Abstract
Aims
To assess the pharmacokinetic and pharmacodynamic effects of co-administration of a combined oral contraceptive (ethinyloestradiol plus levonorgestrel) and lamotrigine.
Methods
Over a period of 130 days, healthy female subjects took lamotrigine (titrated up to 300 mg day−1) and the combined oral contraceptive, either individually or as co-therapy. Plasma ethinyloestradiol and levonorgestrel concentrations were measured in the presence and absence of lamotrigine, and serum lamotrigine concentrations were measured in the presence and absence of the combined oral contraceptive. Serum concentrations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone, oestradiol and sex hormone binding globulin were also determined.
Results
Of the 22 enrolled subjects, 16 had evaluable pharmacokinetic data. The mean (90% CI) ratios of lamotrigine area under the concentration-time curve from 0 to 24 h (AUC(0,24 h)) and maximum observed concentration (Cmax) of lamotrigine when it was given with the combined oral contraceptive and during monotherapy were 0.48 (0.44, 0.53) and 0.61 (0.57, 0.66), respectively. Ethinyloestradiol pharmacokinetics were unchanged by lamotrigine, the mean combined oral contraceptive + lamotrigine : combined oral contraceptive alone ratios (90% CI) of the AUC(0,24 h) and Cmax of levonorgestrel were 0.81 (0.76, 0.86) and 0.88 (0.82, 0.93), respectively. FSH and LH concentrations were increased (by 4.7-fold and 3.4-fold, respectively) in the presence of lamotrigine, but the low serum progesterone concentrations suggested that suppression of ovulation was maintained. Intermenstrual bleeding was reported by 7/22 (32%) of subjects during co-administration of lamotrigine and combined oral contraceptive.
Conclusions
A clinically relevant pharmacokinetic interaction was observed during co-administration of a combined oral contraceptive and lamotrigine. A dosage adjustment for lamotrigine may need to be considered when these agents are co-administered. A modest decrease in the plasma concentration of levonorgestrel was also observed but there was no corresponding hormonal evidence of ovulation.
Keywords: combined oral contraceptive, interaction, lamotrigine, pharmacodynamics, pharmacokinetics
Introduction
Lamotrigine is an anticonvulsant drug for the treatment of partial seizures and primary and secondary generalized tonic-clonic seizures in adults and children [1]. It can be used either as monotherapy or in combination with other drugs. In addition, lamotrigine is approved in some countries for the treatment of bipolar I disorder in adults.
Lamotrigine may be a desirable treatment option for women with epilepsy because it is not associated with weight gain and does not disrupt the menstrual cycle [2]. However, the co-administration of anticonvulsants and oral contraceptives may be associated with drug interactions [3]. Many anticonvulsants have hepatic enzyme-inducing properties [4], and effects that increase clearance or decrease absorption of the active components may lessen the efficacy of oral contraceptives. Similarly, the use of the latter may increase the metabolism of drugs like lamotrigine, whose major route of elimination is by glucuronidation [5–7]. Recent case reports of increased seizure frequency or recurrence of seizures following treatment with, and adverse events after withdrawal of oral contraceptives, were associated with decreased lamotrigine concentrations [8, 9].
Therefore, a study was conducted to examine the effect of co-administration of a combined oral contraceptive (ethinyloestradiol plus levonorgestrel) on the pharmacokinetics of lamotrigine, and also the effect of lamotrigine on the pharmacokinetics of the oral contraceptive.
Methods
Subjects
Females were eligible for enrolment if they were healthy (as reflected by clinical examination, the results of clinical chemistry and haematology tests, and 12-lead electrocardiograms (ECGs) at screening), were aged 18–45 years, and had a body mass index between 19 and 29.9 kg m−2. Subjects must have received a combined oral contraceptive for 3 months before the study and were asked to use an approved alternative method of contraception during the study. Subjects with a history of drug-induced rash were excluded.
Study design and treatments
This open-label, single-sequence study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, at LCG Bioscience (Bourn, Cambridge, United Kingdom). All subjects provided written informed consent following approval by the Huntingdon Local Research Ethics Committee, Papworth Hospital National Health Service Trust, Cambridge, United Kingdom.
The study consisted of a screening visit which occurred up to 21 days before the first drug administration, a treatment period of 130 days, and a follow-up visit within 7 days of the last drug administration. Women entering the study who were taking an oral contraceptive other than ethinyloestradiol 30 µg + levonorgestrel 150 µg (Microgynon 30®, Schering H.C.) were switched to this one. Women already taking it prior to study entry continued to do so.
Day 1 was defined as the first day of a new cycle of combined oral contraception after enrolment. Subjects took the medication in accordance with standard prescribing information (i.e. once-daily for 21 days followed by a 7 day pill-free period). On day 21, serial blood samples were taken for measurement of ethinyloestradiol and levonorgestrel plasma concentrations. After the 7 day pill-free interval, subjects started the next cycle of the combined oral contraceptive and began lamotrigine treatment at a dose of 25 mg once daily. Subjects received lamotrigine at doses titrated from 25 mg day−1 to 300 mg day−1 over 6 weeks, in accordance with dosing recommendations, and continued to receive the combined oral contraceptive at the same dosage. On day 105, when subjects had received lamotrigine (300 mg day−1 for 25 days), and were on day 21 of their fourth combined oral contraceptive cycle, serial blood samples were taken to measure concentrations of ethinyloestradiol, levonorgestrel and lamotrigine. Subjects discontinued the combined oral contraceptive on day 105, but continued to take lamotrigine (300 mg) alone for a further 3 weeks. On day 126, serial blood samples were taken for the measurement of serum lamotrigine concentrations. Subjects then decreased their dose of lamotrigine by 100 mg every 2 days, until the medication was discontinued.
Additional blood samples for the measurement of follicle-stimulating hormone (FSH), luteinizing hormone (LH), oestradiol, sex hormone binding globulin (SHBG) and progesterone concentrations were taken once during the first cycle of the combined oral contraceptive, and again in the fourth cycle, during co-administration of lamotrigine and the combined oral contraceptive.
Blood sampling
Blood samples were drawn pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h post-dose, on days 21 and 105 of treatment for the determination of plasma levonorgestrel and ethinyloestradiol concentrations, and on days 105 and 126 of treatment for the measurement of serum lamotrigine concentrations. Pre-dose blood samples were taken on days 19, 20, 103, 104, 124 and 125 to confirm steady-state conditions, whereas pre-dose concentrations measured on days 108, 110 and 112 were used to ascertain the change in exposure to lamotrigine during the pill-free week.
A total of four blood samples (∼5 ml each) were collected from each subject throughout the study for hormone analysis. Serum FSH, LH, oestradiol and SHBG concentrations were measured in pre-dose samples between days 5–7 (combined oral contraceptive only) and between days 89–91 (days 5–7 of cycle 4 of combined oral contraceptive + lamotrigine). Serum progesterone concentrations were measured in pre-dose samples between days 20–22 (combined oral contraceptive only) and between days 104–106 (days 20–22 of cycle 4 of combined oral contraceptive + lamotrigine).
Drug and hormone analyses
Blood samples (2 ml each) for the measurement of lamotrigine concentrations were collected into serum separation vacutainers, allowed to clot for 20 min at room temperature, and then centrifuged within 60 min at 1500 g for 15 min at 4 °C. The resultant serum was transferred to a screw-cap cryo/polypropylene tube, frozen immediately, and stored at ≤−20 °C prior to analysis. Lamotrigine and the internal standard, [13C215N5] lamotrigine, were extracted from 50 µl aliquots of serum using a solid phase (Oasis HLB) extraction procedure. The residues were reconstituted in 100 µl of 50 : 50 methanol : water and lamotrigine was quantified by turbo ion spray liquid chromatography/tandem mass spectrometry (LC/MS/MS) in the positive ion mode. The assay demonstrated a lower limit of quantification of 4 ng ml−1 and calibration lines were linear over the range 4–4000 ng ml−1. Within and between-run precision and accuracy of quantification during the validation was less than or equal to 5.4% across the lamotrigine concentration range. These assays were performed by Advion Biosciences (15 Catherwood Road, Ithica, NY 14850, USA) from whom further details can be obtained (info@advion.com).
Blood samples (8 ml) for the measurement of plasma ethinyloestradiol and levonorgestrel concentrations were collected into heparin tubes and centrifuged within 30 min at 1500 g for 15 min at 4 °C. The resultant plasma was transferred to a screw-cap cryo/polypropylene tube, frozen immediately, and stored at ≤−20 °C prior to analysis. Ethinyloestradiol and levonorgestrel were solvent extracted from 500 µl aliquots of plasma fortified with internal standards, [ethinyloestradiol-2,4,16,16-d4] and [levonorgestrel-(ethyl-d5)]. Following evaporation and derivatization, the analytes were extracted into hexane and evaporated to dryness. The residues were reconstituted in 300 µl of acetonitrile/water and quantified by LC/MS/MS. The assay had a lower limit of quantification of 2 pg ml−1 for ethinyloestradiol and 50 pg ml−1 for levonorgestrel and calibration graphs were linear over the range 2–500 pg ml−1 for ethinyloestradiol and 50–25000 pg ml−1 for levonorgestrel. Within and between-run precision and accuracy of quantification during the validation was less than or equal to 4.9% and 13.5% over the ethinyloestradiol and levonorgestrel concentration ranges, respectively. These assays were performed by PPD Development (http://www.ppdi.com) from whom further details can be obtained.
Analysis of hormones
Immunoradiometric assays were used to measure FSH (DPC IMMULITE FSH; LLQ = 0.5 IU l−1) and LH (MAIAclone LH; LLQ = 0.5 IU l−1), radioimmunoassays were used to measure oestradiol (Coat-A-Count Oestradiol; LLQ = 73 pmol l−1) and progesterone (DPC Coat-A-Count Progesterone; LLQ = 0.32 nmol l−1) and a solid-phase chemiluminescent immunometric assay was used to measure SHBG (DPC IMMULITE SHBG; LLQ = 0.2 nmol l−1). These assays were performed by LCG Bioscience (Bourn, Cambridge CB3 7TR, UK) from whom further details can be obtained.
Pharmacokinetic analysis
The primary study endpoints were steady-state maximum observed serum concentration (Cmax) and area under the serum concentration-time curve up to 24 h postdose (AUC(0,24 h)) of lamotrigine in the presence and absence of the combined oral contraceptive, and steady-state Cmax and AUC(0,24 h) of ethinyloestradiol and levonorgestrel in the presence and absence of lamotrigine.
Clinical assessment
Adverse events, defined as any untoward medical occurrence regardless of suspected cause, were recorded from the first administration of study medication through to the follow-up visit. All adverse events that subjects reported spontaneously, or in response to an open question at clinic visits, were documented. Vital signs and ECG parameters were assessed at screening and at follow-up, as well as during the treatment period.
Statistical analysis
Assuming a within-subject coefficient of variation of 15%, it was estimated that a sample size of 15 evaluable subjects would provide at least 90% power to demonstrate a lack of effect on the AUC and Cmax of lamotrigine. Using a 22% estimate of within-subject variability, it was estimated that a sample size of 15 evaluable subjects would provide at least 90% power to demonstrate a lack of effect on the AUC and Cmax of ethinyloestradiol and levonorgestrel.
Following loge transformation, the Cmax and AUC(0,24 h) for lamotrigine were analyzed separately by analysis of variance (anova), fitting session as a fixed effect term and subject as a random effect. Point estimates and 90% confidence intervals (CIs) for the differences between lamotrigine taken with the combined oral contraceptive compared with lamotrigine alone were constructed using the residual variance from the anova. These were then exponentially back-transformed to obtain estimates of the ratio of geometric means and 90% CIs for lamotrigine + combined oral contraceptive: lamotrigine alone. Lack of a clinically relevant effect of the combined oral contraceptive on lamotrigine concentrations using an equivalence approach (two, one-sided t-tests expressed as a 90% CI) would have been demonstrated if the 90% CI was completely contained within the range 0.75, 1.333 (i.e. a 25% change). The Cmax and AUC(0,24 h) of ethinyloestradiol and levonorgestrel were compared in the same way, such that lack of a clinically relevant effect of lamotrigine on the combined oral contraceptive concentrations would have been demonstrated if the 90% CI was completely contained within the range 0.7, 1.43 (i.e. a 30% change).
To evaluate whether steady state pharmacokinetics were achieved with each compound, statistical analysis of the predose concentrations for the 2 days prior to, and those on, the pharmacokinetic study day was performed after loge transformation of the data. Separate mixed-effect models were fitted with day as a fixed-effect continuous covariate and subject as a random effect. The coefficient of the slope of the day-effect on the loge scale was calculated to evaluate whether steady state was achieved in each analysis. Using the pooled estimate of variance, the 90% CIs for the slope were calculated and then back-transformed to the original scale. Steady state was reached if the 90% CI limits were within the range of 0.91–1.10. Predose serum lamotrigine concentrations for days 108, 110 and 112 were expressed as a ratio of the individual mean predose lamotrigine concentration on days 103–105 (with the combined oral contraceptive) to those on days 124–126 (no combined oral contraceptive).
Results
Of the 22 subjects enrolled, 16 completed the study. Six subjects (27%) were prematurely withdrawn due to dizziness in association with ataxia (3 subjects), diplopia (1 subject), visual hallucinations (1 subject), or nausea (1 subject). Originally, lamotrigine was to have been titrated to 500 mg day−1 but, following the occurrence of central nervous system adverse events at 400 mg day−1 and the consequent withdrawal of 5 subjects, the maximum lamotrigine dose was decreased to 300 mg day−1. One other subject, receiving 200 mg day−1 of lamotrigine, was also withdrawn. All 16 subjects who completed the study received 300 mg lamotrigine for at least 3 weeks prior to blood sampling on day 105.
Median serum concentration-time profiles for lamotrigine in the presence and absence of the combined oral contraceptive are displayed in Figure 1. Lamotrigine tmax was between 0.5 and 4 h postdose, irrespective of the drug regimen. The mean ratios of lamotrigine Cmax and AUC(0,24 h) for the comparison of lamotrigine + combined oral contraceptive : lamotrigine alone were 0.61 (a 39% decrease) and 0.48 (a 52% decrease), respectively, with the 90% CIs of these ratios being outside of the acceptance range (0.75–1.33) (Table 1). The observed decrease in lamotrigine AUC(0,24 h) translates to an approximate 2.1-fold increase in apparent lamotrigine oral clearance in the presence of the combined oral contraceptive.
Figure 1.
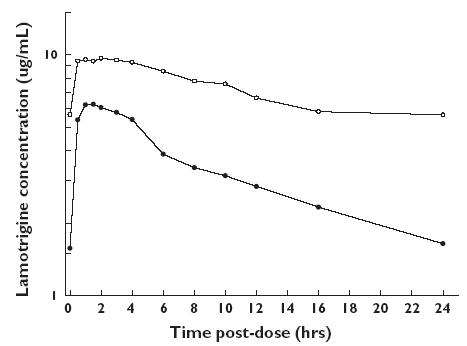
Median (n = 16) serum lamotrigine concentration-time profiles after oral administration of lamotrigine (300 mg) alone or with the combined oral contraceptive. Lamotrigine + combined oral contraceptive (day 105) (•), lamotrigine alone (day 126) (○)
Table 1.
Geometric mean (SD) pharmacokinetic parameters (n = 16) and statistical comparisons (ratios [90% CI]) for lamotrigine (LTG), ethinyloestradiol and levonorgestrel after administration of lamotrigine (300 mg) alone and with the combined oral contraceptive (COC)
| Effect of COC on lamotrigine (LTG) | |||
|---|---|---|---|
| LTG + COC | LTG | Ratio LTG + COC : LTG [90% CI] | |
| Lamotrigine | |||
| Cmax (µg ml−1) | 6.33 (1.43) | 10.3 (1.91) | 0.61 [0.57, 0.66] |
| AUC(0,24 h) (µg ml−1 h) | 78.8 (31.0) | 163 (42.7) | 0.48 [0.44, 0.53] |
| Effect of lamotrigine (LTG) on COC | |||
|---|---|---|---|
| LTG + COC | COC | Ratio LTG + COC : COC [90% CI] | |
| Ethinyloestradiol | |||
| Cmax (pg ml−1) | 91.7 (37.1) | 89.5 (35.1) | 1.02 [0.95, 1.10] |
| AUC(0,24 h) (pg ml−1 h) | 785 (267) | 849 (265) | 0.93 [0.88, 0.97] |
| Levonorgestrel | |||
| Cmax (pg ml−1) | 6886 (1797) | 7858 (2393) | 0.88 [0.82, 0.93] |
| AUC(0,24 h) (pg ml−1 h) | 79981 (21605) | 98653 (33157) | 0.81 [0.76, 0.86] |
Statistical analyses of predose serum lamotrigine concentrations indicated attainment of steady state on day 105 and on day 126. A stepped and marked rise in predose serum lamotrigine concentrations was observed during the pill-free week (days 106–112), with concentrations by day 112 being approximately 2-fold higher than during the combined oral contraceptive co-administration period (Table 2). Normalisation of lamotrigine clearance was incomplete by the end of the pill-free week, with predose concentrations on day 112 being approximately 80% of those achieved during the lamotrigine monotherapy period (Table 2).
Table 2.
Mean (range) of predose serum lamotrigine concentrations and of individual ratios of predose concentrations during the pill-free week relative to combined oral contraceptive co-administration (days 103–105) or lamotrigine monotherapy (days 124–126)
| Predose concentration (µg ml−1) | Ratio relative to co-administration period | Ratio relative to monotherapy period | |
|---|---|---|---|
| Days 103–105 | 2.02 (0.64–5.50), | – | – |
| Day 108 | 2.78 (0.99–7.12), | 1.27 (0.92–1.63), | 0.47 (0.21–0.84) |
| Day 110 | 3.15 (0.91–7.52), | 1.63 (2.58–2.17), | 0.60 (0.16–0.88) |
| Day 112 | 4.05 (1.79–8.02), | 2.16 (1.39–2.94), | 0.77 (0.47–0.98) |
| Days 124–126 | 5.30 (2.44–8.98) |
Days 108, 110 and 112 = Days 3, 5 and 7 of pill-free week, respectively.
The median plasma concentration-time profiles of ethinyloestradiol and levonorgestrel administered with and without lamotrigine are shown in Figure 2. Peak concentrations were observed between 1.0 and 3 h postdose for ethinyloestradiol and 0.5 and 3 h postdose for levonorgestrel, irrespective of the drug regimen. All 90% CIs for ethinyloestradiol and levonorgestrel Cmax and AUC(0,24 h) for the ratio lamotrigine + combined oral contraceptive : combined oral contraceptive alone were inside the acceptance range 0.70–1.43 (Table 1). However, there was a mean 12% decrease in Cmax and 19% decrease in AUC(0,24 h) for levonorgestrel when the combined oral contraceptive was co-administered with lamotrigine. Statistical analysis of predose ethinyloestradiol and levonorgestrel concentrations indicated that steady-state conditions had been attained for each compound prior to the pharmacokinetic profiling.
Figure 2.
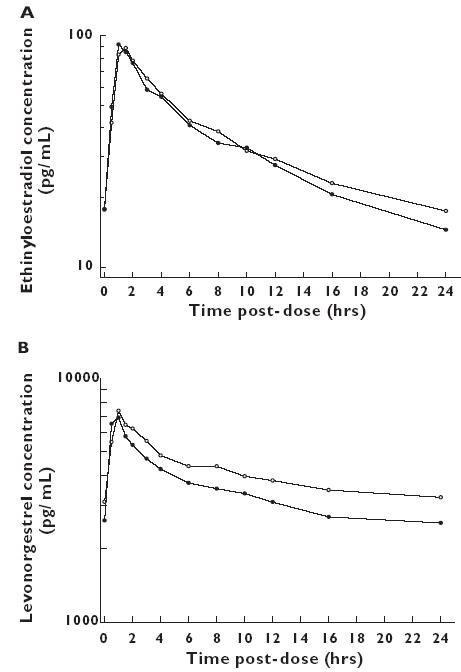
Median (n = 16) plasma ethinyloestradiol alone (day 21) (○), ethinyloestradiol + LTG (day 105) (•) (A) and levonorgestrel alone (day 21) (○), levonorgestrel + LTG (day 105) (B) concentration-time profiles after oral administration of the combined oral contraceptive alone (n = 16) or with lamotrigine
During co-administration of lamotrigine with the combined oral contraceptive there was, on average, a 4.7-fold increase in serum FSH, a 3.4-fold increase in serum LH and a relatively small increase in serum oestradiol, compared with combined oral contraceptive therapy alone (Table 3a). Individual values for serum FSH and LH increased in most subjects, whereas individual values for serum oestradiol remained below 73 pmol l−1 in 9 of the 16 subjects during lamotrigine co-administration. There was a marginal increase in serum oestradiol in 6 subjects, to a maximum of 118 pmol l−1, and, in one subject, there was a larger increase, to 279 pmol l−1. There was a poor correlation between changes in LH, FSH, oestradiol and changes in levonorgestrel exposure (data not shown). During co-administration of lamotrigine and the combined oral contraceptive there was, on average, a slight decrease in serum progesterone (Table 3b). There was no clear pattern to individual changes in serum progesterone.
Table 3.
Serum hormone concentrations following administration of the combined oral contraceptive (COC) alone or with lamotrigine (300 mg)
| (a) FSH, LH, oestradiol and SHBG | ||||
|---|---|---|---|---|
| Day 5 to day 7 (COC alone) (n = 22) | Day 89 to day 91 (COC + lamotrigine 300 mg) (n = 16) | |||
| Hormone | Geometric mean | Range | Geometric mean | Range |
| FSH (IU l−1)a | 1.02 | <0.50–5.20 | 4.80 | 0.94–12.5 |
| LH (MIU l−1)a | 0.70 | <0.50–3.20 | 2.40 | <0.5–8.90 |
| Oestradiol (pmol l−1)b | <73.0c | <73.0c | 86.8 | <73.0–279 |
| SHBG (nmol l−1) | 158 | 50.0–375 | 115 | 57.0–242 |
| (b) Progesterone | ||||
|---|---|---|---|---|
| Day 20 to day 22 (COC alone) (n = 22) | Day 104 to day 106 (COC + lamotrigine 300 mg) (n = 16) | |||
| Hormone | Geometric mean | Range | Geometric mean | Range |
| Progesterone (nmol l−1)a | 1.47 | <1.00–2.90 | 1.28 | <1.00–2.20 |
For the purposes of summary statistics, values < 0.50 IU l−1were set at 0.50 IU l−1
For the purposes of summary statistics, values < 73.0 pmol l−1were set at 73.0 pmol l−1
All values < 73.0 pmol l−1FSH, follicle stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone binding globulin. Normal ranges: FSH (IU l−1) = 3.0–10.2; LH (IU l−1) = Follicular: 1.8–13.4, Mid-cycle: 15.6–79, Luteal: 0.7–19.4; Oestradiol (pmol l−1) = Follicular: 37–184, Mid-cycle: 41–1377, Luteal: 55–422; SHBG (nmol l−1) = 18–114.
For the purposes of summary statistics, values < 1.00 nmol l−1were set at 1.00 nmol l−1. Normal ranges: Progesterone (nmol l−1) = Follicular: 0.5–4.5, Luteal: 5.1–67, Mid-luteal: 17.0–73.0.
There was, also, a slight decrease in the geometric mean and maximum values for serum SHBG, during co-administration of lamotrigine with the combined oral contraceptive, compared with administration of the combined oral contraceptive alone (Table 3a). However, there was no detectable change in the minimum values for serum SHBG.
Data are reported from the 22 subjects who initially enrolled in the study. The most frequently reported adverse events (>2 subjects) were headache, dizziness, skin rash, nausea, upper respiratory tract infection, fatigue, metrorrhagia, diplopia, ataxia, insomnia and gastroenteritis. None of the rashes was severe, and no subjects discontinued the study medication for this reason. Dizziness, diplopia and ataxia occurred primarily in subjects who received lamotrigine 400 mg day−1. Intermenstrual bleeding (metrorrhagia or vaginal haemorrhage), which was only observed during the period of co-administration of lamotrigine and the combined oral contraceptive, was reported by 7/22 (32%) of subjects; there was no clear relationship between the dose of lamotrigine, or the increase in FSH and LH serum concentrations, and the observed episodes of intermenstrual bleeding.
Discussion
Serum concentrations of lamotrigine decreased (by, an average, 39% in Cmax and 52% in AUC(0,24 h)) during co-administration with the combined oral contraceptive. We consider that this halving of systemic exposure is clinically important with direct implications for the dosing of lamotrigine in women who start or stop a combined oral contraceptive. Thus, a dosage adjustment for lamotrigine may be necessary when these agents are co-administered.
The effect of the combined oral contraceptive on lamotrigine disposition in the present prospective study is in close agreement with the earlier findings of Sabers et al.[8, 9]. In a retrospective report of 7 patients in whom plasma lamotrigine concentrations were decreased (by a mean of 49%), it was concluded that the interaction between oral contraceptives and lamotrigine was clinically relevant and associated with changes in seizure frequency/recurrence upon addition, or adverse effects following withdrawal, of an oral contraceptive [8]. Subsequently, Sabers et al. reported that the mean plasma lamotrigine concentration was 13 µmol l−1 (≈ 3.3 µg ml−1) in 22 women taking lamotrigine plus an oral contraceptive, compared with 28 µmol l−1 in 30 women on lamotrigine alone [9].
The two-fold increase in lamotrigine clearance in the presence of the combined oral contraceptive is similar to the effects of known hepatic enzyme-inducing drugs [10] and is consistent with reports that oral contraceptives can increase the metabolism of glucuronidated drugs by induction of the uridine diphosphate glucuronosyltransferase system [5–7].
Predose serum lamotrigine concentrations increased in a fairly rapid and linear manner during the ‘pill-free’ week, with concentrations at the end of that week being, on average, approximately two-fold higher than during co-administration of the combined oral contraceptive. However, complete normalization of lamotrigine clearance was not attained by the end of the ‘pill-free’ week.
A previous study has shown no effect of lamotrigine on the disposition of the components of a combined oral contraceptive. However, no formal pharmacokinetic evaluation was undertaken and only the 12 h postdose concentrations were compared [14]. In the present study the Cmax and AUC(0,24 h) of ethinyloestradiol were found to be comparable when administered with and without lamotrigine. However, those of levonorgestrel were 12% and 19% lower, respectively, during lamotrigine co-administration. The magnitude of these changes did not meet the predefined criteria for clinical relevance. Levonorgestrel AUC has been shown to be decreased by 42% by phenytoin [11], 40% by carbamazepine [11] and 32% by oxcarbazepine [12]. In another report, the anticonvulsant agent felbamate was shown to cause a 42% decrease in the AUC(0,24 h) of gestodene but did not have a clinically relevant effect on exposure to ethinyloestradiol [13].
Although the mechanism of the selective effect of lamotrigine on levonorgestrel is unknown, a modest induction of clearance appears to be the most plausible explanation. Direct glucuronidation of levonorgestrel is a relatively minor route of its metabolism [15]. Thus, the present findings would indicate that direct glucuronidation of levonorgestrel is either greater than has been previously established or that lamotrigine has an effect on the hydroxylation and sulphation of levonorgestrel. Based on results of drug interaction studies conducted with lamotrigine [16], a lack of effect on urinary 6β-hydroxycortisol excretion [17], and on the relatively minor effect of lamotrigine on ethinyloestradiol exposure (present study), lamotrigine is probably not an inducer of cytochrome P450 enzymes.
A small decrease in mean serum progesterone was observed on co-administration of lamotrigine (300 mg) with the combined oral contraceptive, compared with administration of the combined oral contraceptive alone. Serum progesterone values exceeding 5.1 nmol l−1 would have indicated that ovulation had probably occurred. For all subjects studied, and in both pill cycles, serum progesterone values remained well below 5.1 nmol l−1, indicating maintenance of contraceptive efficacy during the period of co-administration.
Large increases in serum FSH and LH were observed on co-administration of lamotrigine (300 mg) with the combined oral contraceptive. A relatively small increase in serum oestradiol was also observed. These changes may have occurred as a consequence of the lower plasma concentrations of levonorgestrel observed during the period of coadministration of lamotrigine and the combined oral contraceptive, although there was a poor correlation between changes in LH, FSH and oestradiol and those in levonorgestrel exposure. Taken together, the observed changes in serum FSH, LH and oestradiol may indicate the potential for some loss of suppression of the hypothalamic-pituitary-ovarian axis during the period of co-administration of lamotrigine and the combined oral contraceptive. However, complete suppression of ovarian follicular activity does not necessarily occur with oral contraceptive use [18, 19].
Intermenstrual bleeding was reported by 32% of subjects during the period of co-administration of lamotrigine and combined oral contraceptive, which may indicate some loss of cycle control. However, there was no clear pattern to the intermenstrual bleeding with respect to the day of the pill cycle on which it started, or the duration of the bleeding. The absence of the any clear relationship between the dose of lamotrigine at which episodes of intermenstrual bleeding occurred, and the lack of a placebo control group with which to compare the incidence of intermenstrual bleeding, makes it difficult to draw conclusions about this observation. Furthermore, there appears to be no direct evidence to relate less effective cycle control to increased ovarian activity [20] or impaired contraceptive efficacy [21]. Any future evaluation of these hormonal findings would benefit from a more extensive temporal evaluation of the pharmacodynamic parameters, and a closer examination of changes in menstrual pattern by using a placebo group.
In general, lamotrigine was well-tolerated at doses of up to 300 mg day−1 when co-administered with the combined oral contraceptive, and the observed adverse events were typical of previous studies of lamotrigine conducted in healthy subjects. An increased incidence of central nervous system adverse events was observed when the dose was increased from 300 mg to 400 mg, characterized primarily by dizziness. The effects of lamotrigine on the central nervous system are well-recognized, and typically occur when the dose is being increased. The severity of symptoms experienced by some subjects may have been the consequence of increasing the dose of lamotrigine to 400 mg on the same day as starting a pill-free week, when the normalization of lamotrigine clearance is occurring.
In conclusion, a clinically relevant effect of a combined oral contraceptive on the pharmacokinetics of lamotrigine was observed, and a dosage adjustment for lamotrigine may be necessary when this combination is administered. Lamotrigine had a modest effect on the pharmacokinetics of levonorgestrel and, although there may have been some loss of suppression of ovarian activity during co-administration, no subjects showed hormonal evidence of ovulation.
Acknowledgments
GlaxoSmithKline sponsored the research described in this manuscript. All the authors are employees of GlaxoSmithKline and hold stock in the company.
References
- 1.Choi H, Morrell MJ. Review of lamotrigine and its clinical applications in epilepsy. Expert Opin Pharmacother. 2003;4:243–51. doi: 10.1517/14656566.4.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Morrell MJ, Isojarvi J, Taylor AE, Dam M, Ayala R, Gomez G, O'Neill F, Tennis P, Messenheimer J. Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Res. 2003;54:189–99. doi: 10.1016/s0920-1211(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 3.Crawford P. Interactions between antiepileptic drugs and hormonal contraception. CNS Drugs. 2002;16:263–72. doi: 10.2165/00023210-200216040-00005. [DOI] [PubMed] [Google Scholar]
- 4.Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472–84. doi: 10.2165/00003088-199018060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Shenfield GM, Griffin JM. Clinical pharmacokinetics of contraceptive steroids. An update. Clin Pharmacokinet. 1991;20:15–37. doi: 10.2165/00003088-199120010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Miners JO, Mackenzie PI. Drug glucuronidation in humans. Pharmacol Ther. 1991;51:347–69. doi: 10.1016/0163-7258(91)90065-t. [DOI] [PubMed] [Google Scholar]
- 7.Walle T, Fagan TC, Walle UK, Topmiller MJ. Stimulatory as well as inhibitory effects of ethinylestradiol on the metabolic clearances of propranolol in young women. Br J Clin Pharmacol. 1996;41:305–9. doi: 10.1046/j.1365-2125.1996.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabers A, Buchholt JM, Uldall P, Hansen EL. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151–4. doi: 10.1016/s0920-1211(01)00305-9. [DOI] [PubMed] [Google Scholar]
- 9.Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61:570–1. doi: 10.1212/01.wnl.0000076485.09353.7a. [DOI] [PubMed] [Google Scholar]
- 10.Hachad H, Ragueneau-Majlessi I, Levy RH. New antiepileptic drugs: review on drug interactions. Ther Drug Monit. 2002;24:91–103. doi: 10.1097/00007691-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Crawford P, Chadwick DJ, Martin C, Tija J, Back DJ, Orme M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br J Clin Pharmacol. 1990;30:892–6. doi: 10.1111/j.1365-2125.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klosterskov Jensen P, Saano V, Haring P, Svenstrup B, Menge GP. Possible interaction between oxcarbazepine and an oral contraceptive. Epilepsia. 1992;33:1149–52. doi: 10.1111/j.1528-1157.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 13.Saano V, Glue P, Banfield C, Reidenberg P, Colucci RD, Meehan JW, Haring P, Radwanski E, Nomeir A, Lin C-C, Jensen PK, Affrime MB. Effects of felbamate on the pharmacokinetics of a low-dose combination oral contraceptive. Clin Pharmacol Ther. 1995;58:523–31. doi: 10.1016/0009-9236(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 14.Holdich T, Whiteman P, Orme M, Back D, Ward S. Effect of lamotrigine on the pharmacology of the combined oral contraceptive pill. Epilepsia. 1991;32(Suppl 1):96. [Google Scholar]
- 15.Stanczyk F, Roy S. Metabolism of levonorgestrel, norethindrone, and structually related contraceptive steroids. Contraception. 1990;42:67–96. doi: 10.1016/0010-7824(90)90093-b. [DOI] [PubMed] [Google Scholar]
- 16.Lamictal® (lamotrigine) Tablets Prescribing Information. Research Triangle Park, NC: GlaxoSmithKline; 2003. June. [Google Scholar]
- 17.Posner J, Webster H, Yuen WC. Investigation of the ability of lamotrigine, a novel antiepileptic drug, to induce mixed function oxygenase enzymes. Br J Clin Pharmacol. 1991;32:658. [Google Scholar]
- 18.Fitzgerald C, Elstein M, Spona J. Effect of age on the response of the hypothalamo-pituitary-ovarian axis to a combined oral contraceptive. Fertil Steril. 1999;71:1079–84. doi: 10.1016/s0015-0282(99)00146-6. [DOI] [PubMed] [Google Scholar]
- 19.Spona J, Feichtinger W, Kinderman C, Wünsch C, Brill K. Inhibition of ovulation by an oral contraceptive containing 100 µg levonorgestrel in combination with 20 µg ethinylestradiol. Contraception. 1996;54:299–304. doi: 10.1016/s0010-7824(96)00183-7. [DOI] [PubMed] [Google Scholar]
- 20.Van der Vange N. Chamberlain G. Contemporary Obstetrics and Gynaecology. London: Butterworths; 1988. Ovarian activity during low-dose oral contraceptives; pp. 315–26. [Google Scholar]
- 21.Åkerlund M, Rode A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 µg desogestrel and either 30 µg or 20 µg ethinyl estradiol. Br J Obstet Gynaecol. 1993;100:832–8. doi: 10.1111/j.1471-0528.1993.tb14309.x. [DOI] [PubMed] [Google Scholar]


