Abstract
Aim
To compare in humans the effects of ivabradine and propranolol on cardiac and systemic haemodynamics at rest, during tilt and exercise.
Methods
Nine healthy volunteers randomly received single oral doses of ivabradine (Iva, 30 mg), propranolol (Propra, 40 mg) or placebo (Plac) during a double-blind cross-over study. Doses were selected to be equipotent in heart rate (HR) reduction. HR, systolic and diastolic blood pressure (SBP, DBP), cardiac index (CI, bioimpedance), rate pressure product (RPP), plasma epinephrine (E) and norepinephrine (NE), were measured at rest at baseline, before and after two tilt and exercise tests, started 2 and 5 h after drug intake. Heart rate variability (low to high frequency ratio LF/HF) was evaluated at rest and at 5th minute of tilt.
Results
At rest, HR and RPP decreased similarly with Iva and Propra (both P < 0.01). During tilt, HR increased less with Iva than Propra (P < 0.01), LF/HF decreased after Iva (P < 0.03), SBP and mean blood pressure decreased after Propra (both P < 0.01), RPP decreased similarly after Iva and Propra (both P < 0.01) and CI decreased to a greater exent with Propra than with Iva or Plac (both P < 0.04). During exercise, Iva and Propra similarly decreased HR (both P < 0.01) and RPP (P < 0.01).
Conclusions
These results demonstrate that for a similar decrease in HR at rest and during sympathetic stimulation, acute administration of ivabradine, a selective heart rate-lowering agent, decreased myocardial oxygen demand to the same extent as a reference β-blocker, propranolol, but without evidence of depressant effect on cardiac function.
Keywords: catecholamines, exercise, haemodynamic, ivabradine, propranolol, tilt
Introduction
There is now substantial evidence to suggest that heart rate (HR) is a powerful predictor of mortality in both normal individuals [1, 2] and in patients with coronary events [3, 4]. Reducing HR both decreases myocardial oxygen demand and improves endocardial blood supply, so there is considerable interest in agents that exhibit this property. β-Blockers are widely used in angina pectoris and after myocardial infarction, partly for their HR-lowering properties. However, these agents can have an undesirable negative inotropic action and can cause a paradoxical vasoconstriction of large epicardial coronary arteries at rest and during exercise, both in animals [5, 6] and in humans [7]. Additionally, β-blockers may cause bronchoconstriction in patients with obstructive airway disease [8] and may have negative metabolic effects, including a reduction in insulin sensitivity [9]. A selective bradycardic agent, which does not produce these undesirable effects, could thus be useful to assess the effect of HR lowering alone and therefore could be of therapeutic interest.
Ivabradine (S 16257) is a member of a new class of selective and specific HR-lowering agents that act by inhibiting the pacemaker current, If, in the sino-atrial node. If is a hyperpolarization-activated mixed sodium/potassium inward current that is important during diastolic pacemaker depolarization [10, 11]. Ivabradine induces a selective, specific and use-dependent inhibition of the If current [12, 13] and has a pronounced bradycardic effect, both at rest and during exercise, in experimental animals [6, 14, 15] and in humans [16], which could be beneficial in angina pectoris [17]. The objectives of the present study were to investigate the haemodynamic and neurohumoral effects of ivabradine in healthy volunteers, at rest, during orthostatic tilt tests and during exercise, and to compare these effects with a reference β-blocker, propranolol, and with placebo. Doses of the two drugs were selected to be equipotent in terms of HR reduction at rest and during exercise and orthostatic tilt tests.
Methods
Subjects and study design
Nine male volunteers, mean (± SEM) age 21 ± 1 years (range 18–30), with a normal body mass index (22 ± 1 kg m−2, range 20–25) took part in the study. They were considered healthy based on their previous medical history, physical examination, normal electrocardiogram and routine laboratory tests. In addition, they performed a satisfactory exercise tolerance test during the screening period. Exclusion criteria included smoking, alcoholism or illicit drugs use, regular intake of caffeinated beverages, or participation in competitive endurance sports. The study was performed in accordance with the Declaration of Helsinki, the study protocol was approved by the relevant Consultative Committee for the Protection of Persons Engaged in Biomedical Research (CCPPRB de Haute-Normandie) and all participants gave written informed consent.
Under double-blind conditions the subjects were orally administered a single dose of ivabradine 30 mg, propranolol 40 mg or placebo, in a random order at 1-week intervals. Thus, each participant received three capsules of identical appearance of either placebo, ivabradine or propranolol and the treatment sequences were allocated randomly in blocks of three participants according to a 3 × 3 Latin square design.
Parameters investigated
The following parameters were evaluated before and during an 8-h period after drug administration: HR and systolic and diastolic blood pressures (SBP, DBP) were measured using a brachial cuff sphygmomanometer. Mean blood pressure (MBP) was calculated as MBP = DBP + [(SBP-DBP)/3] and rate-pressure product (RPP) as RPP = SBP × HR. Cardiac index (CI) was measured by bioimpedance (Bomed NCCOM3; Novacor, Rueil-Malmaison, France) [18–20]. This method of cardiac output measurement has been previously validated in comparison with thermodilution and Doppler echocardiography methods demonstrating a good compatibility [19, 20]. Moreover, in healthy subjects the coefficient of reproducibility using the Bland and Altman method [21] was 1.8, 1.2 and 2.8 l min−1, respectively, at rest, 5-min tilt and exercise [19, 20]. The coefficient of reproducibility achieved by us during two assessments of basal CI separated by 8 h was 0.65 l min−1 m2. Under the same conditions the coefficient of reproducibility among two determinations of changes from baseline in CI at 5-min tilt and exercise during two tests separated by 4 h was, respectively, 1.27 and 2.17 l min−1 m2. Total peripheral resistance index (TPRI) was calculated as TPRI = (MBP/CI) × 80. Finger blood pressure and pulse intervals were measured on the left hand using a non-invasive photoplethysmographic finger device (Finapres 2300; Ohmeda, BOC Group, Englewood, CO, USA) [22]. The cuffed finger was maintained at the heart level during the continuous measurement period and the device was connected to a PC by means of an analogue-to-digital converter (Anapres v1.2; Notocord Systems, Versailles, France) allowing data acquisition (0.5 Hz), storage and analysis (500 Hz). This 0.5-Hz sampling is suited to patients whose hearts usually beat within a 55–85 bpm range. Heart rate variability was evaluated by mean of spectral analysis using a fast Fourier transform algorithm on 512-point stationary time series. This corresponds to a 4-min 26-s period at 0.5-Hz sampling rate. The frequency of oscillation scale was analysed up to 400 mHz. The total integrated amplitude was calculated over 40–400 mHz (T), the low-frequency component over 40–150 mHz (LF) and the high-frequency component over 150–400 mHz (HF), and expressed in beats min−1 Hz−1/2. High- and low-frequency spectral density estimates were expressed in percentage value of total integrated amplitude (T) and the LF/HF ratio was used as an index of sympathovagal balance [23, 24]. Plasma norepinephrine (NE) and epinephrine (E) and plasma concentrations of study drugs and S 18982, the active metabolite of ivabradine, were determined using high-performance liquid chromatography [16, 25].
Experimental procedure
No tea, coffee, alcoholic beverage or smoking were allowed during the entire trial period. After a light breakfast at 07.00 h, the subjects arrived at the laboratory at 08.00 h. An indwelling intravenous catheter with a heparinized lock was inserted into a forearm vein, chest electrodes for cardiac index measurements were placed and the subjects rested for 1 h in the supine position. At 09.00 h basal values of HR, arterial pressure, CI and HR variability were recorded and blood samples for determination of biological parameters were collected, after which the treatment was administered (at 09.30 h). A tilt test, consisting of a passive standing up from a supine position, was performed 2 h after drug administration. Finger blood pressure was measured continuously from baseline until the 10 min of tilt. HR, CI, brachial blood pressures, HR variability indices and plasma catecholamines were obtained before and at the 5th minute of tilt. At 2.15 h, after drug administration, when subjects had recovered from the tilt test, an exercise test, consisting of a 10-min exercise on a bicycle ergometer at increasing workloads (3 min at 50 W, 3 min at 100 W and 4 min at 150 W) was performed. Clinical measurements were made at baseline and at the end of each exercise step. Additional blood samples for catecholamine plasma level determinations were drawn at rest and at the end of the maximal exercise. During exercise, HR was measured by continuous ECG monitoring until 2.45 h after drug intake, corresponding to the 20th min of recovery. A light meal was served immediately after the exercise test and the volunteers rested for 2 h in the supine position. Tilt and exercise tests were then repeated, 5 h after treatment, according to the same procedure, followed by an additional 2-h rest period with clinical measurements performed at rest 8.00 h after the drug intake.
Statistical analysis
Values are mean ± SEM. Statistical analysis was performed using the systat package (systat 5.2.1; SPSS, Chicago, IL, USA). A three-way analysis of variance (treatment, subject, period) with repeated measurements was performed on the changes over time for all the parameters measured. The effect of treatment on parameters measured during the stimulation tests was assessed using a four-way analysis of variance [treatment, subject, period and timing of the test (2nd or 5th hour after treatment)]. In the case of a significant treatment effect, differences between treatments were examined two by two and at each separate time, using a contrast analysis. In addition, the least significance difference between means of repeated measures at rest and during tests (LSD) for each parameter was also calculated for a 5% significance level for all important endpoints for the study. The corresponding 95% confidence interval of mean difference (d) for each comparison was obtained as d ± LSD. The effect of treatment on the relationships between plasma catecholamines and HR, cardiac output or MBP was evaluated using a multiple linear model with subject, period and treatment group as factors and plasma values of catecholamine obtained at different measurement times as cofactors. The treatment–catecholamine interaction was used to indicate a treatment effect on this relationship and followed by a contrast analysis when significant. In parallel, a linear regression model was used to illustrate the within-group relationships in presence of placebo, ivabradine and propranolol. A value of P ≤ 0.05 was considered to be significant.
Results
At rest (Table 1)
Table 1.
Resting haemodynamic and biological parameters
| Parameters | Treatment | T0 | T2 h | T5 h | T8 h |
|---|---|---|---|---|---|
| Heart rate (bpm) | Placebo | 63 ± 2 | 65 ± 3 | 72 ± 4‡ | 67 ± 4 |
| Ivabradine | 66 ± 4 | 54 ± 3* | 60 ± 4*‡ | 62 ± 2 | |
| Propranolol | 66 ± 3 | 59 ± 3* | 62 ± 3*‡ | 60 ± 4 | |
| Systolic blood pressure (mmHg) | Placebo | 124 ± 3 | 123 ± 3 | 120 ± 2 | 121 ± 2 |
| Ivabradine | 124 ± 4 | 116 ± 4* | 115 ± 3* | 115 ± 3*† | |
| Propranolol | 122 ± 3 | 114 ± 3* | 113 ± 3* | 121 ± 2 | |
| Diastolic blood pressure (mmHg) | Placebo | 64 ± 3 | 68 ± 2 | 61 ± 1 | 65 ± 2 |
| Ivabradine | 67 ± 2 | 64 ± 3 | 63 ± 3 | 64 ± 1 | |
| Propranolol | 65 ± 3 | 64 ± 3 | 61 ± 4 | 65 ± 4 | |
| Mean blood pressure (mmHg) | Placebo | 84 ± 3 | 86 ± 2 | 81 ± 1 | 84 ± 2 |
| Ivabradine | 86 ± 2 | 81 ± 3 | 80 ± 3 | 81 ± 1 | |
| Propranolol | 84 ± 3 | 81 ± 3 | 78 ± 3 | 84 ± 3 | |
| Rate pressure product (103 mmHg min−1) | Placebo | 7.77 ± 0.27 | 7.91 ± 0.52 | 8.70 ± 0.56‡ | 8.13 ± 0.43 |
| Ivabradine | 8.20 ± 0.48 | 6.24 ± 0.40* | 6.92 ± 0.46*‡ | 7.14 ± 0.28* | |
| Propranolol | 8.08 ± 0.37 | 6.78 ± 0.26* | 6.96 ± 0.44*‡ | 7.31 ± 0.51 | |
| Cardiac index (l m−1 m−2) | Placebo | 4.5 ± 0.3 | 4.1 ± 0.3 | 4.9 ± 0.4‡ | 4.5 ± 0.3 |
| Ivabradine | 4.8 ± 0.4 | 4.0 ± 0.3 | 4.9 ± 0.4‡ | 4.7 ± 0.4 | |
| Propranolol | 4.6 ± 0.3 | 3.8 ± 0.3 | 4.4 ± 0.4‡ | 4.3 ± 0.3 | |
| Total peripheral resistance index (dynes s cm−5) | Placebo | 1523 ± 100 | 1475 ± 110 | 1380 ± 119‡ | 1540 ± 97 |
| Ivabradine | 1536 ± 163 | 1690 ± 137 | 1385 ± 106‡ | 1429 ± 104 | |
| Propranolol | 1482 ± 70 | 1815 ± 193 | 1563 ± 173‡ | 1621 ± 102 | |
| Epinephrine (pg ml−1) | Placebo | 30 ± 0 | 30 ± 0 | 30 ± 0 | – |
| Ivabradine | 30 ± 0 | 30 ± 0 | 30 ± 0 | – | |
| Propranolol | 30 ± 0 | 34 ± 2 | 34 ± 4 | – | |
| Norepinephrine (pg ml−1) | Placebo | 183 ± 18 | 231 ± 23 | 232 ± 25 | – |
| Ivabradine | 190 ± 23 | 250 ± 27 | 246 ± 22 | – | |
| Propranolol | 196 ± 11 | 265 ± 39 | 221 ± 21 | – |
Values are mean ± SEM. Parameters are obtained at rest, before (T0) and, respectively, 2 (T2 h), 5 (T5 h) and 8 h (T8 h) after treatment administration.
P < 0.05 vs. placebo
P < 0.05 Ivabradine vs. Propranolol
P < 0.05 T5 h vs. T2 h.
There was no significant difference between the basal values of the different parameters obtained before treatment administration during each study period.
After ivabradine and propranolol resting HR decreased similarly compared with placebo [treatment effect: P < 0.002; Iva vs. Plac P < 0.01, Propra vs. Plac P < 0.01, Iva vs. Propra P < 0.31 (95% confidence interval Iva vs. Plac 4.5, 12.8; 95% confidence interval Propra vs. Plac 1.6, 10.0; 95% confidence interval Iva vs. Propra −1.3, 7.1)].
SBP decreased similarly after ivabradine and propranolol compared with placebo [treatment effect: P < 0.007; Iva vs. Plac P < 0.05, Propra vs. Plac P < 0.04, Iva vs. Propra P < 0.33 (95% confidence interval Iva vs. Plac 0.8, 8.2; 95% confidence interval Propra vs. Plac 3.1, 10.5; 95% confidence interval Iva vs. Propra −1.4, 6.0)]. DBP and MBP were not altered by any treatment.
Resting RPP decreased similarly with both active drug treatments compared with placebo [treatment effect: P < 0.002; Iva vs. Plac P < 0.003, Propra vs. Plac P < 0.005, Iva vs. Propra P < 0.62 (95% confidence interval Iva vs. Plac 0.7, 2.0; 95% confidence interval Propra vs. Plac 0.5, 1.8; 95% confidence interval Iva vs. Propra −0.5, 0.8)].
There were no significant treatment effects on CI and TPRI.
At 5 h, during the postprandial period, HR (P < 0.006), CI (P < 0.001) and RPP (P < 0.04) were increased and TPRI (P < 0.001) decreased when compared with the morning, but there was no significant treatment effect related to these changes.
Plasma levels of epinephrine and norepinephrine did not change at rest.
Tilt test (Table 2, Figure 1)
Table 2.
Haemodynamic and biological parameters obtained during tilt and exercise tests
| Parameters | Treatment | Tilt1 | Exercise 1 | Tilt2 | Exercise 2 |
|---|---|---|---|---|---|
| Heart rate (bpm) | Placebo | 74 ± 4 | 153 ± 7 | 77 ± 4‡ | 156 ± 7§ |
| Ivabradine | 59 ± 2*† | 121 ± 4* | 60 ± 3*†‡ | 124 ± 5*§ | |
| Propranolol | 65 ± 2* | 121 ± 3* | 69 ± 3*‡ | 129 ± 5*§ | |
| Systolic blood pressure (mmHg) | Placebo | 123 ± 3 | 146 ± 2 | 125 ± 3 | 141 ± 7 |
| Ivabradine | 126 ± 5† | 143 ± 4 | 121 ± 4† | 151 ± 4 | |
| Propranolol | 112 ± 4* | 144 ± 2 | 114 ± 3* | 148 ± 3 | |
| Diastolic blood pressure (mmHg) | Placebo | 71 ± 3 | 106 ± 5 | 68 ± 2 | 109 ± 9 |
| Ivabradine | 71 ± 4 | 110 ± 5 | 70 ± 3 | 94 ± 6 | |
| Propranolol | 64 ± 3 | 107 ± 3 | 65 ± 2 | 102 ± 6 | |
| Mean blood pressure (mmHg) | Placebo | 88 ± 3 | 120 ± 4 | 87 ± 2 | 120 ± 9 |
| Ivabradine | 89 ± 4† | 121 ± 5 | 87 ± 3† | 113 ± 4 | |
| Propranolol | 80 ± 3* | 119 ± 3 | 81 ± 2* | 117 ± 4 | |
| Rate pressure product (103 mmHg min−1) | Placebo | 9.12 ± 0.63 | 22.43 ± 1.14 | 9.62 ± 0.64‡ | 22.33 ± 1.85§ |
| Ivabradine | 7.33 ± 0.37* | 17.30 ± 0.83* | 7.26 ± 0.55*‡ | 18.71 ± 0.78*§ | |
| Propranolol | 7.26 ± 0.38* | 17.43 ± 0.51* | 7.96 ± 0.44*‡ | 19.04 ± 0.84*§ | |
| Cardiac index (l m−1 m−2) | Placebo | 3.7 ± 0.2 | 11.0 ± 0.7 | 4.7 ± 0.3‡ | 12.2 ± 0.9§ |
| Ivabradine | 3.7 ± 0.3† | 10.8 ± 0.7 | 4.7 ± 0.5†‡ | 13.0 ± 1.2§ | |
| Propranolol | 3.2 ± 0.2* | 10.0 ± 1.0 | 4.1 ± 0.4*‡ | 12.1 ± 1.0§ | |
| Total peripheral resistance index (dynes s cm−5) | Placebo | 2021 ± 116 | 898 ± 71 | 1522 ± 91‡ | 845 ± 106§ |
| Ivabradine | 2021 ± 188 | 944 ± 79 | 1579 ± 131‡ | 738 ± 62§ | |
| Propranolol | 2011 ± 146 | 1042 ± 119 | 1687 ± 127‡ | 824 ± 86§ | |
| Epinephrine (pg ml−1) | Placebo | 41 ± 8 | 80 ± 13 | 30 ± 0‡ | 57 ± 10§ |
| Ivabradine | 33 ± 3 | 103 ± 13 | 30 ± 0‡ | 59 ± 8§ | |
| Propranolol | 51 ± 9 | 149 ± 28*† | 31 ± 1‡ | 90 ± 13§*† | |
| Norepinephrine (pg ml−1) | Placebo | 325 ± 24 | 985 ± 99 | 352 ± 41 | 997 ± 123 |
| Ivabradine | 358 ± 27 | 1133 ± 146 | 348 ± 31 | 989 ± 133 | |
| Propranolol | 333 ± 24 | 1223 ± 154 | 338 ± 27 | 1021 ± 111 |
Values are mean ± SEM. Parameters are obtained at 5 min, during the first (Tilt1) and the second tilt test (Tilt 2) and at 10 min during the first (Exercise 1) and second exercise test (Exercise 2). The two sets of tests were performed, respectively, 2 and 5 h after treatment administration.
P < 0.05 vs. placebo
P < 0.05 Ivabradine vs. Propranolol at each measurement time
P < 0.05 vs. Tilt 1
P < 0.05 vs. Exercise 1.
Figure 1.
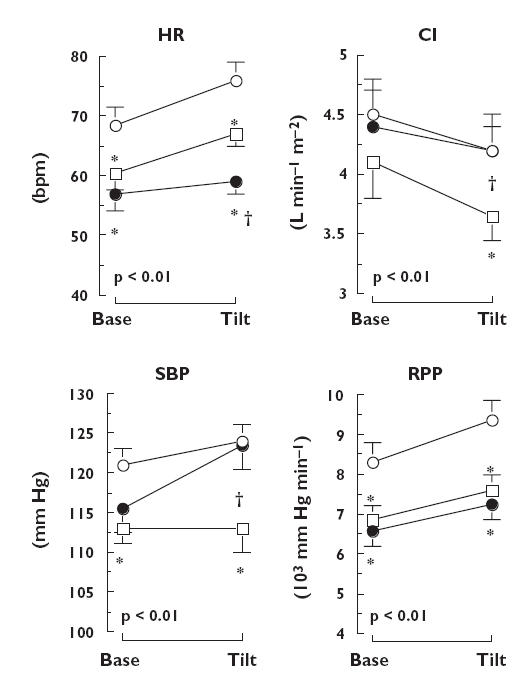
Mean (± SEM) values of heart rate (HR), cardiac index (CI), systolic blood pressure (SBP) and rate pressure product (RPP) obtained 2 and 5 h after administration of placebo (○), ivabradine (•) and propranolol (□) at baseline and at the 5th minute of tilt test. P, Time effect. *P < 0.05 vs. placebo, †P < 0.05 vs. propranolol
During the two tilt tests the HR at 5-min tilt was lower with ivabradine and propranolol than with placebo [treatment effect: P < 0.001; Iva vs. Plac P < 0.001, Propra vs. Plac P < 0.004 (95% confidence interval Iva vs. Plac 10.6, 18.4; 95% confidence interval Propra vs. Plac 4.9, 12.7)]. In addition, HR was significantly lower with ivabradine than with propranolol (Iva vs. Propra P < 0.007, 95% confidence interval 1.9, 9.7).
SBP and MBP differed with the treatment groups (P < 0.001 and P < 0.004, respectively). SBP was similar with ivabradine and placebo (Iva vs. Plac P < 0.72, 95% confidence interval −3.2, 8.7), while remaining significantly lower with propranolol than with ivabradine (Propra vs. Iva P < 0.003, 95% confidence interval 0.3, 12.2) or placebo (Propra vs. Plac P < 0.001, 95% confidence interval 3.1, 15.0). MBP was similar with ivabradine and placebo (Iva vs. Plac P < 0.99, 95% confidence interval −2.8, 6.3) while remaining significantly lower with propranolol than with ivabradine (Propra vs. Iva P < 0.003, 95% confidence interval 0.7, 9.8) or placebo (Propra vs. Plac P < 0.003, 95% confidence interval 2.5, 11.5).
In contrast, ivabradine and propranolol both decreased RPP compared with placebo [treatment effect: P < 0.001; Iva vs. Plac P < 0.001, Propra vs. Plac P < 0.001 (95% confidence interval Iva vs. Plac 0.8, 1.8; 95% confidence interval Propra vs. Plac 0.6, 1.7)]. This treatment effect was similar with ivabradine and propranolol (Iva vs. Propra P < 0.40, 95% confidence interval −0.4, 0.7).
Simultaneously, the decrease in CI was greater with propranolol than with ivabradine (treatment effect: P < 0.05; Propra vs. Iva P < 0.04, 95% confidence interval 0.1, 0.7) or placebo (Propra vs. Plac P < 0.04, 95% confidence interval 0.1, 0.8) and was similar with ivabradine or placebo (Iva vs. Plac P < 0.94, 95% confidence interval −0.3, 0.4).
DBP, TPRI and the plasma levels of epinephrine and norepinephrine increased during the tilt tests (all P < 0.001) without any treatment effect.
During the second tilt test (post prandial) HR (P < 0.006), CI (P < 0.001) and RPP (P < 0.04) were greater and TPRI (P < 0.001) and plasma epinephrine (P < 0.013) were lower when compared with the morning without any treatment effect related to these changes (Table 2).
During exercise (Table 2, Figure 2)
Figure 2.
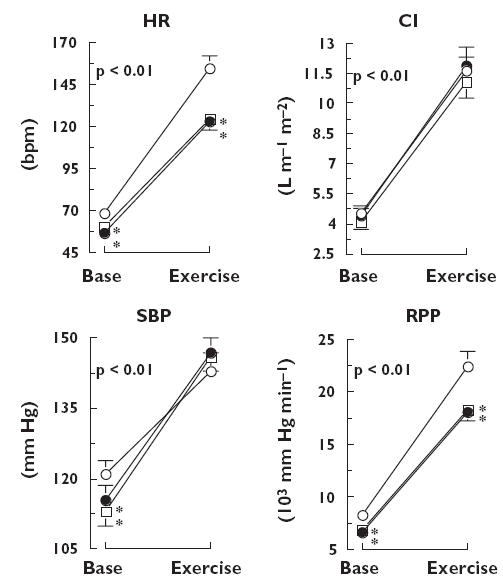
Mean (± SEM) values of heart rate (HR), cardiac index (CI), systolic blood pressure (SBP) and rate pressure product (RPP) obtained 2.25 and 5.25 h after administration of placebo (○), ivabradine (•) and propranolol (□) at baseline and at maximal load during exercise test. P, Time effect. *P < 0.05 vs. placebo, †P < 0.05 vs. propranolol
During the two exercise tests the HR at maximal exercise was similarly lower with ivabradine and propranolol than with placebo [treatment effect: P < 0.001; Iva vs. Plac P < 0.001, Propra vs. Plac P < 0.001, Iva vs. Propra P < 0.376 (95% confidence interval Iva vs. Plac 21.8, 33.7; 95% confidence interval Propra vs. Plac 17.8, 29.7; 95% confidence interval Iva vs. Propra −1.9, 10.0)].
SBP, DBP and MBP increased and TPRI decreased during the exercise tests (all P < 0.001), but these changes did not show any treatment effect.
During the exercise tests RPP was similarly lower with ivabradine and propranolol than with placebo [treatment effect: P < 0.001; Iva vs. Plac P < 0.001, Propra vs. Plac P < 0.001, Iva vs. Propra P < 0.374 (95% confidence interval Iva vs. Plac 2.1, 3.3; 95% confidence interval Propra vs. Plac 1.9, 3.1; 95% confidence interval Iva vs. Propra −0.4, 0.8)].
Simultaneously, CI was slightly greater with ivabradine or placebo than with propranolol, but the difference between the treatments was not significant.
At maximal exercise, norepinephrine was similar in all three groups but epinephrine was higher with propranolol than with ivabradine or placebo [treatment effect: P < 0.001; Propra vs. Iva P < 0.001, Propra vs. Plac P < 0.001 (95% confidence interval Propra vs. Iva 11.2, 39.8, 95% confidence interval Propra vs. Plac 19.2, 47.8)], and similar with ivabradine and placebo (Iva vs. Plac P < 0.287, 95% confidence interval −6.3, 22.3).
During the second exercise test (post prandial) HR, CI and RPP were greater and TPRI and plasma catecholamines were lower when compared with the first exercise test (all P < 0.001). However, there was no significant treatment effect related to these changes.
Relationships between plasma catecholamine levels and HR, CI and MBP (Figure 3)
Figure 3.
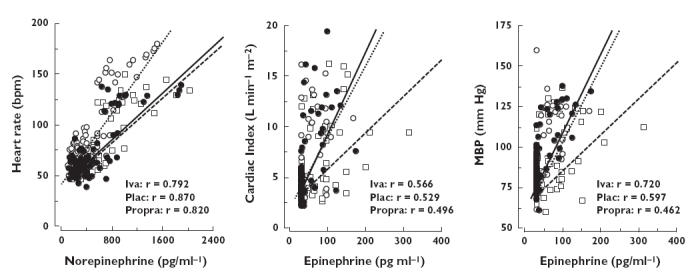
Relationships between plasma norepinephrine and heart rate (left), plasma epinephrine and cardiac index (middle) and plasma epinephrine and mean blood pressure (right), using individual values obtained at rest, during tilt tests and during exercise, after administration of placebo (○), ivabradine (•) and propranolol (□). The r-values correspond to the univariate correlation coefficients obtained for each significant treatment group relationship
There was a significant positive relationship between plasma noreprinephrine and HR (P < 0.001) with a significant shift in the relationship towards lower HR values for given plasma norepinephrine concentrations for both ivabradine (treatment × norepinephrine: P < 0.001) and propranolol (treatment × norepinephrine: P < 0.001) compared with placebo, but no significant difference between the effects of these two drugs (Figure 3, left).
Furthermore, there was a significant positive relationships between plasma epinephrine and CI (P < 0.001) and plasma epinephrine and MBP (P < 0.001), and only propranolol shifted the relationships significantly towards lower values of MBP (treatment × epinephrine: P < 0.01) and CI (treatment × epinephrine: P < 0.004) compared with placebo for given plasma epinephrine levels (Figure 3, middle and right).
Heart rate variability
At rest, the amplitude of the HR high-frequency or low-frequency oscillations and of the low- to high-frequency ratio oscillations were not altered significantly by either drug (Figure 4).
Figure 4.
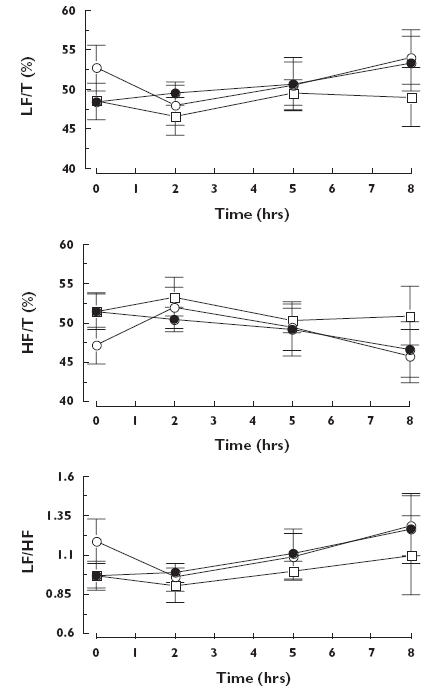
Mean (± SEM) values of low-frequency component (LF/T), high-frequency component (HF/T) and low- to high-frequency ratio (LF/HF) of heart rate spectra obtained at rest before (time 0) and at rest, 2, 5 and 8 h after administration of placebo (○), ivabradine (•) and propranolol (□)
During the two tilt tests (Figure 5), the amplitude of the HR low-frequency oscillations increased (P < 0.007) and the amplitude of the high-frequency oscillations decreased (P < 0.001) but these changes did not show any treatment effect. However, the HR low- to high-frequency ratio oscillations were significantly lower with ivabradine relative to placebo (treatment effect: P < 0.01; Iva vs. Plac P < 0.03, 95% confidence interval 0.02, 0.22) and were lower with propranolol than with placebo, but the difference between propranolol and placebo (Propra vs. Plac P < 0.19, 95% confidence interval −0.10, 0.11) and between the two drugs was not significant (Iva vs. Propra P < 0.39, 95% confidence interval −0.10, 0.11).
Figure 5.
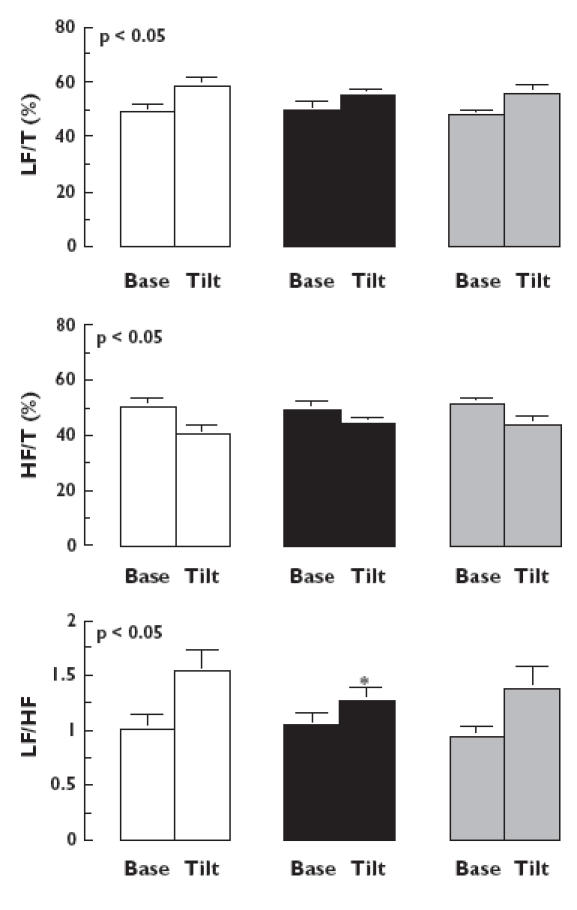
Mean (± SEM) values of low-frequency component (LF/T), high-frequency component (HF/T) and low- to high-frequency ratio (LF/HF) of heart rate spectra obtained 2 and 5 h after administration of placebo (□), ivabradine (▪) and propranolol ( ) at baseline (base) and at the 5th minute of tilt test (tilt). P, Time effect. *P < 0.05 vs. placebo
) at baseline (base) and at the 5th minute of tilt test (tilt). P, Time effect. *P < 0.05 vs. placebo
Pharmacokinetics
The mean maximal plasma concentrations were 82 ± 15, 18 ± 3 and 27 ± 5 ng ml−1 and the time at which the maximal plasma concentration occurred was 137 ± 6, 151 ± 13 and 163 ± 13 min, for, respectively, ivabradine, its active metabolite S 18982, and propranolol. At 2 h and 5 h, just before tilt and exercise tests, the plasma concentrations of ivabradine were, respectively, 75 ± 14 and 25 ± 4 ng ml−1, concentrations of S 18982 were 16 ± 3 and 11 ± 2 ng ml−1 and those of propranolol were 16 ± 4 and 18 ± 4 ng ml−1.
Safety evaluation
There were no major adverse events or premature withdrawals from the study due to adverse events. Five volunteers presented one or more adverse events during the study. Of the eight adverse events reported, six occurred during propranolol periods, two during ivabradine periods and none during placebo periods. The most common adverse event was vagal malaise (nausea, hypotension and bradycardia), which occurred four times in participants receiving propranolol and once in those receiving ivabradine. In each case, the onset of vagal malaise occurred 7–15 min after a tilt test and stopped rapidly after a brief period of rest in the supine position. Finally, one cold left hand lasting 10 min (following propranolol) and intermittent visual disturbances lasting 10 min (following ivabradine) were observed. All adverse events resolved spontaneously.
Discussion
The main result of the present study performed in healthy volunteers was to demonstrate that for a similar decrease in HR at rest and during sympathetic stimulation induced by exercise, acute administration of ivabradine, a new HR-lowering agent that selectively and specifically inhibits the If current, not only decreases myocardial oxygen demand as assessed by the rate pressure product, to the same extent as a reference β-blocker, propranolol, but also acts without any evidence of a depressant effect on cardiac function.
The doses of ivabradine and propranolol were selected to obtain the same decrease in HR at rest and during exercise. Thus, it was possible to compare systemic and cardiac haemodynamic and plasma catecholamines between groups at the same level of sinus node inhibition despite their different mechanisms of action, to study the single effect of HR lowering and to assess the cardiovascular adaptive responses under these conditions. Furthermore, we attempted to compare the kinetics of the treatment effects, at rest and during exercise and tilt tests which were performed twice during each study period, 2 and 5 h after drug administration.
At rest it appears that the decrease in HR was moderate and comparable in intensity and duration when ivabradine was administered at a dose of 30 mg and propranolol at a dose of 40 mg. Under these conditions, MBP, DBP and CI did not change significantly, but only SBP decreased. The decrease in brachial SBP with these two substances, as previously reported after β-blockers, suggests that the HR lowering was directly involved in this effect [26]. The decrease in amplification of the pulse wave between the aorta and peripheral arteries, physiologically associated with the HR lowering, could thus have decreased brachial SBP [27, 28]. However, this mechanism was not investigated in the study. Furthermore, the fact that the decrease in SBP was more marked with propranolol than with ivabradine and that the CI was slightly although nonsignificantly lowered with propranolol alone, also suggests that propranolol has a cardiac depressant effect. Indeed, although not presently investigated, propranolol could have modified ventricular ejection and thus decreased peak systolic pressure [29, 30]. However, it must be stressed in this context that the RPP, which reflects myocardial oxygen demand, was similarly lowered with ivabradine and propranolol.
During the tilt test, the decrease in CI was associated, in all treatment groups, with a mild sympathetic activation resulting from baroreflex deactivation and characterized by an increase in HR, TPRI, blood pressure and plasma catecholamines. In these conditions, the decrease in CI was significantly more marked after propranolol than after placebo or ivabradine despite a lower value of HR after ivabradine. This test, which induces cardiac unloading and depression of preload-dependent contractility by decreasing venous return, thus enables us to demonstrate indirectly the negative inotropic effect of propranolol but not ivabradine. Moreover, because TPRI and DBP, which can be considered as indices of arteriolar tone, were similarly increased after ivabradine and propranolol, the reduced increase in SBP with propranolol resulted from the lower value of CI and stroke volume and thus probably also from the negative inotropic effect of this drug. Simultaneously, the lower value of low- to high-frequency ratio observed after ivabradine and propranolol reflects the inhibitory effect of these treatments on the sinus node during this test [23, 24, 31]. However, in absence of direct measurement of cardiac preload, a lower venous return after β-blockade than If current inhibition could participate in the observed decrease in cardiac output. This is unlikely, because propranolol has probably unmasked a higher α-dependent venous tone than ivabradine, leading to a similar or even higher venous return after β-blockade than If current inhibition. This assessment of interaction of the two drugs with inotropism is supported by the relationships between plasma catecholamines and HR, CI and MBP. According with the norepinephrine–HR relationships, it appears that propranolol and ivabradine had a similar antitachycardic effect [32]. However, the relationships between plasma epinephrine and CI or MBP show a shift toward lower values of CI and MBP for propranolol, but not for ivabradine. This suggests that propranolol has a myocardial depressant effect that was not shown by ivabradine at a dose that was equipotent to that of propranolol in decreasing tachycardia. This finding is consistent with the results of previous studies comparing ivabradine with propranolol in conscious dogs and pigs at rest and during exercise [6, 33]. Finally, it appears that despite these different haemodynamic conditions, the decrease in myocardial oxygen demand during tilt was similar with ivabradine and propranolol, as illustrated by the similar RPP at tilt when compared with placebo.
Several ionic currents operate in the sino-atrial node during the slow diastolic phase of depolarization, including the delayed rectifier current IK, a background sodium current IbNa, a T-type calcium current and If. If is thought to be one of the most important currents in pacemaking in the sino-atrial node [34]. Animal studies indicate that ivabradine is a selective and specific inhibitor of If, and has few effects other than to slow the rate of diastolic depolarization and hence reduce HR [12, 14, 35]. This specificity may explain the absence of significant negative inotropic or lusitropic and vasoactive actions of ivabradine observed in entire animal studies [6, 36–38] and in the present study.
During exercise, which corresponds to a more sustained sympathetic activation characterized by a larger increase in HR, blood pressure and plasma catecholamines, there was, in contrast to the tilt test, an increase in CI and a decrease in TPRI. These changes were associated with a sustained increase in RPP, as expected from the increase in HR and SBP. However, at a dose of propranolol that limits the increase in HR to the same extent as ivabradine, the decrease in the RPP observed at exercise was similar with the two drugs. This decrease in this index of myocardial oxygen demand was associated with an increase in the myocardial diastolic perfusion time and thus probably with an increased subendocardial perfusion.
Furthermore, in contrast to the tilt test, ivabradine and propranolol have comparable effects on CI and SBP at exercise, even if CI tends to be slightly and nonsignificantly lower after propranolol than after ivabradine. Thus, in the presence of strong sympathetic stimulation, the doses of ivabradine and propranolol we administered had similar depressant effects on HR and RPP, thus indicating, although obtained in healthy young subjects and in a relatively small study, a similar beneficial effect on the balance between the myocardial oxygen demand and supply in these conditions.
Finally, concerning the kinetics of the treatment effects, the fact that the effects of ivabradine were similar 2 and 5 h after drug administration, when plasma values of the drug were close to three times higher at 2 than at 5 h, suggests that these pharmacological effects are related to a sustained tissular effect of the substance due to a long-lasting tissular binding possibly associated with the presence of an active metabolite [16].
In conclusion, our study demonstrates that selective HR reduction with no evidence of depressant effect on cardiac function decreases the index of myocardial oxygen demand to the same extent as the reference β-blocker, propranolol, when used at doses providing equivalent decreases in HR. These results and studies concerning experimental heart failure [38] and patients with stable angina [17] suggest that ivabradine could be of potential interest in patients with ischaemic heart disease [39]. In addition, it could also be used particularly when β-blockers are contraindicated or badly tolerated particularly because of their negative inotropic effect.
Acknowledgments
This study was supported by a grant from the Laboratoires Servier, France. The authors thank Richard Medeiros (Medical Editor, Rouen University Hospital, Rouen, France) for his advice in editing the manuscript.
Conflict of interest
The study was partly supported by a grant from the Institut de Recherches Internationales Servier. G.L. is an employee of the Institut de Recherches Internationales Servier.
References
- 1.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 2.Palatini P, Casiglia E, Pauletto P, Staessen J, Kaciroti N, Julius S. Relationship of tachycardia with high blood pressure and metabolic abnormalities. A study with mixture analysis in three populations. Hypertens. 1997;30:1267–73. doi: 10.1161/01.hyp.30.5.1267. [DOI] [PubMed] [Google Scholar]
- 3.Hjalmarson A, Gilpin EA, Kjekshus G, Nicod P, Henning H, Ross J., Jr Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol. 1990;1:547–53. doi: 10.1016/0002-9149(90)91029-6. [DOI] [PubMed] [Google Scholar]
- 4.Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Supplements. 1999;1(Suppl. H):H64–H69. [Google Scholar]
- 5.Berdeaux A, Drieu La Rochelle C, Richard V, Giudicelli JF. Opposed responses of large and small coronary arteries to propranolol during exercise in dogs. Am J Physiol. 1991;261:H265–H270. doi: 10.1152/ajpheart.1991.261.2.H265. [DOI] [PubMed] [Google Scholar]
- 6.Simon L, Ghaleh B, Puybasset L, Giudicelli J-F, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–66. [PubMed] [Google Scholar]
- 7.Bortone AS, Hess OM, Gaglione A, Suter T, Nonogi H, Grimm J, Krayenbuehl HP. Effect of propranolol on coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. Circulation. 1990;81:1225–35. doi: 10.1161/01.cir.81.4.1225. [DOI] [PubMed] [Google Scholar]
- 8.Tattersfield AE. Respiratory function in the elderly and the effects of beta blockade. Cardiovasc Drugs Ther. 1991;4(Suppl. 6):1229–32. doi: 10.1007/BF00114225. [DOI] [PubMed] [Google Scholar]
- 9.Lithell H, Pollare T, Vessby B. Metabolic effects of pindolol and propranolol in a double-blind crossover study in hypertensive patients. Blood Pressure. 1992;1:92–101. doi: 10.3109/08037059209077499. [DOI] [PubMed] [Google Scholar]
- 10.Di Francesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986;324:470–3. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- 11.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- 12.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–7. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine. J General Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardiner SM, Kemp PA, March JE, Bennett T. Acute and chronic cardiac and regional haemodynamic effects of the novel bradycardic agent, S16257, in conscious rats. Br J Pharmacol. 1995;115:579–86. doi: 10.1111/j.1476-5381.1995.tb14971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragueneau I, Laveille C, Jochemsen R, Resplandy G, Funck-Brentano C, Jaillon P. Pharmacokinetic–pharmacodynamic modeling of the effects of ivabradine, a direct sinus node inhibitor, on heart rate in healthy volunteers. Clin Pharmacol Ther. 1998;64:192–203. doi: 10.1016/S0009-9236(98)90153-9. [DOI] [PubMed] [Google Scholar]
- 17.Borer JS, Fox K, Jaillon P, Lerebours G Ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–23. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG, Reines MD, Crawford FA., Jr Comparison of bioimpedance and thermodilution methods for determining cardiac aoutput: experimental and clinical studies. Ann Thorac Surg. 1988;45:421–5. doi: 10.1016/s0003-4975(98)90016-9. [DOI] [PubMed] [Google Scholar]
- 19.Ng HW, Walley T, Tsao Y, Breckenridge AM. Comparison and reproducibility of transthoracic bioimpedance and dual beam Doppler ultrasound measurement of cardiac function in healthy volunteers. Br J Clin Pharmacol. 1991;32:275–82. doi: 10.1111/j.1365-2125.1991.tb03899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northridge DB, Findlay IN, Wilson J, Henderson E, Dargie HJ. Non-invasive determination of cardiac output by Doppler echocardiography and electrical bioimpedance. Br Heart J. 1990;63:93–7. doi: 10.1136/hrt.63.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 22.Parati G, Casadei R, Gropelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension Dallas. 1989;82:647–55. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- 23.Elghozi JL, Laude D, Janvier F. Clonidine reduces blood pressure and heart rate oscillations in hypertensive patients. J Cardiovasc Pharmacol. 1991;17:935–40. doi: 10.1097/00005344-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Paolisso G, Manzella D, Rizzo MR, Ragno E, Barbieri M, Varricchio G, Varricchio M. Elevated plasma fatty acid concentrations stimulate the cardiac autonomic nervous system in healthy subjects. Am J Clin Nutr. 2000;72:723–30. doi: 10.1093/ajcn/72.3.723. [DOI] [PubMed] [Google Scholar]
- 25.Sagnol M, Claustre J, Cottet-Emard JM, Pequignot JM, Fellmann N, Coudert J, Peyrin L. Plasma free and sulphated catecholamines after ultra-long exercise and recovery. Eur J Appl Physiol. 1990;60:91–7. doi: 10.1007/BF00846027. [DOI] [PubMed] [Google Scholar]
- 26.Thuillez C, Richer C, Duhazé P, Bergougnan L, Giudicelli JF. β-Adrenoreceptor blocking effects and plasma levels of bornaprolol and propranolol in man. Eur J Clin Pharmacol. 1985;29:405–11. doi: 10.1007/BF00613453. [DOI] [PubMed] [Google Scholar]
- 27.Laurent P, Albaladejo P, Blacher J, Rudnichi A, Smulyan H, Safar ME. Heart rate and pulse pressure amplification in hypertensive subjects. Am J Hypertens. 2003;16:363–70. doi: 10.1016/s0895-7061(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 28.O'Rourke MF. Influence of ventricular ejection on the relationship between central aortic and brachial pressure pulse in man. Cardiovasc Res. 1970;4:291–300. doi: 10.1093/cvr/4.3.291. [DOI] [PubMed] [Google Scholar]
- 29.Clifton GD, Harrison MR, DeMaria AN. Influence of beta-adrenergic blockade upon hemodynamic response to exercise assessed by Doppler echocardiography. Am Heart J. 1990;120:579–85. doi: 10.1016/0002-8703(90)90014-o. [DOI] [PubMed] [Google Scholar]
- 30.Harrison MR, Smith MD, Nissen SE, Grayburn PA, DeMaria AN. Use of exercise Doppler echocardiography to evaluate cardiac drugs: effects of propranolol and verapamil on aortic blood flow velocity and acceleration. J Am Coll Cardiol. 1988;11:1002–9. doi: 10.1016/s0735-1097(98)90058-4. [DOI] [PubMed] [Google Scholar]
- 31.Girard A, Laude D, Japundzic N, Elghozi JL. Effects of chronic beta-adrenoceptor blockade on variability in blood pressure and heart rate: a non-invasive spectral study. J Hypertens Suppl. 1991;9:S350–S351. [PubMed] [Google Scholar]
- 32.Ohnishi A, Minegishi A, Ishizaki T. Effect of beta-adrenoreceptor blockade on exercise-induced plasma catecholamine concentration–heart rate response relationship. J Cardiovasc Pharmacol. 1987;10:667–74. doi: 10.1097/00005344-198712000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Vilaine JP, Bidouard JP, Lesage L, Reure H, Peglion JL. Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol. 2003;42:688–96. doi: 10.1097/00005344-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Di Francesco D. The contribution of the ‘pacemaker’ current (If) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camm AJ, Lau CP. Electrophysiological effects of a single intravenous administration of ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R D. 2003;4:83–9. doi: 10.2165/00126839-200304020-00001. [DOI] [PubMed] [Google Scholar]
- 36.Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308:236–40. doi: 10.1124/jpet.103.059717. [DOI] [PubMed] [Google Scholar]
- 37.Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282:H672–H679. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- 38.Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, Renet S, Lerebours G, Mahlberg-Gaudin F, Thuillez C. Long-term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–9. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 39.Purcell H. Heart rate as a therapeutic target in ischaemic heart disease. Eur Heart J. 1999;1(Suppl. H):H58–H63. [Google Scholar]


