Abstract
Aim
To investigate the possible effects of pentoxifylline metabolites on retinal blood flow in humans.
Methods
A randomized, placebo-controlled, four-period cross-over study that was observer blinded and partly blinded for the eight participants. On one occasion a placebo was given as an intravenous (i.v.) infusion over 100 min. On the other three occasions pentoxifylline was administered as i.v. infusions over 100 min at a rate of 3 mg min−1. Before two of the pentoxifylline infusions the subjects were pretreated with either ciprofloxacin or rifampicin. Retinal blood flow was measured by scanning laser doppler flowmetry (SLDF) in a selected area of the central temporal retina before, during and until 5 h after the end of infusion. Blood samples for concentration analyses of pentoxifyllin, R-M1, S-M1, M4 and M5 were taken serially and areas under the curves (AUCs) were calculated. Linear mixed models were used for the statistical analyses.
Results
Mean AUCs (ng h ml−1) were significantly increased for pentoxifylline (1964 vs. 1453) and S-M1 (5804 vs. 4227), but not R-M1 when pentoxifylline was co-administered with ciprofloxacin. The mean AUC for M5 was significantly reduced when subjects were pretreated with rifampicin (2041 vs. 3080). Pentoxifylline with and without pretreatment with rifampicin significantly increased retinal blood flow assessed as mean flow, pulsation (i.e. 1-systole/diastole), and diastolic flow (but not during systole), compared with placebo. The increases over placebo were more pronounced on diastolic flow, 9.7% (95% confidence interval 4.2, 15.5) than on mean flow, 4.6% (1.1, 8.3) after pentoxifylline administration. With pentoxifylline after rifampicin pretreatment the corresponding differences were 11.7% (5.8, 17.9) and 5.1% (1.4, 7.8) over placebo, respectively. After co-administration of pentoxifylline and ciprofloxacin we saw only a nonsignificant trend towards increased flow during diastole, but a significant decrease in pulsation. When AUCs for pentoxifylline and its metabolites were used as regressor variables to retinal mean flow we found that pentoxifylline, R-M1 and M5 had coefficients with a positive sign indicating that they enhanced the retinal blood flow. In contrast, S-M1 and M4 had coefficients with negative sign and thus appeared to decrease the blood flow in subjects treated with pentoxifylline.
Conclusion
The R-M1 and M5 metabolites of pentoxifylline contributed significantly to the effects of pentoxifylline on retinal blood flow.
Keywords: ciprofloxacin, metabolite effects, pentoxifylline, pharmacokinetics, retinal blood flow, rifampicin
Introduction
Pentoxifylline, 3,7-dimethyl-1(5′-oxo-hexyl)xanthine, is a haemorheological drug widely used for the treatment of intermittent claudication [1–3]. However, the clinical efficacy of the drug for this indication is still controversial. It has also been suggested that pentoxifylline may be used in diseases affecting retinal blood flow, such as diabetic retinopathy [4–7] or macular degeneration [8]. The therapeutic effect of pentoxifylline in these conditions would be to increase capillary blood flow by increasing deformability of both erythrocytes and leucocytes as well as by a possible direct vasodilatory effect.
Pentoxifylline is metabolized in humans into at least seven metabolites, denoted M1–M7 [9–12]. Reduction of the 5′-oxo group gives the hydroxy metabolite M1, 3,7-dimetyl-1(5′hydroxyhexyl)xanthine. This creates a chiral centre in the molecule, yielding S-M1 as a major and R-M1 as minor metabolite [13, 14]. This metabolism takes place both in the liver [13] and in the erythrocytes [14, 15] and is reversible [10, 13, 14]. Thus, during treatment with pentoxifylline the plasma concentrations of the parent drug, R-M1 and S-M1 rapidly attain equilibrium. Hepatic metabolism produces the carboxylic acid metabolites M4 and M5; M5 is the major excreted form [9–12]. Both M1 (as the racemic form) and M5 have been shown to influence erythrocyte deformability and platelet aggregation in vitro and may thus contribute to the in vivo haemorheological effects of pentoxifylline [16].
The aim of this study was to investigate the possible contributions of the metabolites (M1, M4 and M5) of pentoxifylline to its haemorheological effect, assessed as retinal blood flow, in humans. Scanning laser doppler flowmetry (SLDF) [17] in a selected area of the central temporal retina was used to investigate drug effects on blood flow in small capillaries. When pentoxifylline is administered there is considerable co-variation between the concentrations of pentoxifylline and its metabolites [14, unpublished]. This makes it very difficult to separate any effects of the metabolites from that of the parent compound. In this study we therefore co-administered pentoxifylline and an inhibitor of CYP1A2 (ciprofloxacin) on one occasion [18, 19], and gave pentoxifylline after pretreatment with an inducer of several enzymes of the cytochrome P450 system (rifampicin) on another occasion [20], in order to create different blood concentration ratios between the metabolites and pentoxifylline in the same subject. The study was placebo controlled with a cross-over design using four treatment arms.
Methods
Study design
The study was approved by the Ethics Committee of Lund University and by the Swedish Medical Products Agency. After giving written informed consent, eight healthy, nonsmoking volunteers (six males, 24–42 years, 73–100 kg, and two females, 21–24 years, 65–66 kg), who were free of medication and had no history of allergy to drugs, were included in the study. Each subject passed a prestudy ophthalmic examination, where inclusion criteria were normal ocular findings, no previously known eye pathology, full visual acuity and ametropia less than 3 diopters. The study was randomized, placebo controlled, observer blinded, and partly blinded for the subject in a four-period cross-over design.
All subjects were given the four treatments in random order. During one session they were given placebo (0.9% saline solution) by intravenous (i.v.) infusion over 100 min. During the three other sessions they were given pentoxifylline (Trental, Hoechst Marion Roussel) by i.v. infusion over 100 min at a rate of 3 mg min−1. The volumes infused and the concentrations of pentoxifylline in the solution were measured. In one session the subjects were pretreated with a 750-mg ciprofloxacin tablet (Ciproxin, Bayer) 1 h before start of the infusion. Before another they took one rifampicin 600-mg tablet (Rimactan, Swedish Orphan) daily for 7 days, the last dose being taken approximately 24 h before the start of the pentoxifylline infusion.
The pretreatments with ciprofloxacin and rifampicin could not be blinded but the pentoxifylline and placebo administrations were.
The subjects had fasted since 22.00 h the evening before the study day and were not allowed to take any other medication except for occasional paracetamol 48 h preceding a study day and during the pretreatment with rifampicin. They were given a light meal 4 h after the start of the infusion. The study periods were separated by at least 1 week and after the pretreatment with rifampicin by 2 weeks. Adverse events were assessed before and during the study days by open-ended questions.
Analysis of pentoxifylline and its metabolites
Blood was sampled from an indwelling venous catheter (in the opposite arm from the infusion) into sodium heparin Venoject® tubes. The sampling times were: before and at 20, 45 and 85 min during the infusion period and at 0.5, 1, 2, 3, 4 and 5 h after its termination (in the first four subjects also at 6 h). In order to minimize ex vivo interconversion of pentoxifylline and M1 the samples were immediately centrifuged at 4 oC and the plasma was collected and frozen [14].
Concentrations of pentoxifylline and metabolites R-M1, S-M1, M4 and M5 in plasma were determined by high-performance liquid chromatography (HPLC) as previously described [14]. From the plasma concentration data, the terminal half-lives of pentoxifylline and its metabolites were estimated using the RSTRIP software (MicroMath, Salt Lake City, UT, USA) and used for extrapolation of the areas under the curves (AUCs). The AUCs were calculated by the logarithmic trapezoidal method from 0 to infinity using the MKMODEL software (Biosoft, Cambridge, UK).
Measurement and analysis of retinal blood flow
Retinal blood flow was assessed using the SLDF at 670 nm (Heidelberg Retina Flowmeter, Heidelberg Engineering) [17, 21]. The method of SLDF provides a high-definition tomographic image of perfused retinal vessels with simultaneous evaluation of blood flow using an optical Doppler effect. The measurements were performed in a selected area of the central temporal retina, and one picture included an area of 2.7 × 0.7 mm, at baseline, at 45 and 85 min during the infusion period and at 0.5, 1, 2, 3, 4 and 5 h after termination (in the first four subjects also at 6 h). At all the time points for measurements four pictures of the same area were taken and the three technically best ones were assessed.
Quantification of capillary retinal blood flow was stated in arbitrary units (AUs) describing the product of mean flow velocity and mean quantity of blood cells in a standardized volume. The mean values from each time point were used in the calculations. The readings were analysed using the AFFPIA software (automatic full field perfusion image analyser program) [21]. The variables used were mean blood flow, blood flow during systole, blood flow during diastole, and pulsation (i.e. 1-diastole/systole).
Data presentation and statistical considerations
The number of subjects recruited was based on the following assumptions. In an earlier study on 10 healthy subjects [7] an i.v. infusion over 90 min of 200 mg of pentoxifylline gave an increase by 17 ± 9% (mean ± SD between subjects) and the 400-mg dose gave an increase by 27 ± 12% in ocular fundus pulsation. We anticipated that a 300-mg dose over 100 min would give a 23 ± 10% increase in ocular fundus pulsation between subjects, compared with placebo. Since, in the current study, we compare the effects within the same subject, we made a series of power calculations assuming different within-subject correlations, number of subjects, and detectable differences, e.g. using eight subjects we would be able to detect a difference of 8% with a power of at least 80%, at a two-tailed P-value <0.05, if the intrasubject correlation was 0.75. This was calculated for a single measurement of maximum effect. When calculations were based on integrated effects (i.e. several measurements over time) the power to detect differences were anticipated to be even better.
The volumes infused and the concentrations of pentoxifylline in the solution were used for calculation of the actual doses given. The resulting concentrations were re-calculated to a nominal dose of 300 mg. In the pharmacokinetic analyses we similarly used AUCs recalculated to a nominal dose of 300 mg. However, AUCs calculated from the observed plasma concentrations were used as input in the concentration–effect analyses described below. For statistical analysis the MIXED procedure in SAS (version 8.2; SAS Institute, Cary, NC, USA) was used. In the analysis of the AUCs we used the treatments given as fixed effects and subjects were entered as random effects.
We found that there were no significant changes over time within each study day. Thus, we used all observations after dose in a repeated measures design. Further, the observed effects were rather low, limiting the possibility for detection of deviations from linear relations between effect and regressor variables. Two main statistical models were tested after an initial check for lack of period effects. The first one comprised only the treatments given as fixed effects, thus disregarding the obtained plasma concentration data. In the second model treatments were not included; instead, the AUCs of pentoxifylline and its metabolites were used as regressor variables. Such a model can be successfully used only if the correlations between the regressors are low. Both models used subjects as random effects, and a repeated measures spatial exponential covariance structure design.
Since one subject dropped out, the study was not completely balanced and for both the kinetic and the dynamic analyses least-square means were obtained from the mixed model, thus providing the marginal means that would be expected had the study been completely balanced with no missing data. Arithmetic least-square means and 95% confidence limits are given, except when relative changes in the effect model comprising treatments as fixed effects are presented, in which case geometric least-square means and 95% confidence limits are given. Statistical significance was accepted at P < 0.05 (two-tailed).
Results
The treatments were generally well tolerated, but with some exceptions. Thus, in one subject (with no pretreatment) we stopped the infusion of pentoxifylline after 50 min due to nausea. All observations have been included in the analysis as appropriate (i.e. actual AUCs in the effect analysis). In another subject the rifampicin pretreatment was stopped after only one dose due to diarrhoea, and the planned infusion of pentoxifylline was therefore not given. Yet another subject did not receive placebo due to logistic reasons. All other drug treatments were administered as scheduled.
Plasma concentration curves of pentoxifylline, R-M1, S-M1, M4 and M5 after i.v. administration of the drug alone and after pretreatment with ciprofloxacin or rifampicin are shown in Figure 1. Observed plasma concentrations were used for the calculation of the AUC values that are given in Table 1. AUC for pentoxifylline and S-M1 were significantly higher after pretreatment with ciprofloxacin compared with pentoxifylline administration alone. The effect on R-M1 was not statistically significant. However, the R : S plasma concentration ratio remained unchanged at mean values of 0.019 after pentoxifylline alone and 0.020 after pentoxifylline and ciprofloxacin (P = 0.72 for the difference between ratios). The AUC for M5 was significantly lower after pretreatment with rifampicin compared with pentoxifylline alone.
Figure 1.

The plasma concentrations (±S.D.) of pentoxifylline (upper left panel) and its metabolites: M1 (upper right panel: R-M1, lower set of curves; S-M1, upper set of curves), M4 (lower left panel) and M5 (lower right panel) during and after intravenous infusion of 300 mg pentoxifylline alone or in combination with ciprofloxacin 750-mg tablet or pretreatment with rifampicin 600-mg tablets once daily for 7 days. The concentrations have been re-calculated to correspond to a nominal dose of 300 mg of pentoxifylline. Circles pentoxifylline alone, triangles pentoxifylline + ciprofloxacin, squares pentoxifylline + rifampicin
Table 1.
Mean [min–max] observed AUC of pentoxifylline (Ptx) and its metabolites (R-M1, S-M1, M4 and M5) after administration of pentoxifylline 300 mg intravenous infusion alone or in combination with a ciprofloxacin (PtxC) 750-mg tablet or pretreatment with rifampicin (PtxR) 600-mg tablets once daily for 7 days
| AUC Ptx, ng h ml−1 | AUC RM1, ng h ml−1 | AUC SM1, ng h ml−1 | AUC M4, ng h ml−1 | AUC M5, ng h ml−1 | ||
|---|---|---|---|---|---|---|
| Observations | Ptx | 1453 | 84 | 4227 | 406 | 3221 |
| [743–2278] | [36–163] | [2402–5911] | [193–833] | [1048–6226] | ||
| PtxC | 1964 | 117 | 5804 | 484 | 3080 | |
| [698–2556] | [53–173] | [4322–7470] | [252–735] | [1986–4044] | ||
| PtxR | 1529 | 78 | 4134 | 281 | 2041 | |
| [961–2427] | [62–125] | [3260–5039] | [212–366] | [1336–2715] | ||
| Least-square estimates | Ptx | 1536 | 86 | 4370 | 430 | 3366 |
| (1045, 1028) | (59, 113) | (3453, 5287) | (293, 567) | (2531, 4201) | ||
| PtxC | 1968 | 116 | 5781 | 488 | 3078 | |
| (1476, 2459) | (90, 143) | (4864, 6698) | (350, 625) | (2243, 3913) | ||
| PtxR | 1434 | 78 | 4065 | 287 | 2078 | |
| (928, 1940) | (49, 106) | (3131, 4999) | (141, 434) | (1193, 2962) | ||
| Differences between the least-square estimates | PtxC-Ptx | 431* | 31 | 1411* | 58 | –288 |
| (56, 809) | (−3, 64) | (858, 1964) | (−132, 247) | (−1265, 690) | ||
| PtxR-Ptx | −103 | −8 | −306 | −143 | −1288* | |
| (−497, 291) | (−43, 27) | (−887, 275) | (−339, 53) | (−2308, 269) | ||
| PtxR-PtxC | −534* | −39* | −1716* | −200* | −1000 | |
| (−928, 140) | (−73, 4) | (−2297, 1135) | (−396, 4) | (−2020, 19) | ||
| Ratios of the least-square estimates | PtxC/Ptx | 1.314* | 1.48* | 1.36* | 1.19 | 1.01 |
| (1.05, 1.64) | (1.02, 2.14) | (1.18, 1.57) | (0.79, 1.78) | (0.76, 1.33) | ||
| PtxR/Ptx | 0.938 | 1.00 | 0.96 | 0.73 | 0.67* | |
| (0.74, 1.19) | (0.68, 1.46) | (0.83, 1.12) | (0.48, 1.12) | (0.50, 0.90) | ||
| PtxR/PtxC | 0.714 | 0.68 | 0.71* | 0.62 | 0.66* | |
| (0.56, 0.90) | (0.46, 1.00) | (0.61, 0.83) | (0.40, 0.93) | (0.49, 0.89) |
Least-square estimates of means (95% confidence intervals) and their differences and ratios for AUC of pentoxifylline and its metabolites after administration of pentoxifylline 300 mg intravenous infusion alone or in combination with a ciprofloxacin 750-mg tablet or pretreatment with rifampicin 600-mg tablets once daily for 7 days. Additionally, for the least-square estimates the AUCs have been re-calculated to correspond to a nominal dose of 300 mg pentoxifylline.
P < 0.05.
The correlation matrix for the AUCs of pentoxifylline and its metabolites is given in Table 2. Generally, the absolute values of the correlations were low, with the exception of that between M4 and M5.
Table 2.
Correlation matrix between observed AUC values for pentoxifylline (Ptx) and its metabolites (R-M1, S-M1, M4, and M5) after administration of pentoxifylline 300 mg intravenous infusion alone and in combination with ciprofloxacin 750-mg tablet and pretreatment with rifampicin 600-mg tablets once daily for 7 days, on three separate occasions
| Intercept | AUC Ptx | AUC R-M1 | AUC S-M1 | AUC M4 | AUC M5 | |
|---|---|---|---|---|---|---|
| Intercept | 1 | −0.04013 | 0.02184 | −0.00039 | 0.02069 | −0.02392 |
| AUC Ptx | −0.04013 | 1 | −0.4204 | −0.5214 | −0.4152 | 0.2249 |
| AUC R-M1 | 0.02184 | −0.4204 | 1 | −0.3811 | 0.07453 | −0.04717 |
| AUC S-M1 | −0.00039 | −0.5214 | −0.3811 | 1 | 0.2451 | −0.3722 |
| AUC M4 | 0.02069 | −0.4152 | 0.07453 | 0.2451 | 1 | −0.8062 |
| AUC M5 | −0.02392 | 0.2249 | −0.04717 | −0.3722 | −0.8062 | 1 |
Retinal blood flow measured as mean flow, flow during systole and diastole, as well as pulsation, before, during and after the four treatments are shown in Figure 2. The corresponding mean values during and after the treatments are given in Table 3, as are least-square means of absolute and relative estimates after the different treatments. Least-square mean values of relative differences between the treatments are given in Table 4. In comparison with placebo, pentoxifylline alone and after pretreatment with rifampicin significantly increased retinal blood flow during diastole (P = 0.0014 and P = 0.0004, respectively) but not during systole (P = 0.1476 and P = 0.4366, respectively). This was reflected in a rise of mean flow and decreased pulsations. Pentoxifylline after pretreatment with ciprofloxacin resulted only in a nonsignificant trend (P = 0.0604) towards increased flow during diastole, but caused a significant (P = 0.0096) decrease in pulsation.
Figure 2.
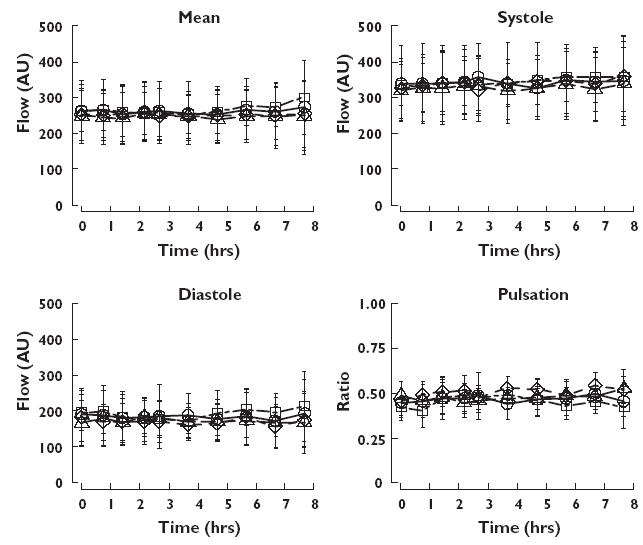
Retinal blood flow (±S.D.) during and after intravenous infusion of 300 mg pentoxifylline alone or in combination with ciprofloxacin 750-mg tablet or pretreatment with rifampicin 600-mg tablets once daily for 7 days, and after intravenous infusion of placebo. Circles pentoxifylline alone, triangles pentoxifylline + ciprofloxacin, squares pentoxifylline + rifampicin, diamonds placebo. Mean flow (upper left panel), flow during systole (upper right panel), flow during diastole (lower left panel), and pulsation (lower right panel)
Table 3.
Mean [min–max] observed retinal blood flow (arbitrary units, AU), measured as mean flow, systole, diastole and pulsation after administration of placebo or pentoxifylline (Ptx) 300 mg intravenous infusion alone or in combination with a ciprofloxacin (PtxC) 750-mg tablet or pretreatment with rifampicin (PtxR) 600-mg tablets once daily for 7 days
| Mean flow AU | Systole AU | Diastole AU | Pulsation | ||
|---|---|---|---|---|---|
| Observations | Placebo | 252 | 339 | 169 | 0.50 |
| [140–472] | [173–614] | [73–395] | [0.23–0.72] | ||
| Ptx | 260 | 343 | 183 | 0.46 | |
| [158–504] | [197–651] | [80–431] | [0.21–0.74] | ||
| PtxC | 248 | 328 | 172 | 0.47 | |
| [118–467] | [204–597] | [73–387] | [0.17–0.71] | ||
| PtxR | 265 | 344 | 188 | 0.45 | |
| [170–452] | [221–606] | [81–358] | [0.16–0.70] | ||
| Geometric least-square estimates | Placebo | 238 | 322 | 157 | 0.50 |
| (199, 284) | (272, 380) | (129, 191) | (0.45, 0.55) | ||
| Ptx | 249 | 331 | 172 | 0.46 | |
| (208, 298) | (280, 390) | (142, 209) | (0.42, 0.50) | ||
| PtxC | 239 | 318 | 165 | 0.46 | |
| (200, 285) | (270, 376) | (136, 200) | (0.42, 0.51) | ||
| PtxR | 250 | 327 | 176 | 0.47 | |
| (209, 299) | (277, 386) | (145, 213) | (0.41, 0.49) | ||
| Arithmetic least-square estimates | Placebo | 245 | 331 | 164 | 0.50 |
| (194, 297) | (271, 391) | (123, 205) | (0.46, 0.55) | ||
| Ptx | 259 | 342 | 181 | 0.47 | |
| (207, 310) | (282, 401) | (140, 222) | (0.439, 0.51) | ||
| PtxC | 247 | 328 | 172 | 0.47 | |
| (195, 298) | (268, 388) | (131, 213) | (0.43, 0.51) | ||
| PtxR | 256 | 332 | 182 | 0.45 | |
| (204, 307) | (273, 392) | (141, 223) | (0.41, 0.50) |
Geometric and arithmetic least-square estimates of means (95% confidence intervals) of retinal blood flow, measured as mean flow, systole, diastole and pulsation after administration of placebo or pentoxifylline 300 mg intravenous infusion alone or in combination with a ciprofloxacin 750-mg tablet or pretreatment with rifampicin 600-mg tablets once daily for 7 days.
Table 4.
Relative differences between least-square estimates of means (95% confidence intervals) of retinal blood flow, measured as mean flow, systole, diastole and pulsation after administration of placebo or pentoxifylline (Ptx) 300-mg intravenous infusion alone or in combination with a ciprofloxacin (PtxC) 750-mg tablet or pretreatment with rifampicin (PtxR) 600-mg tablets once daily for 7 days
| Mean flow % | Systole % | Diastole % | Pulsation % | |
|---|---|---|---|---|
| Ptx–placebo | 4.6* | 2.7 | 9.7* | −7.8* |
| (1.1, 8.3) | (−1.0, 6.7) | (4.2, 15.5) | (−12.3, 3.1) | |
| PtxC–placebo | 0.4 | −1.1 | 5.0 | −6.6* |
| (−3.0, 3.9) | (−4.6, 2.7) | (−0.2, 10.6) | (−11.2, 1.8) | |
| PtxR–placebo | 5.1* | 1.5 | 11.7* | −10.2* |
| (1.4, 7.8) | (−2.4, 5.6) | (5.8, 17.9) | (−14.8, 5.4) | |
| PtxC–Ptx | −4.1* | −3.6 | −4.2 | 1.2 |
| (−7.1, −0.9) | (−7.0, 0.01) | (−8.8, 0.6) | (−3.5, 6.3) | |
| PtxR–Ptx | 0.5 | −1.2 | 1.8 | −2.6 |
| (−2.9, 3.9) | (−4.8, 2.6) | (−3.3, 7.2) | (−7.4, 2.4) | |
| PtxR–PtxC | 4.7* | 2.6 | 6.3* | −3.8 |
| (1.2, 8.4) | (−1.2, 6.5) | (0.9, 11.9) | (−8.5, 1.1) |
P < 0.05.
The estimated intercepts and coefficients from the mixed model where the AUCs are used as regressor variables are shown in Table 5. Overall, for the flow parameters, pentoxifylline, R-M1 and M5 had coefficients with positive signs, indicating that they enhanced the retinal blood flow. In contrast, S-M1 and M4 had coefficients with negative signs and thus appeared to decrease the blood flow in subjects treated with pentoxifylline. The results were consistent for each molecular species as regards systolic, diastolic and mean flows, even though some of the coefficients had 95% confidence intervals that included zero.
Table 5.
Estimates (95% confidence intervals) of the intercept and gradients from the regression analysis of the influence from pentoxifylline (Ptx) and its metabolites (R-M1, S-M1, M4, and M5) on retinal blood flow measured as mean flow, systole, diastole and pulsation after administration of placebo and pentoxifylline 300 mg intravenous infusion alone and in combination with ciprofloxacin 750-mg tablet and pretreatment with rifampicin 600-mg tablets once daily for 7 days, on four separate occasions
| Mean flow | Systole | Diastole | Pulsation* 1000 | |
|---|---|---|---|---|
| Intercept | 245 | 327 | 167 | 490 |
| AU† | (186, 304) | (256, 398) | (122, 211) | (440, 540) |
| AUC Ptx | 17.1* | 36.4* | 3.47 | 33.9 |
| AU†/(µg−1 h−1 ml) | (4.31, 29.9) | (17.6, 55.3) | (−10.6, 17.6) | (−0.37, 68.1) |
| AUC R-M1 | 303* | 168 | 301* | −367 |
| AU†/(µg−1 h−1 ml) | (147, 459) | (−62.6, 399) | (128, 474) | (−804, 71.0) |
| AUC S-M1 | −9.58* | −12.1* | −4.66* | −7.51 |
| AU†/(µg−1 h−1 ml) | (−13.6, 5.52) | (−18.1, 6.05) | (−9.15, 0.158) | (−18.7, 3.7) |
| AUC M4 | −54.5* | −96.1* | −280 | −49.7 |
| AU†/(µg−1 h−1 ml) | (−93.4, 15.5) | (−154, 38.4) | (−71.2, 15.2) | (−159, 59.3) |
| AUC M5 | 6.66* | 9.62* | 3.52 | 4.04 |
| AU†/(µg−1 h−1 ml) | (0.675, 12.6) | (0.762, 18.5) | (−3.12, 10.2) | (−12.7, 20.8) |
P < 0.05.
Except for pulsation.
Discussion
This study was planned to investigate the possible contributions of the metabolites of pentoxifylline to the haemorheological effect in humans, and measurement of retinal blood flow was chosen as a convenient experimental model. However, the findings in the retina may also be of interest in themselves in view of the proposed use of pentoxifylline in retinal vascular disease. The co-variation in plasma concentration between pentoxifylline and its metabolites was successfully diminished through pretreatment of the subjects with ciprofloxacin and rifampicin, allowing the use of a multiple regression concentration–effect model.
Pharmacokinetic interactions between ciprofloxacin and methylxanthines [18], and later between ciprofloxacin and pentoxifylline [19], have been described in the literature. Ciprofloxacin is an inhibitor of cytochrome P450 (CYP) 1A2 [18, 19]. Indirect evidence for metabolism of M1 by CYP1A2 was obtained from a comparative study on pentoxifylline pharmacokinetics in smokers and nonsmokers [22]. The smokers had a significantly lower mean AUC for M1 compared with nonsmokers, with a trend in the same direction observed for the parent drug. Smoking is known to induce CYP1A2 [23]; thus, these differences were probably due to increased metabolism of M1 by this isoenzyme. Since the metabolism of pentoxifylline to M1 is reversible, depletion of M1 also lowered the AUC of the parent drug. In addition, since M1 in the circulation is >96% S-form [14] it is evident that the effect involved this enantiomer, while no conclusions can be drawn about the metabolism of R-M1. Direct evidence for a CYP1A2-mediated inhibitory interaction between ciprofloxacin and pentoxifylline was presented more recently [19]. It seems likely that CYP1A2 catalyses xanthine 7-demethylation of pentoxifylline to M6 and of M1 to M7.
In agreement with the cited findings we found that the AUCs for pentoxifylline and S-M1 were significantly increased by pretreatment with ciprofloxacin, with a trend in the same direction for R-M1. The apparent lack of effect on the R : S-M1 concentration ratio contrasts with an earlier finding [24] that the plasma concentration of R-M1 was 6–17% of total M1 when pentoxifylline was co-administered with ciprofloxacin to patients with renal cell carcinoma. This is much higher than the 1–4% in this and in our previous study [14]. However, the cancer patients also received interleukin-2. Treatment with interleukins may inhibit or downregulate CYP450 [25] and can thus also interfere with drug metabolism. Effects of the disease and of treatment with other drugs (ranitidine, paracetamol and indomethacin) cannot be ruled out either.
Rifampicin is a known inducer of several of the CYP450 enzymes [20] and was therefore used as pretreatment in this study. The effects of smoking and probable induction of CYP1A2 (see above) on the plasma concentrations of pentoxifylline and M1 were not reproduced after treatment with rifampicin, however. The reason may be that rifampicin is a poor inducer of CYP1A2, in particular in comparison with its effects on the CYP2 and CYP3 families [20]. Instead, the mean AUC of M5 was significantly reduced by the treatment with rifampicin. Normally, M5 is excreted without further metabolism [9–12]. The lower AUC could tentatively be explained by activation of metabolic pathways (e.g. 7-demethylation) that enhance the clearance of M5 and/or compete with its formation.
Pentoxifylline alone and after rifampicin pretreatment significantly increased retinal blood flow measured as mean flow, diastole and pulsation, but not systole compared with placebo. The increases over placebo were more pronounced in diastolic flow than in mean flow. After pentoxifylline alone diastolic flow increased by 9.7% (4.2, 15.5), and mean flow by 4.6% (1.1, 8.3). The corresponding increases from pentoxifylline after rifampicin pretreatment were 11.7% (5.8, 17.9) and 5.1% (1.4, 7.8) compared with placebo. Pentoxifylline in combination with ciprofloxacin did not affect retinal blood flows compared with placebo. Thus, pentoxifylline seems to increase diastolic low-velocity flow more than the higher flow rate occurring during systole.
The increases in ocular blood flow observed in this study are smaller than those reported by Schmetterer and co-workers [7]. In their study, 200 mg and 400 mg pentoxifylline increased ocular fundus pulsation by 17% and 27% in the macula, by 15% and 26% in the peripheral region and by 11% and 13% in the optic disk, over baseline. Some of the differences might be explained by different methods of measurement [17, 26]. The main differences between the methods is that SLDF measures retinal blood flow in the temporal area of retina whereas laser interferometer measures blood flow as fundus pulsation amplitude in the macula, peripheral region and optic desk. Fundus pulsations in the macula and peripheral region are predominantly influenced by choroidal blood flow, whereas fundus pulsation in the optic desk is a mixture of choroidal and retinal blood flow. Retinal blood flow is lower than choroidal blood flow (15% compared with 85% of total chorioretinal flow), but has a higher level of oxygen extraction compared with choroidal blood flow [27]. The retinal but not the choroidal blood flow is subject to autoregulation [27], therefore making the retinal blood flow less affected by systemic factors than the choroidal blood flow. Another advantage with the SLDF is that it scans an area of 2.7 × 0.7 mm and measures all flow in this area. Thus, more capillaries are found and the influence of erroneous values is smaller. With SLDF it is also easier to find the same area for all the measurements compared with laser interferometer that measures in one point. Pentoxifylline increases both choroidal and retinal blood flow but the increase is more pronounced in choroidal blood flow, possibly because retinal blood flow is autoregulated.
This study was performed in healthy volunteers partly because pentoxifylline is not approved for general use in Sweden and partly because we needed to co-administer pentoxifylline with ciprofloxacin and rifampicin in order to create different concentration ratios between pentoxifylline and its metabolites. It is difficult to make assumptions about the magnitude of the effect in a patient group with reduced retinal flow after administration to healthy volunteers, but we have shown that an increase in retinal blood flow is obtainable. This has not been shown previously either in patients or in healthy volunteers. In a study by Kruger et al., ocular fundus pulsation and retinal blood flow were measured by laser interferometry and SLDF in patients with age-related macular degeneration after administration of pentoxifylline or placebo, 400 mg three times daily for 3 months [8]. They found that pentoxifylline increased ocular fundus pulsation amplitude up to 28% after 3 months’ treatment but could not find any effects on retinal blood flow. In the present study we investigated the effects of pentoxifylline and metabolites on healthy eyes. Thus, our findings are not dependent on pathophysiological changes of the retinal vascular bed. In addition, SLDF combined with the AFFPIA software analysis used in the present study resulted in better reproducibility and less bias compared with the technique in Krueger's study. Our results are in agreement with previous studies, using the subjective blue field entoptic phenomenon computer simulation technique, that have shown an increase in capillary blood flow velocity in healthy volunteers and diabetes patients [4, 5].
A previous study has shown a clear time dependency of the effects on blood flow after a single dose of pentoxifylline [7]. We found no such time dependency (Figure 2). We cannot explain this, but we measured retinal blood flow, whereas other investigators have measured a total of retinal and the more abundant choroidal blood flow. In order to study the contributions from pentoxifylline and its metabolites we needed to diminish the naturally occurring high correlations between these compounds. The correlation matrix (Table 2) shows that we were successful, possibly with the exception of a remaining rather high correlation between M4 and M5. Applying a simple linear AUC–effect model, we found that pentoxifylline, R-M1 and M5 had coefficients with positive signs indicating that they all contribute to the effects. It is particularly noticeable that R-M1 exerts a significant effect in spite of being present in concentrations that are two orders of magnitude lower than those of pentoxifylline and M5. The high potency of this compound is also reflected in the values of the coefficients (Table 5), which are accordingly one to two orders of magnitude greater than those of the other compounds. Two of the metabolites, S-M1 and M4, showed negative coefficients. This should not necessarily be interpreted as these substances, per se, having a negative effect on blood flow, only that they, in the mix of substances obtained after pentoxifylline administration, tend to modify the effects in a direction opposite that of pentoxifylline.
Our results showing biological activity of R-M1 and M5 are to some extent in agreement with those of previous studies. The haemorheological effects of pentoxifylline and its metabolites have been investigated in vitro [16]. As regards enhancement of erythrocyte filterability, there was little difference between pentoxifylline, racemic M1 and M5. In a test for adenosine diphosphate-induced platelet aggregation M5 was not quite as effective as the parent drug; however, racemic M1 was approximately 10 times more potent than pentoxifylline. R-M1 has been investigated as an immunomodulatory drug, under the generic name of lisofylline. In this context R-M1 was shown to be 800 times more potent than pentoxifylline for inhibiting release of inflammatory mediators from monocytic leukaemia cells [28]. All these results, in conjunction with our own, point to R-M1 as a biologically very active molecule.
In conclusion, using a linear multiple-regression model we were able to demonstrate that R-M1 and M5 metabolites of pentoxifylline contribute significantly to the haemorheological effects of pentoxifylline in humans.
Acknowledgments
Johnny Ring, Department of Ophthalmology, Malmö University Hospital, obtained the SLDF registrations and Louise Goldberg, Department of Ophthalmology, Malmö University Hospital analysed the SLDF registrations. The project has received funds from the Anna and Edwin Berger Foundation and the Faculty of Medicine, Lund University.
References
- 1.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Samlaska CP, Winfield A. Pentoxifylline. J Am Acad Dermatol. 1994;30:603–21. doi: 10.1016/s0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Pham B, Ausejo M, Saenz A, Hood S, Barber GG. Pharmacological management of intermittent claudication. Drugs. 2000;59:1057–70. doi: 10.2165/00003495-200059050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sonkin PL, Sinclair SH, Hatchell DL. The effect of pentoxifylline on retinal capillary blood flow velocity and whole blood viscosity. Am J Ophthalmol. 1993;115:775–80. doi: 10.1016/s0002-9394(14)73647-5. [DOI] [PubMed] [Google Scholar]
- 5.Sonkin PL, Kelly LW, Sinclair SH, Hatchell DL. Pentoxifylline increases retinal capillary blood flow velocity in patients with diabetes. Arch Ophthalmol. 1993;111:1647–52. doi: 10.1001/archopht.1993.01090120069024. [DOI] [PubMed] [Google Scholar]
- 6.Sebag J, Tang M, Brown S, Sadun AA, Charles MA. Effects of pentoxifylline on choriodal blood flow in nonproliferative diabetic retinopathy. Angiology. 1994;45:429–33. doi: 10.1177/000331979404500603. [DOI] [PubMed] [Google Scholar]
- 7.Schmetterer L, Kemmler D, Breiteneder RN, Alschinger C, Koppensteiner R, Lexer F, Fercher AF, Eichler H-G, Wolzt M. A randomized, placebo-controlled, double-blind crossover study of the effects of pentoxifylline on ocular fundus pulsation. Am J Ophthamol. 1996;121:169–76. doi: 10.1016/s0002-9394(14)70581-1. [DOI] [PubMed] [Google Scholar]
- 8.Kruger A, Matulla B, Woltz M, Pieh S, Strenn K, Findl O, Eichler H-G, Schmetterer L. Short-term oral pentoxifylline use increases choriodal blood flow in patients with age related macular degeneration. Arch Ophthalmol. 1998;116:27–30. [PubMed] [Google Scholar]
- 9.Hinze HJ, Bedessem G, Söder A. Struktur der Ausscheidungsprodukte des 3,7-Dimetyl-1-(5-oxo-hexyl)-xantins (BL 191) beim Menschen. Arzneimittelforschung. 1972;22:1144–51. [PubMed] [Google Scholar]
- 10.Hinze HJ. Zur Pharmakokinetik von 3,7-Dimetyl-1-(5-oxo-hexyl)-xantin (BL 191) am Menchen. Arzneimittelforschung. 1972;22:1492–5. [PubMed] [Google Scholar]
- 11.Smith RV, Walker ES, Doluisio JT, Bauza MT, Puri SK, Ho I, Lassman HB. Pharmacokinetics of orally administered pentoxifylline in humans. J Pharm Sci. 1986;75:47–52. doi: 10.1002/jps.2600750111. [DOI] [PubMed] [Google Scholar]
- 12.Bryce TA, Hillbeck CD, Maceonald CM. Metabolism and pharmacokinetics of 14C-pentoxifylline in healthy volunteers. Arzneim-Forsch/Drug Res. 1989;3:512–7. [PubMed] [Google Scholar]
- 13.Lillibridge JA, Kalhorn TF, Slattery JT. Metabolism of lisofylline and pentoxifylline in human liver and cytosol. Drug Metab Disp. 1996;24:1174–9. [PubMed] [Google Scholar]
- 14.Nicklasson M, Björkman S, Roth B, Jönsson M, Höglund P. Stereoselective metabolism of pentoxifylline in vitro and in vivo in humans. Chirality. 2002;14:643–52. doi: 10.1002/chir.10121. [DOI] [PubMed] [Google Scholar]
- 15.Ings RMJ, Nüdemberg F, Burrows JL, Bryce TA. The pharmacokinetics of oxpentifylline in man when administered by constant intravenous infusion. Eur J Clin Pharmacol. 1982;23:539–43. doi: 10.1007/BF00637503. [DOI] [PubMed] [Google Scholar]
- 16.Ambrus JL, Stadler S, Kulaylat M. Hemorrheologic effects of metabolites of pentoxifylline (Trental) J Med. 1995;26:65–75. [PubMed] [Google Scholar]
- 17.Michelson G, Schmasuss B. Two dimensional mapping of the perfusion of the retina and optic nerve head. Br J Ophthamol. 1995;79:1126–32. doi: 10.1136/bjo.79.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhr U, Anders EM, Mahr G, Sörgel F, Staib AH. Inhibitory potency of quinolone antibacterial agents against cytochrome P4501A2 activity in vivo and in vitro. Antimicrob Agents Chemother. 1992;36:942–8. doi: 10.1128/aac.36.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson TC, Peterson MR, Wornell PA, Blanchard MG, Gonzalez FJ. Role of CYP 1A2 and CYP 2E1 in the pentoxifylline ciprofloxacin drug interaction. Biochem Pharmacol. 2004;68:395–402. doi: 10.1016/j.bcp.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 21.Michelson G, Welzenbach J, Pal I, Harzny J. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthamol. 1998;82:1294–300. doi: 10.1136/bjo.82.11.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauro VF, Mauro LS, Hageman JH. Comparison of pentoxifylline pharmacokinetics between smokers and nonsmokers. J Clin Pharmacol. 1992;32:1054–8. doi: 10.1002/j.1552-4604.1992.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 23.Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425–38. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JA, Bianco JA, Benyunes MC, Neubauer MA, Slattery JT, Fefer A. Phase 1b trial of pentoxifylline and ciprofloxacin in patients treated with interleukin-2 and lymphokine-activated killer cell therapy for metastatic renal cell carcinoma. Cancer Res. 1994;54:3436–41. [PubMed] [Google Scholar]
- 25.Piscitelli SC, Reiss WG, Figg WD, Petros WP. Pharmacokinetic studies with recombinant cytokines. Scientific issues and practical considerations. Clin Pharmacokinet. 1997;32:368. doi: 10.2165/00003088-199732050-00003. [DOI] [PubMed] [Google Scholar]
- 26.Schmetterer LF, Lexer F, Unfried CJ, Sattmann H, Fercher AF. Topical measurment of fundus pulsations. Opt Engineering. 1995;34:711–6. [Google Scholar]
- 27.Delaey C, Voorde JVD. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 28.Rice GC, Brown PA, Nelson RJ, Bianco JA, Singer JW, Bursten S. Protection from endotoxic shock in mice by pharmacologic inhibition of phosphatidic acid. Proc Natl Acad Sci USA. 1994;91:3857–61. doi: 10.1073/pnas.91.9.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]


