Abstract
Aims
To characterize the demographic and pharmacogenetic factors that influence interpatient variability in the plasma concentrations of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz.
Methods
Data from all samples analyzed for efavirenz in our TDM service in 2002 and 2003 were reviewed. Information on gender, age, body weight, height, race, hormonal contraceptive use (in a subset of patients), and time between sampling and last intake was recorded. PCR-restriction fragment length polymorphism analysis was performed to detect the cytochrome P450 2B6 (CYP2B6) C1459T variant (present in CYP2B6*6 and CYP2B6*7) which is associated with low CYP2B6 activity.
Results
A total of 255 patients were included in this analysis. The median plasma efavirenz concentration was 2.50 (interquartile range: 1.85–3.55) mg l−1. Eight patients (3.1%) were considered to have a subtherapeutic plasma concentration (<1.0 mg l−1) and 48 (18.9%) a toxic efavirenz concentration (>4.0 mg l−1). Gender, time after last intake, and race were the only factors that were significantly related to plasma efavirenz concentration in a multivariate analysis. No influence was observed for body weight, hormonal contraceptive use, and the presence of the CYP2B6 C1459T polymorphism.
Conclusions
Gender and race are important factors in determining interpatient variability in plasma efavirenz concentrations which were unaffected by the presence of the CYP2B6 C1459T polymorphism. Physicians should be particularly alert for signs of efavirenz-induced toxicity in females and non-Caucasian patients.
Keywords: CYP2B6 polymorphism, efavirenz, ethnicity, gender, HIV infection, non-nucleoside reverse transcriptase inhibitor, pharmacokinetics
Introduction
The HIV non-nucleoside reverse transcriptase inhibitor (RTI) efavirenz is one of the preferred agents, combined with two nucleoside RTIs, for the initial treatment of patients with HIV infection [1]. Several studies have demonstrated the potent antiviral activity of efavirenz in this combination, leading to more than 80% of patients having an HIV-1 viral load below the detection limit of 50 copies ml−1[2–4].
The pharmacokinetics of efavirenz are associated with extensive protein binding (>99%), hepatic clearance by cytochrome P450 (CYP) 2B6 and 3A4 enzymes, and a long elimination half-life (40–55 h) [5]. The pharmacokinetics of efavirenz are also subject to substantial interpatient variability [6–8], the clinical relevance of which translates into variable response in HIV-infected patients. Thus, patients with low exposure to efavirenz have an increased risk of virological failure [6, 7, 9–11], whereas those with high exposure to the drug suffer from adverse reactions, mostly affecting the central nervous system [9, 12]. Based on this information, a therapeutic range for efavirenz of 1.0–4.0 mg l−1 has been recommended [13].
Given the presence of a concentration-effect relationship for efavirenz and the large interpatient variability in its pharmacokinetics, it is important to identify the factors that influence the pharmacokinetic behaviour of this agent. As a result, treatment with efavirenz could be more individualized, and treatment failure, either due to an inadequate virological response or to CNS toxicity, can be prevented. In a preliminary analysis of patients from our Therapeutic Drug Monitoring (TDM) service, we found that female gender was a significant risk factor for the development of toxic plasma efavirenz concentrations [14]. In the present study, we investigated potential factors associated with abnormal efavirenz plasma concentrations in a larger number of female patients.
Methods
Patients
Patients were selected on the basis of samples that were submitted to our national TDM service for efavirenz from six different sites in 2002 and 2003. Only the first sample from each patient was included to avoid potential bias from repeated sampling. Patients were receiving the standard dose of efavirenz of 600 mg once daily. Subjects who were suspected of nonadherence to therapy (indicated by the physician on the application form) and subjects whose samples contained no detectable efavirenz (<0.2 mg l−1) were excluded from the analysis. Also data from samples withdrawn more than 24 h after the last dose were omitted.
All HIV-infected patients in the Netherlands are followed as a national cohort by the HIV Monitoring Foundation (SHM) and recruited patients gave informed consent for this study. This protocol was approved by all the institutional review boards of the 22 Dutch treatment centres for HIV-infected patients.
Drug analysis
Plasma efavirenz concentrations were determined by a validated high-performance liquid chromatographic assay, as described previously [15]. The maximum intra- and inter-day precision of the assay was 2.6% and 2.8%, respectively. Plasma efavirenz concentrations were defined as either subtherapeutic (<1.0 mg l−1), therapeutic (1.0–4.0 mg l−1), or toxic (>4.0 mg l−1) [13].
Genetic analysis
The CYP2B6 C1459T single nucleotide polymorphism (SNP) was selected for genotyping because it is associated with decreased protein expression in the liver [16]. DNA was isolated from 200 µl of plasma using the MagNA Pure LC (Roche Diagnostics GmbH, Mannheim, Germany). PCR-restriction fragment length polymorphism analysis was used to detect the C1459T variant of the CYP2B6 gene. The method employed was a modification of the assay described previously by Lang et al.[14] using the forward primer 5′-CTGTTG CAGTGGACATTTG-3′ and the reverse primer 5′-ATCTCACTCCTGCACTCAC-3′. The absence or presence of the nucleotide change C1459T results in either wild-type CC (WT), heterozygous variant CT, or homozygous variant TT.
Statistical analysis
Differences in plasma efavirenz concentrations between subgroups were compared by analysis of variance. Univariate and multivariate regression were applied to identify factors affecting plasma efavirenz concentrations. Test results with a P value < 0.05 were considered statistically significant.
Results
A total of 255 patients were selected from the six different study sites. Patient demographics are listed in Table 1. The median plasma efavirenz concentration was 2.50 mg l−1 with an interquartile range from 1.85 to 3.55 mg l−1. The distribution of concentrations over the 24 h dose interval is depicted in Figure 1. Out of these 255 patients, eight (3.1%) were considered to have a subtherapeutic concentration (<1.0 mg l−1) and 48 (18.9%) to have a toxic concentration (>4.0 mg l−1). Consequently, the remaining 199 subjects (78.0%) had a plasma efavirenz concentration within the therapeutic range (1.0–4.0 mg l−1).
Table 1.
Demographic information about the study population
| Parameter (unit) | % of patients | Median | Interquartile range |
|---|---|---|---|
| Age (years) | 40 | 33–48 | |
| Gender | |||
| Male | 74.1% | ||
| Female | 25.9% | ||
| Weight (kg) | 72 | 63–80 | |
| Height (cm) | 176 | 168–181 | |
| Body surface area (m2) | 1.85 | 1.73–2.00 | |
| Time after intake (h) | 13 | 12–16 | |
| EFV plasma concentration (mg l−1) | 2.5 | 1.9–3.6 | |
| Race- | |||
| Caucasian | 63.5% | ||
| Black | 32.5% | ||
| Asian | 3.9% | ||
| Oral contraceptive use | n = 39 | ||
| Yes | 20.5% | ||
| No | 79.5% | ||
| CYP2B6 genotype at position 1459 | n = 228 | ||
| C/C | 82.9% | ||
| C/T | 14.5% | ||
| T/T | 2.6% | ||
Figure 1.
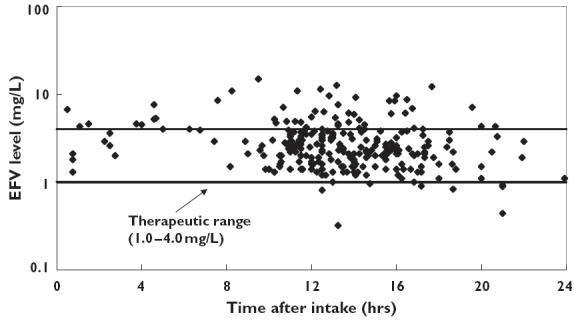
Plasma efavirenz concentration vs. time data from 255 patients
All demographic factors were entered in a univariate regression model to search for potential relationships with the plasma efavirenz concentration. Subsequently, factors that were significantly associated with efavirenz exposure were added stepwise in a multivariate analysis. The results are depicted in Table 2. Gender, time after last intake, and race were the only factors that were significantly associated with concentration (Figure 2). The mean plasma efavirenz concentration in female patients was 4.0 mg l−1 compared with 2.8 mg l−1 in male patients (mean difference: 1.2 mg l−1 (95% confidence interval (CI) 0.6, 1.8 mg l−1; P < 0.001). Three different ethnic groups were present in our study population, namely Asians (n = 10), Blacks (n = 84) and Caucasians (n = 161). Taking Caucasians as the reference group (mean value 2.8 mg l−1), mean differences (+ 95% CI) in plasma efavirenz concentrations were 1.0 mg l−1 (0.43, 1.6 mg l−1; P = 0.001) for Blacks, and 0.51 mg l−1 (−0.53, 1.5 mg l−1; P = 0.34) for Asians, respectively.
Table 2.
Univariate and multivariate analysis of the plasma efavirenz concentration data
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | R | F | P value | R | F | P value |
| Age | 0.093 | 2.22 | 0.137 | |||
| Gender | 0.238 | 15.18 | <0.001 | 0.287 | 17.65 | <0.001 |
| Body weight | 0.217 | 12.15 | 0.001 | |||
| Body surface area | 0.218 | 10.84 | 0.001 | |||
| Height | 0.163 | 6.07 | 0.015 | |||
| Time after intake of medication | 0.158 | 6.45 | 0.012 | 0.350# | 13.65# | <0.001# |
| Race | 0.183 | 8.80 | 0.003 | 0.389## | 11.56## | <0.001## |
| CYP2B6 genotype | 0.132 | 3.99 | 0.047 | |||
In addition to gender
in addition to gender and time after intake of medication. R = regression coefficient; F = Fischer's exact test value.
Figure 2.
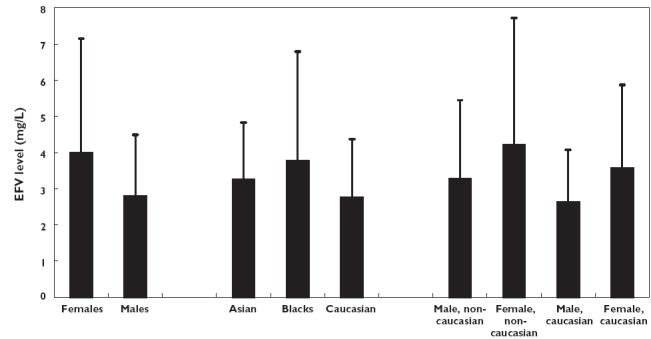
Mean (+ SD) plasma efavirenz concentrations in the various subgroups
As expected, female patients had a lower average body weight than male patients: 65.3 vs. 75.1 kg (mean difference 9.8 kg (95% CI 6.0, 13.6; P < 0.001)). The same was true for non-Caucasians compared with Caucasians: 69.0 vs. 75.9 kg (mean difference 6.9 kg (95% CI: 3.0, 10.8; P = 0.001)). Thus, a lower body weight in female and non-Caucasian patients could be an explanation for the association between gender and race with plasma efavirenz concentrations. However, in a multivariate analysis, body weight was no longer associated with higher concentrations when corrected for gender, time after intake, and race (P = 0.355).
Another possible explanation for the observed effect of female gender on plasma efavirenz concentrations is the use of hormonal contraceptives in a subset of the female subjects. We were able to obtain information on hormonal contraceptive use in 39 of the 66 female patients in our cohort. Eight of these were taking some form of hormonal contraception, but its use was not associated with higher plasma efavirenz concentrations. An opposite trend towards higher plasma efavirenz concentrations was found in females who reported that they did not use hormonal contraceptives compared with those who did (mean values 5.0 vs. 2.7 mg l−1; mean difference: 2.2 mg l−1 (95% CI −0.49, 4.9 mg l−1; P = 0.10).
To investigate a possible genetic basis for the observed differences in plasma efavirenz concentrations between different ethnic groups, we have evaluated the CYP2B6 C1459T polymorphism in the 228 samples for which DNA could be amplified. A large majority of the patients (83%) were identified as wild type (CC) carriers, whereas heterozygous (CT) and homozygous (TT) variants were found in 15 and 2.6% of the patients, respectively (Table 1). A difference in the frequency of the variant allele (CT and TT combined) was observed. Using Caucasians (frequency 22%) as the reference, this was significantly lower in Blacks (mean difference −13%; 95% CI −25.0, −1.7%; P = 0.024) and in Asians (mean difference −22%; 95% CI −29.1, −14.0%; P < 0.001). However, this genetic polymorphism was not linked to differences in plasma efavirenz concentrations. Taking the CC genotype (mean concentration 3.2 mg l−1) as the reference, the mean difference (95% CI) was −0.81 mg l−1 (95% CI −1.7, 0.03 mg l−1; P = 0.058) for the CT genotype, and −0.88 mg l−1 (95% CI −2.8, 1.1 mg l−1; P = 0.37) for the TT genotype.
Discussion
Our study is the largest interpatient comparison of the pharmacokinetics of efavirenz. The most important observations are the consistently higher plasma efavirenz concentrations in female patients and non-Caucasian patients. In a posthoc subgroup analysis, female non-Caucasian patients had 60% higher plasma efavirenz concentrations than male Caucasian patients. Thus, when treating patients with efavirenz, physicians should be aware of a higher risk for drug-induced toxicity in females and non-Caucasian patients [9, 12].
Far fewer patients had subtherapeutic plasma concentrations of efavirenz (<1.0 mg l−1) than those with toxic concentrations (>4.0 mg l−1). This may have been caused by our exclusion criterion for nonadherent patients (based on the opinion of the physician and/or the absence of detectable efavirenz concentration in the TDM sample). The inclusion of data from these samples would have confounded our analysis of the factors influencing plasma efavirenz concentrations. Another reason for the higher proportion of toxic plasma efavirenz concentrations may be selection bias by physicians in the sending of samples for analysis. TDM of efavirenz is recommended in the Netherlands for all patients at week 4 and 24 after starting treatment, and when there is a suspicion of toxicity, suboptimal therapy, drug–drug interaction, or nonadherence. Given the substantial number of patients with toxic plasma efavirenz concentrations, it is important to investigate potential causative factors.
As reported earlier in a preliminary analysis of a smaller cohort in our TDM service [14], female patients had a significantly higher plasma efavirenz concentration than male patients. The consequence might be an increased risk of efavirenz-induced toxicity in female patients. Several cohort studies have demonstrated a 1.5–1.7 fold higher risk of adverse drug reactions in female patients using antiretroviral agents (reviewed in [17]), although details from those using efavirenz are not available. Recently, Spire et al. found that female patients had a 2.2 times (95% CI 1.2, 3.8) higher risk of discontinuing treatment with efavirenz than males [18].
An effect of ethnic group also became apparent in our analyses, although this can partly be explained by a higher proportion of females among non-Caucasian (47%) compared with Caucasian patients (14%). Nevertheless, this effect was significant in a multivariate analysis when corrected for gender and time after drug intake. Non-Caucasian females and males were found to have higher plasma efavirenz concentrations than their Caucasian counterparts. Pfiser et al. also observed a lower hepatic clearance of efavirenz in a combined group of African-Americans/Hispanics compared with white non-Hispanics [6].
We examined several factors that could have contributed to these gender and ethnic effects. First, differences in body weight, height, or body composition are present between females and males, and to some extent between races. However, body weight, height and body surface area were not related to plasma efavirenz concentration when corrected for gender, race and time after intake of medication. Second, the use of hormonal contraceptives by a subgroup of female patients may have led to inhibition of the CYPB6-mediated metabolism of efavirenz, as reported earlier for bupropion, another substrate of this enzyme [19]. However, we could not confirm inhibition of metabolism and even observed a trend towards lower efavirenz exposure in females using hormonal contraceptives. In addition, the Summary of Product Characteristics of Stocrin/Sustiva® states that no effect of a single dose of ethinyl oestradiol was observed on the steady-state pharmacokinetics of efavirenz [20]. It should be noted that the subgroup of female patients using oral contraceptives was relatively small in the present study.
The metabolism of efavirenz is predominantly catalyzed by cytochrome P450 2B6 [26]. By amplification of DNA from plasma we were able to detect the CYP2B6 C1459T polymorphism in 17% of the subjects. This value is comparable with those reported by other groups, although the frequency of this polymorphism varies among races [16, 21–24]. The presence of this allele has been associated with low CYP2B6 protein expression, and thus low catalytic activity in human liver samples [14]. However, we did not observe any relationship between this genotype and plasma efavirenz concentration. Preliminary data from other groups suggest that the presence of the G516T variant in CYP2B6 which is present in haplotypes CYP2B6*6 and CYP2B6*7 is associated with elevated efavirenz concentrations [21, 25]. Unfortunately, we were not able to determine the G516T SNP in DNA from our patients due to technical limitations of the genetic assay.
Differences in comedication between subjects may cause variability in exposure to efavirenz. However, information on comedication is usually not provided on requests for TDM. Inhibitors of CYP450 enzymes such as ritonavir, ketoconazole, and clarithromycin demonstrate no or minimal effects on plasma efavirenz concentrations [5, 20], confirming the limited contribution of CYP3A4 on efavirenz clearance [26].
In conclusion, this analysis of our TDM service database has demonstrated a significant effect of gender and race on plasma efavirenz concentrations, which was independent of body composition, hormonal contraceptive use, or the CYP2B6 C1459T polymorphism. Our findings indicate an increased risk of efavirenz-induced toxicity in females and non-Caucasian patients. Further research is needed to identify other factors that may influence exposure to efavirenz, allowing further optimization of treatment with this highly potent drug.
Acknowledgments
We would like to thank the following people who helped with data collection: Bert Zomer, Minny Meeuwissen, Iman Padmos, Anja van den Berg, Nienke Langebeek, Piet van der Meulen, and Willemien Dorama.
References
- 1.Panel on clinical practices for treatment of HIV infection, convened by the Department of Health and Human Services DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. 2004. March 23.
- 2.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, Stryker R, Johnson P, Labriola DF, Farina D, Manion DJ, Ruiz NM. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 3.Van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, Cahn P, Lalloo UG, van der Westhuizen IP, Malan DR, Johnson MA, Santos BR, Mulcahy F, Wood R, Levi GC, Reboredo G, Squires K, Cassetti I, Petit D, Raffi F, Katlama C, Murphy RL, Horban A, Dam JP, Hassink E, van Leeuwen R, Robinson P, Wit FW, Lange JM. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA, III, Acosta EP, Schackman BR, Pilcher CD, Murphy RL, Maher WE, Witt MD, Reichman RC, Snyder S, Klingman KL, Kuritzkes DR. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 5.Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin Pharmacokinet. 2001;40:893–905. doi: 10.2165/00003088-200140120-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pfister M, Labbe L, Hammer SM, Mellors J, Bennett KK, Rosenkranz S, Sheiner LB. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003;47:130–7. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundage RC, Yong FH, Fenton T, Spector SA, Starr SE, Fletcher CV. Intrapatient variability of efavirenz concentrations as a predictor of virologic response to antiretroviral therapy. Antimicrob Agents Chemother. 2004;48:979–84. doi: 10.1128/AAC.48.3.979-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 9.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 10.Joshi AS, Barrett JS, Fiske WD, Pieniaszek HJ, Ludden TM, Bacheler LT, Ruiz NM. Population pharmacokinetics of efavirenz in phase II studies and relationship with efficacy. 2000. 39th ICAAC, September 26–29 1999, San Francisco, Califormia, USA, (Abstract) 1201.
- 11.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–70. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Nunez M, Gonzalez dR, Gallego L, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Higher efavirenz plasma levels correlate with development of insomnia. J Acquir Immune Defic Syndr. 2001;28:399–400. doi: 10.1097/00126334-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. Optimising TDM in HIV clinical care. A practical guide to performing therapeutic drug monitoring (TDM) for antiretroviral agents. Available: http://www.HIVpharmacology.com Last visited March 2, 2005.
- 14.Burger DM, La Porte C, Van der Ende M, Miesen J, Koopmans P. Gender-related differences in efavirenz pharmacokinetics. 4th International Workshop on Clinical Phamacology of HIV Therapy; March 27–29 2003; Cannes, France. [Google Scholar]
- 15.Aarnoutse RE, Grintjes KJ, Telgt DS, Stek M Jr, Hugen PW, Reiss P, Koopmans PP, Hekster YA, Burger DM. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin Pharmacol Ther. 2002;71:57–67. doi: 10.1067/mcp.2002.121424. [DOI] [PubMed] [Google Scholar]
- 16.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Gender differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 18.Spire B, Carrieri P, Garzot MA, L’henaff M, Group T, Obadia Y. Factors associated with Efavirenz discontinuation in a large community-based sample of patients. AIDS Care. 2004;16:558–64. doi: 10.1080/09540120410001716342. [DOI] [PubMed] [Google Scholar]
- 19.Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K. Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2003;74:326–33. doi: 10.1016/S0009-9236(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 20.Stocrin®. Hertfordshire, United Kingdom: Merck Sharp & Dohme Limited; 2002. Summary of Product Characteristics. [Google Scholar]
- 21.Tsuchiya K, Gatanaga H, Tachikawa N, et al. Homozygous CYP2B6*6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–6. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 22.Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, Fackenthal JD, Rogan PK, Ring B, Wrighton SA, Schuetz EG. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther. 2003;307:906–22. doi: 10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- 23.Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Murdter TE, Roots I, Brockmoller J. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–26. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Ingelman-Sundberg M. Human drug metabolising cytochrome P450 enzymes: properties and polymorphisms. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:89–104. doi: 10.1007/s00210-003-0819-z. [DOI] [PubMed] [Google Scholar]
- 25.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, Clifford DB, Hulgan T, Marzolini C, Acosta EP. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 26.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]


