Abstract
Aims
To determine the population pharmacokinetics of chlorproguanil, dapsone and the active metabolite of chlorproguanil, chlorcycloguanil; and to estimate the duration of parasitocidal activity for chlorpoguanil/dapsone against Plasmodium falciparum isolates of varying sensitivity.
Methods
Rich and sparse pharmacokinetic data were collected prospectively from: healthy volunteers (n = 48) and adults (n = 65) and children (n = 68) suffering from P. falciparum malaria. All subjects received 2.0 mg kg−1 of chlorproguanil and 2.5 mg kg−1 of dapsone.
Results
The population pharmacokinetic parameter estimates for chlorproguanil were ka = 00.09 h−1 (intersubject variability was 44%), CL/F = 51.53 l h−1 (57%), CLD/F = 54.67 l h−1, V1/F = 234.40 l (50%) and V2/F = 1612.75 l; for dapsone were ka = 00.93 h−1, CL/F = 1.99 l h−1 (72%) and V/F = 76.96 l (48%); and for chlorcycloguanil were CLm/Fm = 3.72 l h−1 kg−1 (67%) and Vm/Fm = 12.76 l kg−1 (64%). For dapsone, CL/F and V/F were both significantly positively correlated with body weight. For a 10-kg child, the mean duration of parasitocidal activity for chlorproguanil/dapsone against the three most susceptible P. falciparum strains was 4.5 days [5thand 95th percentiles 2.4, 7.3] for W282; 5.9 days (3.6, 9.7) for ItG2F6; and 6.1 days (3.7, 10.1) for K39. For an isolate with the ile-164-leu mutation, V1/S, activity ranged from 0.8 days (0.0, 3.3) for a 10-kg child to 1.8 days (0.0, 4.0) for a 60-kg adult.
Conclusions
Plasmodium falciparum malaria has no effect on the pharmacokinetic parameters for chlorproguanil, dapsone or chlorcycloguanil. Chlorproguanil/dapsone will probably prove to be ineffective against parasite strains with the mutation ile-164-leu, were these to become prevalent in Africa.
Keywords: chlorcycloguanil, chlorproguanil, dapsone, pharmacodynamics, Plasmodium falciparum, population pharmacokinetics
Introduction
The greatest burden of Plasmodium falciparum malaria is found in sub-Saharan Africa; approximately 2 million people die from P. falciparum malaria annually in Africa [1]. For many years chloroquine was the primary antimalarial agent deployed in Africa, but in most areas there is now major resistance to chloroquine and alternative drugs are urgently needed [2]. Sulfadoxine-pyrimethamine has been the first-choice drug for uncomplicated falciparum malaria in much of Africa [3]. However, both pyrimethamine and sulfadoxine are eliminated slowly [4], possibly leading to rapid drug resistance, which has now been observed in many areas of Africa [2].
The antifolate combination chlorproguanil/dapsone (Lapdap; CPG/DDS) has been identified as a possible replacement for sulfadoxine-pyrimethamine. In comparison with sulfadoxine-pyrimethamine, both components of CPG/DDS are eliminated rapidly from the body [5, 6]. CPG/DDS is considerably cheaper than other recently developed antimalarial drugs (public sector price of less than US$0.50/adult 3-day course). Before CPG/DDS is introduced into routine therapy the potential for differences in the pharmacokinetics of CPG/DDS between malaria patients (the target population) and healthy volunteers, and children and adults needs to be investigated. Intensive blood sampling is not always feasible or ethical to perform on malaria patients. However, sparse data (i.e. only a few blood samples per subject) can now be included in pharmacokinetic modelling using the ‘population approach’[7].
The primary aim of the present study was to provide population pharmacokinetic parameters for CPG, DDS and chlorcycloguanil (CCG: the active metabolite of chlorproguanil) and to investigate any associations between the pharmacokinetics of these drugs and their metabolites with P. falciparum malaria, age and body weight. A further aim of the study was to incorporate into the analysis a pharmacodynamic model based on the in vitro parasitocidal activity of CCG/DDS, in order to estimate the duration of parasitocidal activity in vivo for CPG/DDS against P. falciparum isolates of varying sensitivity.
Methods
Study population
Pharmacokinetic data were collected prospectively from three different groups of patients: (i) healthy adults, aged 18–65 years, were recruited in London, UK during 1999 (as a Phase-I clinical trial of the drug); (ii) adults with uncomplicated falciparum malaria were recruited from the Tropical Diseases Research Centre, Ndola, Zambia in 2000 (a Phase-II clinical trial); (iii) children with uncomplicated falciparum malaria were recruited from the Medical Research Council Laboratories, The Banjul, Gambia in 2000 (also a Phase-II trial). All subjects, or their guardians, gave written informed consent. The protocol was approved by the Standing Committee on Research in Human Subjects (SCRIHS) of the World Health Organization, the Joint MRC/Gambia Governments Ethics Committee, and the Institutional Review Board of the Tropical Diseases Research Centre, Ndola, Zambia.
Adults with malaria were aged 18–60 years and were of both sexes (women were either of nonchild-bearing potential or using contraception). Patients had clinically uncomplicated disease and pure P. falciparum parasitaemia at baseline ranging from 2000 to 100 000 µl−1. Exclusion criteria were: severe malaria [8], concomitant infection at the time of presentation, other long-term medical conditions that could interfere with evaluation of the response, history of allergy and treatment within the past 7 days with an antimalarial drug.
Children with uncomplicated malaria were aged 3 months to 12 years. The acceptable range of parasite counts and exclusion criteria were the same as those for adult patients. Safety and efficacy data in children and adults will be reported elsewhere.
Chlorproguanil/dapsone dosing
Target doses of CPG (2.0 mg kg−1) and DDS (2.5 mg kg−1) were identical for all groups. For 24 of the healthy volunteers (part of a bioequivalence study), two ‘adult-sized’ tablets (Lapdap-80: CPG 80 mg and DDS 100 mg per tablet) were given as a single oral dose on day 0. For the remaining 24 healthy volunteers (part of an interaction study), a CPG/DDS solution was given as a single oral dose on day 0 (CPG 2.0 mg kg−1 and DDS 2.5 mg kg−1). The Zambian adults (n = 65) were administered orally ‘adult-sized’ tablets daily over 3 days. The Gambian children (n = 77) were given orally ‘paediatric-sized’ tablets (Lapdap-15: CPG 15 mg and DDS 18.75 mg per tablet) daily for 3 days. Young children unable to swallow the tablets were given a suspension of CPG/DDS prepared by crushing the tablets and mixing them with water on a spoon. The subjects with P. falciparum malaria were observed for post-dose vomiting. If the subject vomited within 0.5 h of dose, the subject was re-dosed. Further vomiting resulted in exclusion from the study. All subjects given a weight-adjusted dose of CPG/DDS had their exact dose calculated (i.e. dose in the units, mg).
Blood sampling
In the case of the healthy adult subjects (n = 48), blood (10 ml) was drawn from an intravenous cannula at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 18, 24, 48, 72, 96 h. For 11 Gambian children with malaria, blood (2 ml) was collected (using a cannula) at 0, 1, 2, 6, 8, 12, 24 h. For the remaining children and adults with malaria, patients were allocated to predefined sampling schedules at recruitment. All were sampled before dosing. Thereafter, each patient was sampled on one further occasion from the following times: 1, 2, 4, 6, 8, 12, 24, 28, 36, 48, 52, 72 and 96 h after the first dose. Blood (2 ml) was drawn by venepuncture. Blood was drawn into lithium heparinized tubes, then centrifuged (1500 g) for 10 min within 1 h of sampling. Plasma was removed and stored at −20 °C in plain polypropylene tubes until assayed.
Drug and metabolite assays
Plasma concentrations of CPG and CCG were determined by liquid chromatography and tandem mass spectrometry (LCMS/MS); DDS was determined by high-performance liquid chromatography (HPLC) with ultraviolet (UV) absorption detection. For the determination of CPG and CCG, 1 ml of plasma was extracted with ethyl acetate (5 ml) after basification with sodium hydroxide (1 ml; 0.1 m). The ethyl acetate extract was removed, evaporated to dryness under nitrogen and the sample reconstituted in mobile phase (200 µl) prior to LCMS/MS. LCMS/MS was performed using a Spectra series P2000 binary pump coupled to an LCQDeca ion trap mass spectrometer (Thermofinnigan). Separation was achieved on a 3-µm hypurity elite C18 column (Thermohypersil) with a mobile phase composition of water/acetonitrile/methanol (60/27/13) containing 0.1% formic acid buffered to pH 2.0 and flowing at 0.4 ml min−1. Under these conditions CCG eluted at 3.2 min, CPG at 8.7 min and the internal standard proguanil (100 ng ml−1) at 4.6 min. Analytes were quantified from their LCMS/MS product ions at 229, 204 and 170 atomic mass unit, respectively. Calibration curves were linear between 0 and 200 ng ml−1 with the lower limit of quantification equal to 5 ng ml−1. Inter- and intra-assay coefficients of variation at 5 ng ml−1, 50 ng ml−1 and 100 ng ml−1 were <15%. For the determination of DDS, 0.5 ml of plasma containing the internal standard (pyrimethamine 500 ng, dissolved in methanol) was extracted with 5 ml of dichloromethane by vortex mixing (20 s). The samples were then centrifuged (1600 g for 10 min), after which the organic (bottom) layer was transferred to a clean tube. This extraction was repeated and the organic phases combined. The pooled organic layers were evaporated to dryness under nitrogen at 30 °C. Samples were reconstituted in 100 µl of mobile phase, of which 50 µl was injected onto the HPLC column. The HPLC system consisted of a spectra series P100 pump, an AS300 autosampler and a UV100 detector. The mobile phase used in this system consisted of acetonitrile (far UV)/water/methanol/triethylamine (10 : 69.9 : 20 : 0.1 v/v), at a flow rate of 1 ml min−1. Separation was achieved on a Phenomenex Synergi 4-µm MAX RP C12 (150 cm × 4.6 mm, 4 µm partical size) column, fitted with a LiChrosphere® (100Rp-18, 5µ guard column). Analytes were detected by UV absorption at 254 nm. Calibration curves were linear in the range 0 up to 2500 ng ml−1 with DDS eluting at 6.0 min and the internal standard at 12.5 min. The lower limit of quantification was 100 ng ml−1 and the inter- and intra-assay coefficients of variation at 150, 750 and 2000 ng ml−1 were <15%.
Pharmacokinetic models
The choice of the pharmacokinetic model was based on a comparison of Akaike's information criterion for fitted one- and two-compartment models with first-order input, as well as visual inspection of goodness-of-fit plots, including observed vs. predicted and residual vs. predicted value.
A two-compartment model with first-order absorption was selected as the kinetic model for CPG. The fundamental parameters used to characterize the two-compartment model were:
ka, absorption rate constant
CL/F, apparent clearance
CLD/F, intercompartmental clearance
V1/F, volume of distribution of the central compartment
V2/F, volume of distribution of the peripheral compartment.
A one-compartment model with first-order absorption and first-order elimination was selected as the kinetic model for DDS. The fundamental pharmacokinetic parameters used to characterize the one-compartment model were:
ka, absorption rate constant
CL/F, apparent clearance
V/F, apparent volume of distribution.
A two-compartment metabolite model was fitted to the CCG data. The apparent volume of distribution for CCG (Vm/Fm) and the apparent clearance of CCG (CLm/Fm) were estimated from the modelling. The posterior individual estimates of the pharmacokinetic parameters obtained from fitting a two-compartment model to the CPG data were treated as data variables in the metabolite model. This technique is known as ‘sequential modelling’.
Data analysis
The NLME procedure [9] of the SPLUS data programme (SPLUS, Insightful Corp., Seattle, WA, USA) was used to calculate estimates of the population pharmacokinetic parameters and their respective intersubject variances. The program also provides estimates of the residual random intrasubject error (ɛ) and the variances of the intersubject error terms. Convergence was achieved when the objective value did not differ by more than some prespecified difference (e.g. 0.0001) and the program returned the final estimates of the population pharmacokinetic parameters. The objective function (minus twice the log-likelihood of the data) was used to determine the model that best fitted the data. Models with different covariance structures (e.g. having different numbers of random effects) and with different distributions for the intrasubject error (e.g. additive vs. lognormal) were compared. A significant drop in the objective function (using the χ2 distribution with degrees of freedom equal to number of parameters which are set equal to a fixed value in the restricted model) from the general model to the restricted model was used to determine the final pharmacokinetic model. The goodness of fit of the final pharmacokinetic model was also determined by the precision of the parameter estimates and examination of the scatter plot of residuals vs. predicted concentrations. The individual pharmacokinetic parameters were calculated using the posterior estimates.
Intersubject variability in the pharmacokinetic parameters and the residual intrasubject error for CPG, DDS and CCG were modelled with log-normal error models.
The magnitude of the intersubject and intrasubject variability was expressed as a coefficient of variation (% CV), approximated by the square root of the variance estimate.
Covariates
The covariates investigated were body weight (kg); healthy volunteers vs. P. falciparum malaria patients; and adults vs. children. Covariates that were continuous variables (i.e. body weight) were centred around their median values so that the population estimates would represent those of an average patient, for example Pi = [P + θ × (wti − wtmed)] × exp(ηPi), where Pi is pharmacokinetic parameter for individual i; wti is the body weight of the ith individual; and P is the pharmacokinetic parameter for an individual with the median body weight (wtmed) of 62.25 kg.
Pharmacodynamic models
Data on the pharmacodynamic (parasitocidal) activity of CCG/DDS were obtained from in vitro studies in which parasite inhibition was measured for four established, well-characterized strains of P. falciparum with a range of concentration combinations [10]. The K39 strain carries the dhfr ser-108-asn mutation and is typical of Kenyan pyrimethamine-resistant parasites. The ItG2F6 strain carries the dhfr ser-108-thr and ala-16-val mutations that confer reduced susceptibility to triazine inhibition [11]. W282 has three dhfr point mutations (asn-51-ile, cys-59-arg and ser-108-asn) and two dhps mutations. V1/S has four dhfr mutations (asn-51-ile, cys-59-arg, ser-108-asn and ile-164-leu) and two dhps mutations. Equation 1 [12] was used to fit the data from the synergy isobologram studies. The axes of the isobologram are normalized according to the respective IC50s of CCG and DDS. Loewe additivity is indicated, meaning that no interaction between CCG and DDS exists, when the coefficient, α, equals zero. Loewe synergism is indicated by positive values of α and loewe antagonism is indicated by negative values of α.
where [DDS] is the concentration of DDS (mg ml−1), [CCG] is the concentration of CCG (ng ml−1), IC50 is the concentration required to reduce control response by 50%, α determines the extent to which the isobolograms concave (synergy), and β determines the degree of asymmetry (around y = x) of the isobolograms.
The duration of parasitocidal activity, defined as the time during which sufficient unbound plasma concentration combinations of CCG and DDS are present to exceed the in vitro derived IC50s, was estimated for each isolate. This was accomplished by performing Monte Carlo simulations with 10 000 replications in Microsoft Excel 2000. The mean and 95% confidence intervals (CI) for expected plasma CCG and DDS concentrations following three-dose regimens (according to the package-insert dosing instructions, Table 1) were calculated using the population pharmacokinetic model, for patients ranging between 5 and 80 kg in body weight. Free drug concentrations were calculated by assuming the unbound fractions for CCG and DDS of 0.721 and 0.321, respectively [6].
Table 1.
Dosing table for chlorproguanil/dapsone (CPG/DDS)
| Body weight (kg) | Dose of CPG (mg day−1) | Dose of DDS (mg day−1) |
|---|---|---|
| <5 | Not recommended | Not recommended |
| 5–7.9 | 15 | 18.75 |
| 8–11.9 | 22.5 | 28.125 |
| 12–15.9 | 30 | 37.5 |
| 16–20.9 | 40 | 50 |
| 21–39.9 | 80 | 100 |
| 40–59.9 | 120 | 150 |
| >60 | 160 | 200 |
Results
Study patients and samples
Nine of the Gambian children were excluded because either they did not receive all three doses of CPG/DDS (n = 6) or were not successfully re-dosed (n = 3). Table 2 gives the number of CPG, DDS and CCG concentrations (excluding 0-h samples), the number of subjects and the number of concentrations per subject available for analysis. A few of the malaria patients did not contribute concentrations for the analysis because their recorded post-dose concentration was not detectable. Data were missing for five of the healthy volunteers who withdrew from the study for personal reasons.
Table 2.
Number of subjects/concentrations available for population pharmacokinetic modelling
| Group | No. of subjects | No. of concentrations | Median no. of concentrations per subject (range) |
|---|---|---|---|
| Healthy volunteers—tablets | |||
| CPG | 20 | 290 | 15 (12–15) |
| DDS | 20 | 295 | 15 (13–15) |
| CCG | 19 | 204 | 10 (7–14) |
| Healthy volunteers—solution | |||
| CPG | 23 | 340 | 15 (13–15) |
| DDS | 23 | 343 | 15 (14–15) |
| CCG | 23 | 238 | 10 (7–13) |
| Zambian adult patients | |||
| CPG | 65 | 65 | 1/subject |
| CPG (pre 2nd dose) | 65 | 35 | 1/subject |
| DDS | 65 | 65 | 1/subject |
| DDS (pre 2nd dose) | 35 | 35 | 1/subject |
| CCG | 58 | 58 | 1/subject |
| CCG (pre 2nd dose) | 28 | 28 | 1/subject |
| Gambian children patients | |||
| CPG | 65 | 118 | 1 (1–7)* |
| CPG (pre 2nd dose) | 41 | 94 | 1 (1–7)* |
| DDS | 66 | 117 | 1 (1–7)* |
| DDS (pre 2nd dose) | 40 | 91 | 1 (1–7)* |
| CCG | 58 | 89 | 1 (1–5) |
| CCG (pre 2nd dose) | 35 | 66 | 1 (1–5) |
One subject had two blood samples recorded at 1 h post dose.
Very few concentrations were recorded post the second and third doses given to the Zambian adults and Gambian children. Therefore, the modelling below includes only the concentrations recorded before the second dose (i.e. up to and including 24 h post dose) was given to the Zambian adults and Gambian children. The drug concentrations recorded post the second and third doses were compared with simulated values.
Chlorproguanil (CPG)
The model that gave the best fit to the data was a two-compartment model with first-order absorption and with ka, CL/F, and V1/F fitted as both fixed and random effects. The data did not support a full variance–covariance matrix for the random effects, so only the variances of the random effects, ka, CL/F and V1/F, were estimated. Figure 1 shows the observed values and population predicted values vs. time. The residual plot indicated no bias in estimation for the concentrations from the healthy volunteers and Zambian adults, whereas for the Gambian children the model slightly over-estimated the higher CPG plasma concentrations at the earlier times and underestimated the lower CPG plasma concentrations at the latter times (data not shown). The individual concentration–time curves were characterized adequately (data not shown). The mean population pharmacokinetic parameters and interindividual variability are presented in Table 3. There were no differences observed between the healthy volunteers and the Zambian adult patients suffering from P. falciparum malaria for the pharmacokinetic parameters ka, CL/F and V1/F. The covariates, ‘children’ and ‘body weight’, improved the objective function significantly when included as fixed effects in the population pharmacokinetic model. The decrease in ka for a child compared with an adult was 00.03 h−1 (95% CI 0.01, 0.05) and the increase in CL/F associated with an increase in 1 kg of body weight was 0.45 l h−1 (95% CI 0.21, 0.69).
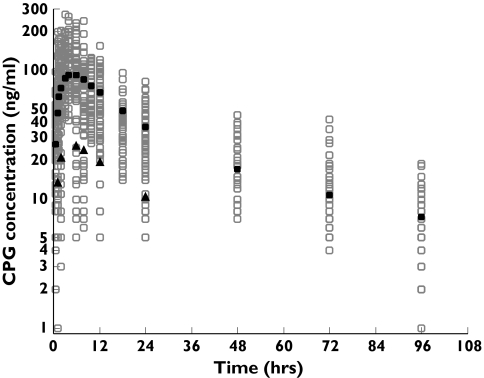
Figure 1.
Observed (○) and population predicted concentrations of chlorproguanil (CPG) vs. time for healthy volunteers, and Zambian adults and Gambian children with Plasmodium falciparum malaria. The population predicted concentrations represent an adult dose of 160 mg (•) and a child dose of 45 mg (▴)
Table 3.
Population pharmacokinetic parameters of chlorproguanil (CPG) and dapsone (DDS) for the base model
| Parameter | Estimate (SE) | Interpatient variability (% CV) |
|---|---|---|
| CPG | ||
| ka (h−1) | 0.09 (0.01) | 44 |
| CL/F (l h−1) | 51.53 (3.81) | 57 |
| CLD/F (l h−1) | 54.67 (5.77) | |
| V1/F (l) | 234.40 (31.82) | 50 |
| V2/F (l) | 1612.75 (149.50) | |
| σɛ* (% CV) | 39 | |
| AUC (ng ml−1 h−1) = | 3105 | |
| dose/CL/F | (for an adult dose of 160 mg) | |
| t1/2 elim (h) | 34.7 | |
| DDS | ||
| ka (h−1) | 0.93 (0.05) | |
| CL/F (l h−1) | 1.99 (0.16) | 72 |
| V/F (l) | 76.96 (3.95) | 48 |
| σɛ* (% CV) | 33 | |
| AUC (ng ml−1 h−1) = | 100503 | |
| dose/CL/F | (for an adult dose of 200 mg) | |
| t1/2 elim (h) | 26.67 | |
Lognormal error model.
Simulated pharmacokinetic profiles, using the population parameter estimates derived in Table 3, lie within the centre of the observed CPG plasma concentrations assayed at times post the second and third doses of CPG (data not shown).
Dapsone (DDS)
The model that gave the best fit to the data was a one-compartment model with first-order absorption and with CL/F and V/F fitted as both fixed and random effects. The variances and covariance of the random effects were estimated from the data. Figure 2 shows a scatter plot of the observed values and population predicted values vs. time. The residual plot indicated no bias in estimation and the individual concentration–time curves were characterized adequately (data not shown). The mean population pharmacokinetic parameters and interindividual variability are presented in Table 3. There were no differences observed between the healthy volunteers and the Zambian adult patients suffering from malaria for the pharmacokinetic parameters CL/F and V/F. When comparing the posterior estimates of CL/F and V/F between the children and adults, children were observed to have significantly lower values for CL/F and V/F. However, the children had significantly lower body weights compared with the adults and body weight was observed to be significantly positively correlated with both the posterior estimates of CL/F and V/F. After performing multiple linear regressions with the dependent variable being either the posterior estimates of CL/F or V/F, body weight was observed to be the significant independent predictor of both CL/F and V/F. Children were now observed to have higher values for CL/F and V/F, although the difference did not reach statistical significance for either CL/F or V/F. The covariate, ‘body weight’, improved the objective function significantly of the population pharmacokinetic model. An increase in V/F of 0.70 l (95% CI 0.48, 0.92) and an increase in CL/F of 0.03 l h−1 (95% CI 0.02, 0.04) were associated with an increase in 1 kg of body weight.
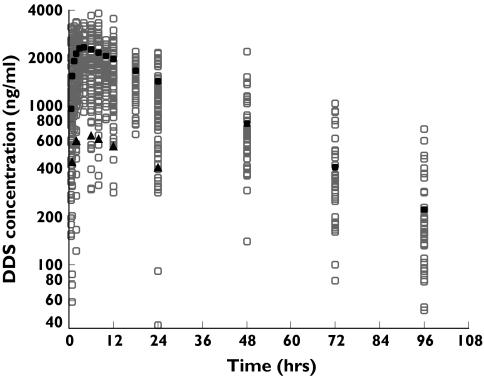
Figure 2.
Observed (○) and population predicted concentrations of dapsone (DDS) vs. time for healthy volunteers, and Zambian adults and Gambian children with Plasmodium falciparum malaria. The population predicted concentrations represent an adult dose of 200 mg (•) and a child dose of 56.25 mg (▴)
Figure 3 presents the observed DDS plasma concentrations vs. time for the Zambian adults and Gambian children, respectively. Superimposed on the graphs are the pharmacokinetic profiles for different dosing regimes where the DDS dose has been given daily over 3 days. The pharmacokinetic profiles were simulated using the population parameter estimates derived in Table 3. The pharmacokinetic profiles do not lie within the centre of the observed DDS plasma concentrations assayed at times post the second and third doses of DDS for the Zambian adults. The observed DDS plasma concentrations are much lower than the predicted values after 24 h. The pharmacokinetic profiles lie within the centre of the observed DDS concentrations assayed at times post the second and third doses of DDS for the Gambian children.
Figure 3.
Observed dapsone (DDS) concentrations and simulated DDS concentrations (using population mean parameter estimates in Table 3) vs. time for the Zambian adults (○) and Gambian children (•). [Dosing of 200 mg for the Zambian adults and 56.25 and 28.1 mg for the Gambian children of chlorproguanil (as part of Lapdap) daily for 3 days. Note, other doses were administered to the Gambian children, for clarification only two doses were presented on the graph.]
Chlorcycloguanil (CCG)
The model that gave the best fit to the data was a two-compartment metabolite model with both CLm/Fm and Vm/Fm fitted as both fixed and random effects. The data did not support a full variance–covariance matrix for the random effects, so only the variances of the random effects were estimated. Figure 4 shows the observed values and population predicted values vs. time. As can be seen, the model underpredicted the observed CCG concentrations at the later time points: 48, 72 and 96 h. The model would not converge when the data from the Gambian children were added if the exact dose (mg) was modelled as opposed to the exact weight-adjusted dose (mg kg−1). The mean population estimates were 3.72 l h−1 kg−1 (intersubject variability was 67%) and 12.76 l kg−1 (64%) for CLm/Fm and Vm/Fm, respectively.
Figure 4.
Observed (○) and population predicted concentrations (•) of chlorcycloguanil (CCG) vs. time for healthy volunteers, and Zambian adults and Gambian children with Plasmodium falciparum malaria. [Note there are different population predicted concentrations at the same time points due to the different exact dosages received and due to the differences in the posterior individual estimates of ka, CL/F and V1/F from modelling of the chlorproguanil (CPG) data.]
There were no differences observed between the healthy volunteers and the Zambian patients suffering from malaria for the pharmacokinetic parameters CLm/Fm and Vm/Fm. When comparing the posterior estimates of CLm/Fm and Vm/Fm between the children and adults, children were observed to have significantly higher values for CLm/Fm and significantly lower values for Vm/Fm. After performing multiple linear regression with both weight and child as the independent variables, neither weight nor being a child was found to be independently associated with the posterior estimates of Vm/Fm, while weight was found to be significantly and independently negatively correlated with the posterior estimates of CLm/Fm. Note, both Vm/Fm and CLm/Fm are already a function of body weight because the dosage of CPG used in the modelling was the weight-adjusted dose (mg kg−1). The covariate ‘body weight’, which improved the objective function significantly of the population pharmacokinetic model, reduced the interindividual variability of CLm/Fm from 67% to 51%.
Simulated pharmacokinetic profiles, using the population parameter estimates for CLm/Fm and Vm/Fm and the population parameter estimates from the previous population pharmacokinetic modelling of CPG (see Table 3), were observed to lie within the centre of the observed CCG concentrations assayed at times post the second and third doses of CPG (data not shown).
Pharmacodynamics
Table 4 presents the IC50 for CCG and DDS (separately) against each P. falciparum isolate, together with parameter estimates for α and β of Equation 1. Figure 5 presents the fitted isobologram synergy plots for CCG/DDS vs. the four P. falciparum isolates. In decreasing order of synergy, as measured by the area under the isobolograms, are ItG2F6, W282, K39 and V1/S. Mean parasitocidal activities, for each parasite strain and according to body weight, are presented in Figure 6. For a 10-kg child, the mean duration of parasitocidal activity for CPG/DDS against the three most susceptible strains of P. falciparum was 4.5 days (5th, 95th percentiles 2.4, 7.3) for W282; 5.9 days (3.6, 9.7) for ItG2F6; and 6.1 days (3.7, 10.1) for K39. For an isolate with the ile-164-leu mutation, V1/S, activity ranged from 0.8 days (0.0, 3.3) for a 10-kg child up to 1.8 days (0.0, 4.0) for a 60-kg adult.
Table 4.
IC50 values for dapsone (DDS) and chlorcycloguanil (CCG) (alone) against K39, ItG2F6, W282 and V1/S strains, obtained from Watkins et al. 1997
| Parasite strain | IC50 DDS alone (mg ml−1) | IC50 CCG alone (ng ml−1) | α (SE) | β (SE) |
|---|---|---|---|---|
| K39 | 0.013 | 1.477 | 8.71 (6.35) | 0.23 (0.09) |
| ItG2F6 | 0.0055 | 4.5* | 13.65 (1.74) | 0.15 (0.02) |
| W282 | 0.0128 | 4.237 | 38.22 (<0.01) | 0.92 (<0.01) |
| V1/S | 0.006 | 41.95 | 3.82 (1.20) | 0.50 (0.08) |
Estimate; SE, standard error. α and β are parameter estimates for Equation 1.
Figure 5.
Chlorcycloguanil/dapsone (CCG/DDS) isobolograms for four different isolates of Plasmodium falciparum: W282 (▪), K39 (•), ItG2F6 (▴) and V1/S (♦)
Figure 6.
Plot of mean parasitocidal activity of chlorproguanil/dapsone (CPG/DDS) vs. four different strains of Plasmodium falciparum, K39 (•), ItG2F6 (▴), W282 (▪) and V1/S (♦)
Discussion
The purpose of this study was to determine the population pharmacokinetics of CPG, CCG and DDS from a mixture of rich and sparse data collected from healthy volunteers and the target population (i.e. patients suffering from P. falciparum malaria) and to use these population pharmacokinetic estimates in conjunction with a pharmacodynamic model based on in vitro parasitocidal activity to determine the duration of parasitocidal activity in vivo for CPG/DDS.
For CPG, the population pharmacokinetic estimates were observed to be: 00.09 h−1 for ka, 51.53 l h−1 for CL/F, 54.67 l h−1 for CLD/F, 234.40 l for V1/F and 1612.75 l for V2/F. These estimates give an elimination half-life for CPG of 34.7 h, similar to that reported by Peterson and coworkers (31–44 h [13]) (these authors report a slightly lower clearance) and longer than the value reported by Winstanley and colleagues [6]. Importantly, the value for half-life reported by Winstanley and colleagues is likely to be an underestimate, given that the limit of quantification for the analysis of CPG is much higher than that reported here. Veenendaal and colleagues [14] report a half-life value closer to Winstanley et al.[6] and a clearance value nearer that quoted here, around 1.3 l h−1 kg−1. Notably, in our study, the last sampling time was at 96 h post the first dose of CPG/DDS (approximately two to three elimination half-lives for CPG), and it would have been better to have the subjects sampled up to 120 h (approximately four elimination half-lives) for characterization of the elimination phase. Our estimate of ka was low, giving an absorption half-life of approximately 7 h, but our model did predict that the maximum concentration occurred around 4 h, consistent with the literature [6, 14]. It is possible that ka and the elimination rate constant of the first phase are confounded in the two-compartment model.
For CCG, which is of greater clinical importance than its parent drug CPG, the population pharmacokinetic parameter estimates were observed to be 3.72 l h−1 kg−1 for CLm/Fm and 12.76 l kg−1 for Vm/Fm. Winstanley and colleagues [6] could not estimate the elimination phase of CCG since CCG was detected in the plasma of only eight children with only one plasma sample available for three of the children. The observed maximum concentration of CCG for their data and our data were similar.
For DDS, the population pharmacokinetic estimates were observed to be 00.93 h−1 for ka, 1.99 l h−1 for CL/F and 76.96 l for V/F. DDS was absorbed quickly, which does not agree with a previous published study conducted in young children [6], who were given a daily dose of 2.4 mg kg−1 of DDS over 3 days. Our pharmacokinetic estimates were calculated from pharmacokinetic data collected over 24 h following an approximate single dose of 2.5 mg kg−1. Derived from the population pharmacokinetic estimates, the elimination half-life for our study was 26.67 h, similar to previous estimates [6, 15]. The population pharmacokinetics of DDS have been determined in children with human immunodeficiency virus infection [16]. The population estimates were 1.40 l kg−1 for V/F, 0.028 l h−1 kg−1 for CL/F and 2.66 h−1 for ka. Our estimates (calculated in corresponding units using the median body weight of our study population) were similar for V/F (1.24 l kg−1) and CL/F (0.03 l h−1 kg−1); however, we observed a slower absorption rate constant (00.93 h−1). This may be in part due to the blood sampling being different between the two studies. We observed greater interpatient variability in the pharmacokinetic parameters CL/F and V/F, which may reflect a dataset consisting of healthy adult volunteers and adults and children suffering from P. falciparum malaria.
The observed DDS concentrations were lower than the predicted values after the second and third doses of CPG/DDS for the Zambian adults. Given that all treatment was directly observed, there are three possible explanations for this observation (rooted in drug metabolism): (i) autoinduction of dapsone hydroxylation (by CYP3A) – dapsone is used as a probe for CYP3A4 and as a marker for induction of this enzyme [17, 18], but we are unaware of reports of autoinduction; (ii) autoinduction of phase II pathways – similarly, dapsone is used as a probe to assess induction of NAT2, but we are unaware of reports of autoinduction; (iii) age-dependent expression of glucuronosyl transferases (UGTs) – dapsone is N-glucuronidated, and there is evidence that many UGTs show differences in expression between livers of children and adults [19] and the UGTs responsible for the metabolism of dapsone are known (UGT1A4 and UGT 1A9) [20]. Certainly, UGT1A9 expression changes with age, but the trend is counter to the present observations.
No differences in any of the pharmacokinetic parameters for CPG, DDS and CCG were observed between adult P. falciparum malaria patients and healthy volunteers. Some differences were observed between the pharmacokinetic profiles of children and adults. However, no differences were observed between children and adults in terms of safety and efficacy of the 3-day dosing regimen [21]. The associations between body weight and the pharmacokinetic parameters of CPG and DDS confirm that the dose for CPG/DDS should be adjusted for body weight. Note the patients in the current dataset had body weights varying from 6 to 98 kg. Therefore the observed findings may not hold for small babies (age <3 months, body weight <6 kg).
The objective of antimalarial treatment is to maintain drug concentrations high enough to maintain a parasite multiplication rate per cycle of <1, until the last viable parasite has been killed. There are generally between 108 and 1013 parasites in the body during symptomatic malaria. Generally, the parasite population is exposed to very high drug concentrations soon after dosing, and 99.9% of the parasite ‘biomass’ may be killed during the first asexual cycle (approximately 48 h). However, the remaining 0.1% of the biomass still represents between 105 and 1010 parasites, which need to be killed in subsequent cycles. Thus, in a patient with a total body parasite load of 1012 (a relatively high biomass corresponding to a parasite count of approximately 100 000 µl−1 or 2% parasitaemia), treated with a drug that causes a 3-log reduction in parasite number per cycle (and CPG/DDS is unlikely to perform better than this), a ‘minimum parasitocidal concentration’ must be maintained for more than four cycles (i.e. >8 days for P. falciparum). The contribution of the immune system is difficult to quantify, but patients with a degree of immunity to local parasite strains are thought to be capable of removing a small residual parasite biomass even after drug concentrations have declined below therapeutic concentrations. In the case of synergistic combinations, such as sulfadoxine-pyrimethamine and CPG/DDS, one cannot speak of a ‘minimum parasitocidal concentration’ and a more complicated model is required. The synergistic model used in this study suggests the following mean durations of CPG/DDS parasitocidal activity: (i) between 5 and 6 days for the K39 isolate, (ii) between 5 and 6 days for ItG2F6, (iii) between 4 and 5 days for W282 and (iv) for the V1/S isolate parasitocidal activity remains below 2 days and is body weight-dependent. These data support the previous findings by Watkins et al.[10] and Winstanley et al.[6]. The K39 isolate has one point mutation of dhfr at position 108 and is therefore similar to around 90% of infections in East Africa. ItG2F6 has two dhfr point mutations, while W282 has three dhfr point mutations and two dhps mutations and is regarded as the current ‘worst-case’ scenario in East Africa. V1/S, which has four dhfr mutations (including ile-164-leu) and two dhps mutations, is not representative of isolates encountered in Africa [22], but is similar to isolates seen in South-east Asia. From the above, CPD/DDS, given as a 3-day monotherapy regimen, will not be effective against a parasite strain with the mutation ile-164-leu and it is likely that there will be recrudescence of parasites with the other three strains. Therefore, our results suggest that the addition of artesunate to CPG/DDS would result in a more efficacious treatment, and probably one that would select mutant parasites less readily. The development of a CPG/DDS and artesunate combination is currently in progress [23]. These results, which are a rigorous attempt to model CPG/DDS pharmacokinetic data with isobologram results obtained using laboratory parasite strains, should be interpreted with caution: (i) they cannot take account of host immunity; (ii) IC50-based estimates of parasitocidal activity are only approximations of the true ‘minimum parasitocidal concentration’-based estimates of parasitocidal activity; and (iii) they must be viewed alongside conventional clinical trial data that demonstrate the efficacy of CPG/DDS in Africa [21].
Acknowledgments
We thank the clinical staff of the Tropical Diseases Research Centre, Ndola, Zambia, and the Medical Research Council Laboratories, Banjul, The Gambia: the clinical trial work that generated the present data were conducted in those two sites. We also thank Atholl Johnson from the Royal London School of Medicine and Dentistry, London, UK, for the data from the healthy volunteers study. The present analysis received financial support from the ‘Lapdap Project’, itself a partnership of WHO-TDR with the UK Department for International Development and GlaxoSmithKline (GSK) Pharmaceuticals; the funding source played no part in the decision to publish, or in the preparation of the manuscript.
Conflict of interest
None of the authors has a conflict of interest, and none has benefited financially from the development of the registered drug Lapdap. P.W., S.W. and W.A.W. are members of the Lapdap Product Development Team.
References
- 1.Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull World Health Organ. 1999;77:624–40. [PMC free article] [PubMed] [Google Scholar]
- 2.East African Network for Monitoring Antimalarial Treatment (EANMAT) The efficacy of antimalarial monotherapies sulphadoxine-pyrimethamine and amodiaquine in East Africa: implications for sub-regional policy. Trop Med Int Health. 2003;8:860–7. doi: 10.1046/j.1360-2276.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Geneva: WHO; 2000. The Use of Antimalarial Drugs: Report of a WHO Informal Consultation, November 13–17, 2000. WHO/CDS/RBM/2001.33. [Google Scholar]
- 4.Winstanley PA, Watkins WM, Newton CRJC, Nevill C, Mberu E, Warn PA, Waruiru CM, Mwangi IN, Warrell DA, Marsh K. The disposition of oral and intramuscular pyrimethamine/sulfadoxine in Kenyan children with high parasitaemia but clinical non-severe falciparum malaria. Br J Clin Pharmacol. 1992;33:143–8. doi: 10.1111/j.1365-2125.1992.tb04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins WM, Chulay JD, Sixsmith DG, Spencer HC, Howells RE. A preliminary pharmacokinetic study of the antimalarial drugs proguanil and chlorporguanil. J Pharmacy Pharmacol. 1987;39:261–9. doi: 10.1111/j.2042-7158.1987.tb06263.x. [DOI] [PubMed] [Google Scholar]
- 6.Winstanley P, Watkins W, Muhia D, Szwandt S, Amukoye E, Marsh K. Chlorproguanil/dapsone for uncomplicated Plasmodium falciparum malaria in young children: pharmacokinetics and therapeutic range. Trans R Soc Trop Med Hyg. 1997;91:322–7. doi: 10.1016/s0035-9203(97)90093-6. [DOI] [PubMed] [Google Scholar]
- 7.Simpson JA, Aarons L, White NJ. How can we do pharmacokinetic studies in the tropics? Trans R Soc Trop Med Hyg. 2001;95:347–51. doi: 10.1016/s0035-9203(01)90178-6. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Severe falciparum malaria. World Health Organization. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1) [PubMed] [Google Scholar]
- 9.Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87. [PubMed] [Google Scholar]
- 10.Watkins WM, Mberu EK, Winstanley PA, Plowe C. The efficacy of antifolate combinations in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Parasitol Today. 1997;13:459–64. doi: 10.1016/s0169-4758(97)01124-1. [DOI] [PubMed] [Google Scholar]
- 11.Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–22. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greco WR, Bravo G, Paesons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–85. [PubMed] [Google Scholar]
- 13.Peterson E, Flachs H, Hogh B, Hanson AP, Bjorkman A, Hvidberg EF. Plasma, erythrocyte and urine concentrations of chlorproguanil and two metabolites in man after different doses. J Trop Med Hyg. 1991;94:199–205. [PubMed] [Google Scholar]
- 14.Veenendaal JR, Edstein MD, Rieckmann KH. Pharmacokinetics of chlorproguanil in man after a single oral dose of Lapudrine. Chemotherapy. 1988;34:275–83. [PubMed] [Google Scholar]
- 15.Mirochnick M, Cooper E, McIntosh K, Xu J, Lindsey J, Jacobus D, Mofenson L, Sullivan JL, Dankner W, Frenkel LM, Nachman S, Wara DW, Johnson D, Bonagura VR, Rathore MH, Cunningham CK, McNamara J. Pharmacokinetics of dapsone administered daily and weekly in human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 1993;43:2586–91. doi: 10.1128/aac.43.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirochnick M, Cooper E, Capparelli E, McIntosh K, Lindsey J, Xu J, Jacobus D, Mofenson L, Bonagura VR, Nachman S, Yogev R, Sullivan JL, Spector SA. Population pharmacokinetics of dapsone in children with human immunodeficiency virus infection. Clin Pharmacol Ther. 2001;70:24–32. doi: 10.1067/mcp.2001.115891. [DOI] [PubMed] [Google Scholar]
- 17.Frye RF, Matzke GR, Adedoyin A, Porter JA, Branch RA. Validation of the five-drug ‘Pittsburgh cocktail’ approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther. 1997;62:365–76. doi: 10.1016/S0009-9236(97)90114-4. [DOI] [PubMed] [Google Scholar]
- 18.Adedoyin A, Stiff DD, Smith DC, Romkes M, Bahnson RC, Day R, Hofacker J, Branch RA, Trump DL. All trans-retinoic acid modulation of drug-metabolizing enzyme activities: investigation with selective metabolic drug probes. Cancer Chemother Pharmacol. 1998;41:133–9. doi: 10.1007/s002800050719. [DOI] [PubMed] [Google Scholar]
- 19.Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, Manns MP. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–65. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green MD, Tephly TR. Glucuronidation of amine substrates by purified and expressed UDP-glucuronosyltransferase proteins. Drug Metab Dispos. 1998;26:860–7. [PubMed] [Google Scholar]
- 21.Alloueche A, Bailey W, Barton S, Bwika J, Chimpeni P, Falade CO, Fehintola FA, Horton J, Jaffar S, Kanyok T, Kremsner PG, Kublin JG, Lang T, Missinou MA, Mkandala C, Odoula AM, Premji Z, Robertson L, Sowunmi A, Ward SA, Winstanley PA. Comparison of chlorproguanil-dapsone with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in young African children: double-blind randomised controlled trial. Lancet. 2004;363:1843–8. doi: 10.1016/S0140-6736(04)16350-2. [DOI] [PubMed] [Google Scholar]
- 22.Bates SJ, Winstanley PA, Watkins WM, Alloueche A, Bwika J, Happi TC, Kremsner PG, Kublin JG, Premji Z, Sibley CH. Rare, highly pyrimethamine-resistant alleles of the Plasmodium falciparum dihydrofolate reductase gene from 5 African sites. JID. 2004;190:1783–92. doi: 10.1086/425078. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Antimalarial Drug Combination Therapy. Geneva: WHO; 2001. Report of a WHO technical consultation. [Google Scholar]