Abstract
Aims
To compare the disposition of fluoxetine and norfluoxetine enanantiomers in the mother, foetus and infant.
Methods
Blood from pregnant women taking fluoxetine (n = 9), during pregnancy was sampled in the third trimester and at delivery (maternal and cord venous blood), and from the infants 48 h after delivery. The subset of these women who were breastfeeding, plus additional subjects recruited in the postpartum period, were studied further, and maternal and infant blood, and breast milk was sampled between 6 days and 11 months (n = 23). Drug and metabolite concentrations were measured using gas chromatography/mass spectrometry or liquid chromatography, tandem mass spectrometry.
Results
There was a high correlation between maternal and foetal (cord blood) fluoxetine and norfluoxetine enantiomers (r2−0.9), the mean foetal/maternal ratios (95% confidence intervals) being 0.91 (0.61, 1.02) and 1.04 (0.93, 1.05), for fluoxetine and norfluoxetine, respectively. In 2 day old infants exposed to the drug in utero, the fluoxetine and norfluoxetine plasma concentrations were the same as in cord blood at delivery. Over the next 2 months, the plasma concentrations in the infants fell progressively. Stereoselective disposition of both the drug and metabolite in the mother, foetus, infant and breast milk was observed. The S : R ratios in the foetus and newborn (∼3) were significantly higher than in the serum (∼2) or breast milk (∼1.9) of the mothers, resulting in greater exposure of the foetus and infants to the biologically active enantiomers, particularly S-norfluoxetine.
Conclusions
Foetal and infant exposure to fluoxetine and norfluoxetine is enhanced by their stereoselective disposition in the mother, foetus, breast milk and infant. Increased exposure may also result from decreased metabolism of the drug in the foetus and neonate.
Keywords: breast milk, depression, fluoxetine, norfluoxetine, postpartum, pregnancy, stereoselective drug disposition
Introduction
Clinical depression occurs in 10–15% of women during pregnancy [1], while postpartum depression occurs in 10–22% of women [2]. The former condition can place both the mother and foetus at risk [1, 3] and there is also abundant evidence for adverse effects of maternal postnatal depression on the cognitive, motor and emotional development of the offspring [2]. Since the introduction of fluoxetine in the 1980s, it and other selective serotonin reuptake inhibitors (SSRIs) are increasingly used for the treatment of depression in pregnancy because of their effectiveness, lower incidence of maternal side-effects and wider safety margin compared with tricyclic antidepressants and monoamine oxidase inhibitors [1, 4]. However, there have been reports that the use of these drugs during pregnancy can increase the incidence of adverse pregnancy and postnatal outcomes [4–10]. This prompted the Federal Drug Administration in the USA to require an addition to the SSRI product inserts warning that use of these agents in the third trimester may result in jitteriness and other withdrawal symptoms in the infants. Similarly, Health Canada has issued advice that newborns may be adversely affected when pregnant women use SRRIs and other newer antidepressants during the third trimester. Given the requirement for effective treatment of maternal depression in pregnancy, these recent decisions by major North American health regulatory agencies point to the urgent need for more information on both the efficacy and adverse effects of SRRIs in pregnancy.
Although all SSRIs have a primary action in inhibiting serotonin reuptake, their potencies differ as does their ability to affect uptake mechanisms for other monoamines [11]. These drugs also differ markedly with respect to their pharmacokinetics and metabolism and in particular to the degree to which they inhibit cytochrome P450 drug metabolizing enzymes [12]. Several of the SSRIs are chiral compounds, but only fluoxetine is marketed as a racemate. Moreover, it possesses an active metabolite, norfluoxetine, which is also chiral [13, 14].
Since the exposure of the foetus and neonate to SSRIs depends on their pharmacokinetics and metabolism in the maternal-foetal unit and in the neonate, knowledge of these parameters is important for assessing the safety of these agents during pregnancy and the postpartum period. The stereoselective disposition of a chiral drug and its metabolite enanantiomers can also affect the degree of foetal and infant exposure. To our knowledge there are no published data on enantioselective pharmacokinetics of fluoxetine in pregnancy and the newborn.
The results presented in the present study were obtained in a prospective investigation of the disposition and effects of SSRIs in clinically depressed pregnant and postpartum women [7, 9, 10]. The aim of the work was to assess the stereoselective disposition of fluoxetine and norfluoxetine in the mother, foetus and neonate and in breast milk.
Methods
Subjects
Pregnant and nursing mothers referred to the Reproductive Mental Health Programs at British Columbia Women's and St. Paul's Hospitals with depressive symptoms were approached for the study. All mothers met DSM-IV criteria for major depression, with or without comorbid anxiety and/or obsessive-compulsive disorders. Criteria for exclusion were suicidal or psychotic symptoms, gross neonatal morphological malformation, premature delivery (<35 week), hospitalization for reasons other than pregnancy/delivery and discontinuation of breast-feeding. Each subject was informed of the available treatment options and the possible risks of antidepressant medication taken during pregnancy and the nursing period by the attending psychiatrist. The maternal and infant serum and breast milk sampling protocols were discussed with each subject. Written informed consent was obtained from all patients. An age-matched control group of pregnant women was recruited for infant biobehavioural assessments; some of these results have been reported previously [8, 10, 11]. The project was reviewed and approved by the University of British Columbia Clinical Research Ethics Board.
Study protocol
All participants were taking fluoxetine for the treatment of depression. The study population consisted of nine patients recruited during pregnancy and 23 (five of these were from the nine pregnant subjects) during the nursing period. All patients recruited during pregnancy had received fixed daily doses of fluoxetine for a minimum of 3 weeks prior to delivery. Maternal venous samples were collected from these patients during the third trimester, when they came into the Reproductive Psychiatry Unit for scheduled appointments. It was not possible to collect the samples at a specified time in relation to the dose because appointment times differed. At delivery, simultaneous umbilical cord blood (umbilical vein) and maternal blood samples (∼5 ml) were collected. An additional blood sample (∼0.5 ml) was taken (by heel-lance) from the newborn at the time for phenylketonuria testing, which is normally performed 1–2 days after birth as a part of routine neonatal care. During the postpartum component of the study, the mothers who had either been studied during pregnancy or had commenced SRRI therapy following delivery, had paired maternal and infant blood plus breast milk sampled. The latter group had been on fixed daily doses of fluoxetine for a minimum of 4 weeks. Postpartum maternal (∼5 ml) and infant (∼1 ml) blood and breast milk (∼10 ml) samples were collected during routine clinical visits and/or study sessions for infant assessments at times ranging from 6 days to 11 months. All blood samples were collected in Vacutainer tubes without additives and allowed to clot for 30 min. The serum was then separated following centrifugation at 3000 g for 10 min and transferred to glass tubes. Milk samples (foremilk) were collected by manual expression or breast pump at the same time as blood sampling and were transferred to glass tubes. All serum and milk samples were stored at −20 °C until analysis.
Drug and metabolite analysis
Fluoxetine and norfluoxetine isomer concentrations were measured using stereoselective gas chromatography-mass spectrometry electron impact ionization (GC/MS) [15] or stereoselective liquid chromatography- tandem mass spectrometry (LC/MS/MS) [16]. The overall intra- and interassay coefficients of variation were <15% at concentrations over the ranges 1 to 400 µg l−1 (GC/MS) and 0.5–500 µg l−1 (LC/MS/MS). The limit of quantification (100 µl sample) was 1.0 µg l−1 for fluoxetine and norfluoxetine with the GS/MS assay and 0.1 and 0.5 µg l−1 for fluoxetine and norfluoxetine, respectively, with the LC/MS/MS method.
Data analysis
Foetal-to-maternal serum (F : M) ratios were calculated by dividing umbilical cord analyte concentration by that measured in maternal serum at delivery. Milk : serum (M : P) ratios were calculated by dividing the breast milk analyte concentration by that measured in maternal serum. The daily doses of fluoxetine and norfluoxetine in breast-fed infants were estimated as follows:
where Cmilk is the analyte concentration in milk, Vmilk is the estimated volume of the milk ingested by the nursing infant (150 ml kg−1 day−1) [17] and FBF is the percentage of breast-feeding reported by mother. The relative infant dose was calculated from the expression:
Data are reported as mean with 95% confidence interval unless otherwise indicated. Statistical analysis was performed using unpaired and paired t-tests. Linear regression analysis was conducted using the least squares method. The level of significance was P < 0.05.
Results
Table 1 gives the clinical details of the women taking fluoxetine (n = 9) during pregnancy. The daily dose of fluoxetine ranged from 10 to 30 mg.
Table 1.
Clinical details of the subjects recruited during pregnancy and the postpartum period (mean and 95% confidence intervals)
| Pregnancy | Postpartum | |
|---|---|---|
| Number | 9 | 23 |
| Maternal age (years) | 32.2 (29.3, 35.1) | 33.0 (31.4, 34.7) |
| Maternal weight (kg) | 74.1 (61.1, 87.2) | 65.3 (61.1, 69.5) |
| Drug dose (mg day−1) | 20.0 (16.7, 23.2) | 21.0 (18.0, 24.0) |
| Duration of therapy (months) | 4.0 (2.0, 6.0) | 6.8 (2.7, 10.9) |
| Third trimester sampling (week) | 32.3 (26.4, 38.1) | |
| Gestation length (weeks) | 39.0 (38.3, 39.7) | |
| Postnatal age (m) | 3.7 (2.7, 4.8) | |
| Infant weight (kg) | 6.4 (5.6, 7.3) | |
| Birth weight (g) | 3370 (3172, 3568) | |
| Time of PKU test (h) | 50.8 (41.4, 60.2) |
Table 2 gives the mean concentrations of fluoxetine and norfluoxetine isomers in maternal venous serum in the third trimester, in maternal and umbilical venous serum at delivery, and in the infants at the time of phenylketonuria testing. In all these groups, the mean norfluoxetine concentration was greater than the fluoxetine concentration and the mean difference between the concentrations was 46.8 µg l−1 (23.5, 70.2). The concentrations of total racemic fluoxetine and norfluoxetine tended to be higher in the third trimester compared with those at delivery. However, the intrasubject variability was great and no differences were statistically significant. At delivery, the total racemic fluoxetine concentration ranged from 4.4 to 101.9 µg l−1 in maternal serum and from below the assay limit in one sample to 94.3 µg l−1 in the foetus.
Table 2.
Mean concentrations (µg l−1) and 95% confidence intervals for the racemates (FX, NFX) and the R and S enantiomers of fluoxetine (RFX, SFX) and norfluoxetine (RNFX, SNFX) in maternal (M) serum during the third trimester and delivery in foetal umbilical serum at delivery and in infant serum 48 h after birth (n = 9). The F : M, S : R and infant : foetus concentration ratios for the isomers and racemates are also presented
| RFX | SFX | FX | S : R ratio | |
|---|---|---|---|---|
| Fluoxetine | ||||
| M (third trimester) | 20.0 (7.3, 32.7) | 41.0 (13.7, 68.3) | 61.0 (21.3, 100.6) | 1.93 (1.47, 2.39)* |
| M (Delivery) | 10.9 (6.3, 15.5) | 27.7 (8.4, 47.0) | 38.5 (15.1, 62.1) | 2.06 (1.31, 2.82)* |
| Foetus (cord blood) | 8.9 (5.2, 12.7) | 32.4 (11.6, 53.10 | 41.3 (16.9, 59.8) | 3.01 (2.05, 3.97)*# |
| F : M ratio | 0.84 (0.61, 1.07) | 1.11 (0.95, 1.27)@ | 0.91 (0.60, 1.02) | |
| Infant at 48 h after birth | 9.0 (4.7, 13.3) | 31.3 (14.6, 48.0) | 36.4 (16.7, 56.0) | 3.24 (2.20, 4.39) |
| Infant : foetus ratio | 0.99 (0.78, 1.19) | 1.01 (0.72, 1.29) | 1.01 (0.79, 1.23) | |
| RNFX | SNFX | NFX | S : R ratio | |
|---|---|---|---|---|
| Norfluoxetine | ||||
| M (third trimester) | 32.6 (19.8, 1.19) | 80.6 (41.8, 119.3) | 113.1 (63.5, 162.8) | 2.48 (1.75, 3.20)* |
| M (Delivery) | 22.4 (15.8, 29.0) | 52.2 (24.4, 80.0) | 74.6 (41.7, 107.4) | 2.20 (1.45, 2.96)* |
| Foetus (cord blood) | 17.5 (13.5, 21.5) | 61.0 (27.3, 94.6) | 78.5 (42.3, 114.6) | 3.25 (1.86, 4.84)*# |
| F : M ratio | 0.84 (0.71, 0.97)* | 1.15 (1.06, 1.25)*† | 1.04 (0.93, 1.15) | |
| Infant at 48 h after birth | 22.7 (17.7, 27.7) | 71.7 (39.3, 104.1) | 94.4 (57.3, 131.4) | 2.90 (2.06, 3.75)* |
| Infant : foetus ratio | 1.28 (1.10, 1.47)* | 1.21 (0.98, 1.44) | 1.23 (1.04, 1.41)* | |
Significantly different from a ratio of 1
significantly different from the maternal values (paired t-test)
significantly different from the corresponding R isomer value (paired t-test).
In both the mother and foetus, the disposition of fluoxetine and norfluoxetine enantiomers was stereoselective, with the S : R ratios all being significantly greater than 1 (Table 2). The relationships between R and S isomers of fluoxetine and norfluoxetine in the individual paired maternal and foetal values at delivery are shown in Figure 1. Overall, there was a strong correlation between the maternal and foetal concentrations of the fluoxetine and norfluoxetine isomers. However, the regression coefficients for the S isomers of fluoxetine (1.00 [0.85, 1.14]) and norfluoxetine (1.21 [1.10, 1.40]) were higher than the corresponding values for the R isomers (R-fluoxetine 0.74 [0.43, 1.04]; R-norfluoxetine 0.52 [0.26, 0.79]), and for norfluoxetine this difference was statistically significant. These findings were consistent with the significantly higher F : M concentration ratios for S-fluoxetine and S-norfluoxetine, compared with the corresponding R isomers (Table 2).
Figure 1.
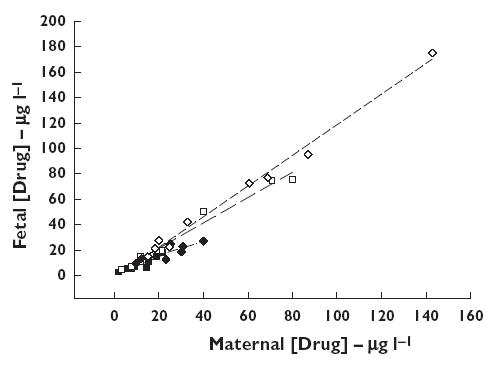
The relationship between maternal venous and foetal cord venous concentrations of fluoxetine (FX) and norfluoxetine (NFX) enantiomers at delivery. RFX (▪), SFX (□), RNFX (♦), SNFX (⋄)
The foetal vs. maternal S : R ratios for fluoxetine and norfluoxetine are shown in Figure 2. There was a strong relationship between the foetal and maternal S : R ratios. The slope of the regression line was 1.61 (1.23, 2.01), which was significantly greater than unity, and the mean foetal minus maternal S : R ratios for both fluoxetine (0.77 [0.34, 1.18]) and norfluoxetine (1.05 [0.13, 1.97]) were significantly greater than zero.
Figure 2.
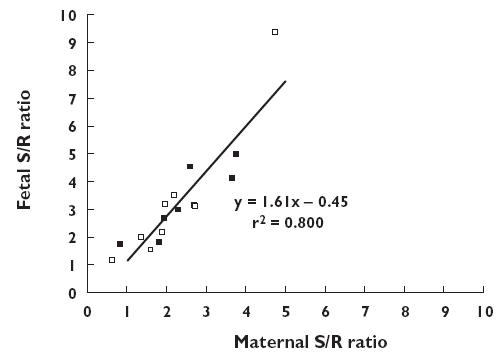
The relationship between the S : R fluoxetine (FX) (▪) and norfluoxetine (NFX) (□) enantiomer ratios in maternal and foetal serum at delivery
Table 2 shows the mean values for the serum fluoxetine and norfluoxetine enantiomer concentrations in the infants at the time of phenylketonuria testing, i.e. ∼48 h after birth. For both drug and metabolite, there was no decrease in the concentration of the enantiomers between birth and 48 h. There was a tendency for the concentration of R and S norfluoxetine to increase, and for the R form the 48 h : cord ratio of 1.28 (1.10, 1.47) was significantly greater than 1. This was associated with a significant increase in the R-norfluoxetine : R-fluoxetine ratio at 48 h after birth (3.88 [1.81, 5.96]) compared with the cord blood samples (2.67 [1.25, 4.49]), the mean difference being 1.09 (0.27, 1.91). For the S-enantiomeric pair, the difference in the S-norfluoxetine : S-fluoxetine ratio between the 48 h and cord blood samples was 0.03 (−0.82, 0.88) which was not statistically significant. The S : R ratios for fluoxetine (3.24 [2.20, 4.39]) and norfluoxetine (2.90 [2.06, 3.75]) in the samples taken 48 h after birth were similar to the values obtained in cord serum (Table 2).
Table 1 shows the clinical details of the mothers studied postnatally. There were 23 subjects, seven of whom were studied on two separate occasions, usually at 1 and 2 months after delivery. The time following birth at which samples were collected ranged from 6 days to 11 months (mean 3.7 [2.7, 4.8]). Table 3 shows the concentrations of the fluoxetine and norfluoxetine enantiomers in maternal serum and breast milk. The concentrations of racemic fluoxetine and norfluoxetine were significantly higher in maternal serum in the postnatal period compared with those obtained at delivery (Table 2), and this was also the case for the enantiomers. The percentage increase in drug concentrations from delivery to the postnatal period was 259% and 106% for fluoxetine and norfluoxetine, respectively. A similar trend was observed in four of the five mothers sampled both during pregnancy and in the nursing period, with concentrations increasing by 310% and 75% for the parent drug and metabolite, respectively. In the remaining subject, the postnatal values were less than those measured during pregnancy. As indicated in Table 3, the mean fluoxetine S : R ratio (2.91 [2.46, 3.36]) was higher than that from the mothers sampled during the third trimester (1.93 [1.47, 2.39]) and at delivery (2.06 [1.31, 2.82]) (Table 1), with the former difference being statistically significant. A significant difference was also observed in the five women sampled during and following pregnancy, with their mean S : R values being 1.86 (1.09, 2.63) and 3.10 (2.58, 3.63), respectively.
Table 3.
Mean concentrations (µg l−1) and 95% confidence intervals for fluoxetine (FX) and norfluoxetine (NFX) enantiomers in maternal serum and breast milk, the milk : serum concentration ratio and estimated infant FX and NFX doses (n = 30)
| RFX | SFX | FX | S : R FX | |
|---|---|---|---|---|
| Fluoxetine | ||||
| Mother | 32.4 (24.3–40.6) | 100.5 (69.3–131.8) | 132.9 (94.9–171.0) | 2.91 (2.46–3.36)* |
| Milk | 26.8 (17.8–35.7) | 50.4 (33.7–67.2) | 77.2 (52.1–102.3) | 1.92 (1.66–2.19)*# |
| Milk : serum ratio | 0.84 (0.67–1.01) | 0.54 (0.45–0.64)* | 0.62 (0.50–0.73)* | |
| Infant dose@ | 0.54 (0.37–0.71) | 1.02 (0.69–1.35)† | 1.56 (1.08–2.04) | |
| Infant dose (%) | 0.34 (0.23–0.45) | 0.63 (0.44–0.81)† | 0.54 (0.37–0.71) | |
| RNFX | SNFX | NFX | S : R NFX | |
|---|---|---|---|---|
| Norfluoxetine | ||||
| Mother | 42.3 (31.6–53.1) | 109.3 (81.4–137.2) | 151.6 (114.7–188.6) | 2.78 (2.28–3.27)* |
| Milk | 32.3 (22.7–41.8) | 49.0 (36.9–61.1) | 81.3 (60.6–101.8) | 1.86 (1.53–2.19)*# |
| Milk : serum ratio | 0.75 (0.68–0.86)* | 0.55 (0.44–0.67)* | 0.60 (0.50–0.71)* | |
| Infant dose@ | 0.68 (0.46–0.90) | 1.03 (0.72–1.33)† | 1.69 (1.18–2.21) | |
| Infant dose (%) | 0.43 (0.30–0.56) | 0.71 (0.50–0.92)† | 0.57 (0.41–0.57) | |
µg kg−1 day−1, %, infant dose as a percentage of the maternal dose
significantly different from unity
significantly different from the value in maternal plasma
significantly different from the corresponding R isomer value.
The mean concentrations of the fluoxetine and norfluoxetine isomers in breast milk were lower than those in serum (Table 3), with the milk : serum (M : P) concentration ratios ranging from 0.54 (0.45, 0.64) to 0.84 (0.67, 1.01). FBF, the percentage of breastfeeding reported by the mothers averaged 91% (89, 97), with 23 of 30 mothers breast-feeding exclusively. As with plasma, stereoselective disposition of fluoxetine and norfluoxetine was observed in breast milk (Table 3). However, the S : R ratios for both the intact drug (1.92 [1.66, 2.19]) and metabolite (1.86 [1.53, 2.19]) were significantly lower than the corresponding values for serum (2.91 [2.46, 3.36] and 2.78 [2.28, 3.27], respectively). The higher concentrations of S-fluoxetine and S-norfluoxetine in breast milk resulted in the daily exposure of these isomers being significantly higher than that of the R isomers (Table 3). The sum of the daily doses of R-fluoxetine, S-fluoxetine and S-norfluoxetine, (the three isomers with the majority of the biological activity [13]), was 2.51 µg kg−1 day−1 (1.82, 3.20) and the total dose of drug and metabolite, as a percentage of the maternal fluoxetine dose averaged 1.11% (0.81, 1.40). Figure 3 shows the relationship between the infant : maternal (I : M) ratios for the sum of R-fluoxetine, S-fluoxetine, and S-norfluoxetine serum concentrations and postnatal age. The data include only those infants with detectable serum concentrations of fluoxetine and/or norfluoxetine. The number of such infants as a proportion of the total decreased with increasing postnatal age, being 6/7 over the first month, 6/8 over the second month and 2/14 thereafter. As shown in Figure 3, for those infants with detectable drug and/or metabolite concentrations, the exposure was greatest in the first month and fell rapidly thereafter. The infant/mother concentration ratio for the sum of these moieties averaged 0.134 (0.069, 0.199). Stereoselective disposition of fluoxetine and norfluoxetine was observed in the infants. Over the first month, the S : R ratio for fluoxetine was 2.7 (0.7, 4.6) and for norfluoxetine 3.3 (1.8, 4.7). Over the second month, the ratios were 2.9 (n = 2) and 2.8 (1.5, 4.2) for parent drug and metabolite, respectively. In infants older than 2 months, only norfluoxetine could be detected and the S : R ratio was 1.4 (n = 2).
Figure 3.
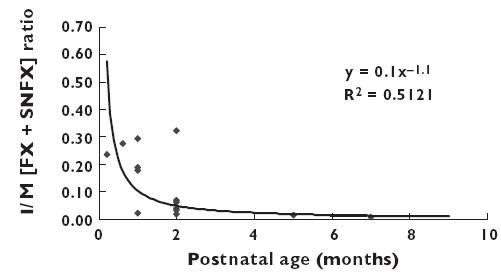
The relationship between the maternal : infant serum concentration ratios for the sum of R-fluoxetine (RFX), S-fluoxetine (SFX) and S-norfluoxetine (SNFX) and postnatal age
Discussion
In the current study, we have found clear evidence for the stereoselective disposition of fluoxetine in pregnancy and the postpartum period in both the mother and infant and in breast milk as well. Previously, we have reported this phenomenon in pregnant sheep [18], with higher maternal and foetal plasma S-fluoxetine concentrations compared with R-fluoxetine being observed, which was associated with a lower maternal systemic clearance and volume of distribution for the S isomer. These differences were attributed largely to differential plasma protein binding of the enantiomers, with the free fractions of R-fluoxetine being 4.8 (2.1, 7.5) and 10.3 (7.9, 12.7) in the ewe and foetus, respectively, and those of S-fluoxetine being 2.2 (1.0, 3.4) and 5.8 (3.1, 8.5). The free fractions of these enantiomers were higher in the foetus than in the ewe because of lower plasma protein concentrations. We have also observed a similar difference in the S : R ratio for fluoxetine protein binding in serum samples from non-pregnant women studied in vitro (J. Kim, D. Rurak, K. Riggs, unpublished data). Whether a comparable difference occurs in the human foetus and neonate has not yet been determined. In the human and sheep, the protein binding of R-norfluoxetine and S-norfluoxetine also differs to a similar extent. The stereoselective disposition of these compounds in plasma presumably explains their stereoselective disposition in breast milk.
Stereoselective metabolism may be partially responsible for the differences in serum drug concentrations of fluoxetine enantiomers. The hepatic metabolism of fluoxetine to norfluoxetine, the primary metabolite, is catalyzed by CYP2D6 and CYP2C9/2C19 [19, 20]. Fluoxetine disposition is stereoselective in humans following a single oral dose, but only in subjects with a deficiency in CYP2D6 [21, 22]. However, fluoxetine and norfluoxetine are inhibitors of CYP2D6, with the S isomers being more potent than the R forms [22, 23]. Thus, the fluoxetine and/or norfluoxetine-mediated inhibition of this enzyme would decrease the metabolism of S-fluoxetine, since CYP2D6 is primarily responsible for its metabolism [20]. In contrast, the metabolism of R-fluoxetine would be minimally affected since it is a substrate for CYP2C9/2C19 [20], resulting in a higher clearance and lower serum concentrations compared with the S-isomer.
The current study provides some evidence that decreased or absent CYP2D6 activity may enhance the stereoselective disposition of fluoxetine in both the mother and offspring. Maternal serum concentrations of fluoxetine and norfluoxetine were higher in the postnatal period than during pregnancy. A similar finding has been recently reported by Heikkinen et al.[24]. The lower maternal drug concentrations during pregnancy could be due to several of the physiological changes that occur, including increased blood volume, hepatic and renal blood flows, increased glomerular filtration rate, and decreased plasma protein concentrations [25], as well as induction of CYP2D6 and other drug metabolizing enzymes during gestation [26]. That latter process is consistent with the significant increase in racemic fluoxetine concentrations and in the S : R isomer ratio in the postpartum period compared with pregnancy.
The amounts of CYP2C and CYP2D6 are low or nondetectable in foetal liver, but increase progressively after birth [27]. Our findings of the nearly identical maternal and foetal fluoxetine enantiomer concentrations at delivery, in the cord and 48 h later, are consistent with a limited capacity of the foetus and neonate to eliminate the drug, even when the long elimination half-life of fluoxetine in adults (1–4 days) [12] is considered. However, the increase in R-norfluoxetine concentration and the R-norfluoxetine : R-fluoxetine ratio in the offspring between delivery and 48 h of life suggest that some CYP2C activity may be present in late gestation and the neonatal period, but might only become evident after birth, when the offspring become free from maternal influences. The presence of CYP2C may also have contributed to the elevated fluoxetine S : R ratio in the foetus and newborn compared with the mother. Our results for fluoxetine and norfluoxetine in the neonate are similar to those reported by Heikkinen et al.[24], who found 48 h : cord concentration ratios of 1.06 and 1.03, respectively. In addition, in three infants who were exposed to fluoxetine in utero and sampled at delivery and 5 days after birth, the decrease in plasma drug concentrations between these sampling times averaged only 46%[28].
Previous studies of maternal and foetal fluoxetine and norfluoxetine concentrations at delivery have reported lower values in the foetus compared with the mother, with the F : M concentration ratios ranging from 0.61 to 0.68 for fluoxetine and from 0.65 to 0.71 for norfluoxetine [24, 28, 29]. All these values are lower than those obtained in the current study, a difference which is unexplained. All the estimates were based upon maternal and cord blood samples collected at a single time point in each subject, i.e. at delivery. Foetal and maternal serum drug concentrations can vary markedly with time after drug administration, especially when they are not at steady-state [30]. However, during chronic fluoxetine dosing, there is a minimal variation in plasma drug concentrations during the day, even with a once daily dosing regimen [14, 31]. Thus, near steady-state conditions apply, and fluctuations in the foetal and maternal drug concentrations with time cannot explain the differing estimates of foetal exposure to fluoxetine and norfluoxetine between our study and those of others [24, 28, 29]. All studies involved relatively small numbers of subjects (4–15), and thus these differences might be due to intersubject variability. However, the data overall indicate significant exposure of the human foetus to both fluoxetine and norfluoxetine, which is consistent with the lipophilic nature of the drug [32].
Stereoselective disposition of fluoxetine and norfluoxetine in breast milk was also observed and is likely to be due to the differential protein binding of the enantiomers in maternal plasma. The S : R ratios for the drug and metabolite in milk were significantly lower than the corresponding values for plasma. Differences in the extent of protein binding of the enantiomers in milk compared with plasma could underlie this discrepancy. However, the degree to which fluoxetine and norfluoxetine are bound to the protein components of breast milk has not been determined.
Our estimates of the amount of drug present in breast milk are within the ranges reported by others [24, 33–37]. The excretion of drugs into breast milk delivers relatively small doses, both in absolute terms and in comparison with the maternal dose. The amounts for fluoxetine, norfluoxetine and combined drug and metabolite are somewhat lower than previously published values (fluoxetine plus norfluoxetine ∼3%[24], fluoxetine alone 3.4%[33] and 6.5%[36]). Our estimates of the infant dose are corrected for the percentage of breast-feeding (FBF) reported by the mothers, whereas this was not the case in the previous reports. However, as FBF averaged 91% (84, 97), this factor cannot explain our lower estimate for the relative infant dose of fluoxetine. One possibility is that variations in milk collection protocols may explain the differences.
Overall, the stereoselective disposition of fluoxetine has a larger influence on the foetus and infant than on the mother with respect to the potential pharmacological actions of the drug. Owing to stereoselective disposition in the mother, the foetus and infant receive higher doses of S-fluoxetine and S-norfluoxetine than of the R isomers. This difference is further increased by stereoselective disposition in the foetus and infant, which may involve differences in the free fractions of the R and S isomers, and perhaps some preferential metabolism of the R isomer. Thus, the S : R ratios for both fluoxetine and norfluoxetine in the foetus are significantly higher than in the mother, resulting in increased exposure to the biologically active S-norfluoxetine, as well as to the enantiomers of fluoxetine both of which are active.
In conclusion, the results of this study confirm the extensive placental transfer of fluoxetine and norfluoxetine in pregnancy and the persistence of the drug and metabolite in newborn serum following birth. Based on the correlation between the drug concentrations in maternal serum and breast milk, the infant dose will be largely determined by the concentration of the maternal drug, and this in part will depend upon the maternal dose. In addition, there is stereoselective disposition of fluoxetine and norfluoxetine, resulting in increased concentrations of the biologically active enantiomers, particularly S-norfluoxetine in the foetus and infant compared with the mother.
Acknowledgments
The authors gratefully acknowledge financial support from BC Medical Services Foundation and thank Annie Kuan, Yoon-hee Kim, Karen Lo and Wendy Lee for their assistance during the study.
References
- 1.Nonacs R, Cohen LS. Depression during pregnancy: diagnosis and treatment options. J Clin Psychiatry. 2002;63(Suppl 7):24–30. [PubMed] [Google Scholar]
- 2.Cooper PJ, Murray L. Postnatal depression. BMJ. 1998;316:1884–6. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson C, Sydsjö G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol. 2004;104:459–66. doi: 10.1097/01.AOG.0000136087.46864.e4. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159:2055–61. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010–5. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 6.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of selective serotonin reuptake inhibitors during pregnancy on serotoninergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–6. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 7.Oberlander TF, Misri S, Fitzgerald C, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65:230–7. doi: 10.4088/jcp.v65n0214. [DOI] [PubMed] [Google Scholar]
- 8.Sanz E, De-Ias-Cuevas C, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:2005. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 9.Oberlander TF, Grunau RE, Fitzgerald C, Ellwood AL, Misri S, Rurak D, Riggs KW. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediat Res. 2002;51:443–53. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Oberlander TF, Ruth Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–25. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- 11.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders – I. Basic pharmacology. J Psychopharmacol. 1998;12(Suppl B):S5–S20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 12.Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 13.Fuller RW, Snoddy HD, Krushinski JH, Robertson DW. Comparison of norfluoxetine enantiomers as serotonin reuptake inhibitors in vivo. Neuropharmacol. 1992;31:997–1000. doi: 10.1016/0028-3908(92)90100-4. [DOI] [PubMed] [Google Scholar]
- 14.De Vane CL. Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cell Molec Neurobiol. 1999;19:443–66. doi: 10.1023/A:1006934807375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Axelson JE, Kerns GL, Yin W, Rurak DW. Stereoselective determination of fluoxetine and norfluoxetine by gas chromatography with mass selective detection. Pharm Res. 1995;12:S22. [Google Scholar]
- 16.Kim J, Burton R, Rurak DW, Riggs KW. Stereoselective quantitation of fluoxetine and norfluoxetine in plasma by LC/MS/MS. Pharm Sci. 1998;1:S185. [Google Scholar]
- 17.Pons G, Rey E, Matherson I. Excretion of psychotropic drugs into breast milk. Clin Pharmacokinet. 1994;27:270–89. doi: 10.2165/00003088-199427040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Riggs KW, Rurak DW. Stereoselective pharmacokinetics of fluoxetine and norfluoxetine enantiomers in pregnant sheep. Drug Metab Disp. 2004;32:1–10. doi: 10.1124/dmd.32.2.212. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZQ, Shu Y, Wang LS, He N, Zhou HH. Effect of CYP2C19 genotype and CYP2C9 on fluoxetine N-demethylation in human liver microsomes. Acta Pharmacol Sin. 2001;22:85–90. [PubMed] [Google Scholar]
- 20.Ring BJ, Eckstein JA, Gillespie JS, Binkley SN, Vanderbranden M, Wrighton SA. Identification of the human cytochromes P450 responsible for in vitro formation of R- and S- norfluoxetine. J Pharmacol Ther. 2001;297:1044–50. [PubMed] [Google Scholar]
- 21.Fjordside L, Jeppensen U, Eap CB, Powell K, Baumann P, Brosen K. The stereoselective metabolism of fluoxetine in poor and extensive metabolizers of sparteine. Pharmacogenetics. 1999;9:55–60. doi: 10.1097/00008571-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hamelin BAJ, Turgeon F, Vallee Belanger PM. The disposition of fluoxetine but not sertraline is altered in poor metabolizers of debrisoquin. Clin Pharmacol Ther. 1996;60:512–21. doi: 10.1016/S0009-9236(96)90147-2. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JC, Wrighton SA. Interaction of the enantiomers of fluoxetine and norfluoxetine with human liver cytochromes P450. J Pharmacol Exp Ther. 1993;226:964–71. [PubMed] [Google Scholar]
- 24.Heikkinen T, Ekblad U, Palo P, Laine K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73:330–7. doi: 10.1016/s0009-9236(02)17634-x. [DOI] [PubMed] [Google Scholar]
- 25.Bocking A. Maternal adaptations to pregnancy. In: Harding R, Bocking A, editors. Fetal Growth, Development. Cambridge UK: Cambridge University Press; 2001. pp. 224–40. [Google Scholar]
- 26.Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–7. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 27.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: Phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–60. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 28.Rampono J, Proud S, Hackett LP, Kristensen JH, Ilett KF. A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmacol. 2004;7:329–34. doi: 10.1017/S1461145704004286. [DOI] [PubMed] [Google Scholar]
- 29.Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 30.Rurak DW, Wright MR, Axelson JE. Drug disposition and effects in the fetus. J Dev Physiol. 1991;15:33–44. [PubMed] [Google Scholar]
- 31.Gupta S, Banfield C, Kantesaria B, Flannery B, Herron J. Pharmacokinetics/pharmacodynamics of desloratadine and fluoxetine in health volunteers. J Clin Pharmacol. 2004;44:1252–9. doi: 10.1177/0091270004269518. [DOI] [PubMed] [Google Scholar]
- 32.Drayton CJ. Cumulative Subject Index and Drug Compendium. 1. Vol. 6. Oxford, UK: Pergamon Press; 1990. Comprehensive Medicinal Chemistry. [Google Scholar]
- 33.Kristensen JH, Ilett KF, Hackett LP, Yapp R, Paech M, Begg EJ. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol. 1999;48:521–7. doi: 10.1046/j.1365-2125.1999.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida K, Smith B, Craggs M, Kumar RC. Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br J Psychiatry. 1998;172:175–9. doi: 10.1192/bjp.172.2.175. [DOI] [PubMed] [Google Scholar]
- 35.Suri R, Stowe N, Hendrick V, Hostetter A, Widawski M, Altshuler LL. Estimated of nursing infant daily dose of fluoxetine in breast milk. Biol Psychiatry. 2002;52:446–51. doi: 10.1016/s0006-3223(02)01368-9. [DOI] [PubMed] [Google Scholar]
- 36.Taddio A, Ito S, Koren G. Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol. 1996;36:42–7. doi: 10.1002/j.1552-4604.1996.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 37.Hendrick V, Stowe ZN, Altshuler LL, Mintz J, Hwang S, Hostetter A, Suri R, Leight K, Fukuchi A. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50:775–82. doi: 10.1016/s0006-3223(01)01197-0. [DOI] [PubMed] [Google Scholar]


