003;43:23–8. doi: 10.1177/0091270002239702. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen F, Nielsen KK, Brøsen K. Determination of quinidine, dihydroquinidine, (3S)-3-hydroxyquinidine and quinidine N-oxide in plasma and urine by high-performance liquid chromatography. J Chrom B. 1994;660:103–10. doi: 10.1016/0378-4347(94)00259-2. [DOI] [PubMed] [Google Scholar]
- 28.Strange P, Schwartz SL, Graf RJ, Weston I, Marbury TC, Huang W, Goldberg RB. Pharmacokinetics, pharmacodynamics, and dose-response relationship of repaglinide in type 2 diabetes. Diabetes Tech Ther. 1999;1:247–56. doi: 10.1089/152091599317143. [DOI] [PubMed] [Google Scholar]
- 29.Martínez C, García-Martín E, Blanco JG, Gamito FJG, Ladero JM, Agúndez JAG. The effect of the cytochrome P450 CYP2C8 polymorphism on the disposition of (R)-ibuprofen enantiomer in healthy subjects. Br J Clin Pharmacol. 2004;59:62–8. doi: 10.1111/j.1365-2125.2004.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soyama A, Saito Y, Hanioka N, Murayama N, Nakajima O, Katori N, Ishida S, Sai K, Ozawa S, Sawada J. Non-synonymous single nucleotide alterations found in the CYP2C8 gene result in reduced in vitro paclitaxel metabolism. Biol Pharm Bull. 2001;24:1427–30. doi: 10.1248/bpb.24.1427. [DOI] [PubMed] [Google Scholar]
- 31.Soyama A, Hanioka N, Saito Y, Murayama N, Ando M, Ozawa S, Sawada S. Amiodarone N-deethylation by CYP2C8 and its variants, CYP2C8 3 and CYP2C8 P404A. Pharmacol Toxicol. 2002;91:174–8. doi: 10.1034/j.1600-0773.2002.910404.x. [DOI] [PubMed] [Google Scholar]
- 32.Walsky RL, Gaman EA, Obach RS. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol. 2005;45:68–78. doi: 10.1177/0091270004270642. [DOI] [PubMed] [Google Scholar]
- 33.Klose TS, Blaisdell J, Goldstein JA. Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol. 1999;13:289–95. doi: 10.1002/(sici)1099-0461(1999)13:6<289::aid-jbt1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Edwards DJ, Fitzsimmons ME, Schuetz EG, Yasuda K, Ducharme MP, Warbasse LH, Woster PM, Schuetz JD, Watkins P. 6′,7′-Dihydroxybergamottin in grapefruit juice and seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin Pharmacol Ther. 1999;65:237–44. doi: 10.1016/S0009-9236(99)70102-5. [DOI] [PubMed] [Google Scholar]
- 35.Parker RB, Yates R, Soberman JE, Laizure SC. Effects of grapefruit juice on intestinal P-glycoprotein: evaluation using digoxin in humans. Pharmacotherapy. 2003;23:979–87. doi: 10.1592/phco.23.8.979.32881. [DOI] [PubMed] [Google Scholar]
- 36.Romiti N, Tramonti G, Donati A, Chieli E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004;76:293–302. doi: 10.1016/j.lfs.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468–78. doi: 10.1016/j.clpt.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–72. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- 39.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]