Abstract
Aims
The Transdermal Delivery System (TDS®) is a liquid formulation that can be applied to the skin via a metered pump spray to deliver drug to the systemic circulation. The aims of this study were to assess the ability of the TDS® preparation to deliver testosterone systemically, and to characterize the pharmacokinetic profiles of the hormone in healthy males.
Methods
An open label, comparative, randomized placebo controlled study involving three treatments and three periods with a minimum of a 1 week washout period was conducted. Twelve healthy males received 50 mg TDS®-testosterone, TDS®-placebo, and 50 mg of a commercially available topical testosterone preparation (Androgel®, 1% topical testosterone gel).
Results
The mean AUC(0,12 h) was higher following application of TDS®-testosterone (61.8 ng ml−1 h), compared with Androgel® (57.7 ng ml−1 h) and TDS®-placebo (50.7 ng ml−1 h. The mean Cmax (0,12 h) was similar for TDS®-testosterone (6.6 ng ml−1) and Androgel® (6.5 ng ml−1) and these values were higher than those for TDS®-placebo (5.7 ng ml−1). Analysis of variance showed that the 90% confidence intervals on the relative difference of the ratio for the AUC(0,12 h) and the Cmax (0,12 h) between TDS®-testosterone and Androgel®, were contained within the bioequivalence limit (80, 125%) (Cmax 89.2, 112.3% and AUC 93.5, 120.5%). Serum testosterone concentrations were lower following TDS®-Placebo and were not bioequivalent either to the gel or spray.
Conclusions
The TDS® preparation was shown to deliver testosterone systemically to humans and the concentrations of the hormone in the 12 h following TDS® administration were bioequivalent to an existing topical delivery gel.
Keywords: bioequivalence, pharmacokinetics, TDS®, testosterone, transdermal delivery
Introduction
Testosterone (17α-hydroxyandrost-4-ene-3-one), is the most important androgen secreted into the blood. About 95% of the circulating testosterone present in men is secreted by the testes, which produce between 3 and 10 mg of the hormone per day. Testosterone is responsible for the development of secondary male sex characteristics (e.g. increased growth of body hair, beard, and sexual libido). Hormone concentrations are higher during puberty, but decline with age [1] and testosterone replacement therapy may be indicated, especially for hypogonadal (testosterone deficiency) men.
A number of testosterone preparations have been tested for replacement therapy. These include subcutaneous implants [2], scrotal transdermal patches [3], non scrotal transdermal patches [4], oral and sublingual preparations [5, 6], testosterone gel [7–9], and the testosterone esters, enanthate and cypionate [10]. With the exception of the transdermal preparations, none is suitable for replacement therapy. Oral administration of testosterone leads to absorption into the hepatic circulation and rapid metabolism by the liver [11]. Behre [12] reported that oral administration of testosterone gives rise to wide fluctuations with high within and between individual variability in serum testosterone concentrations. It has been reported that methyltestosterone can cause hepatic toxicity and adversely affect cholesterol concentrations following long-term usage [13–15].
Sublingual preparations of testosterone result in rapid increases in serum concentrations, which decline to below the normal range after 2 h [6]. Occasional mild redness or itching is common with transdermal patches. Although scrotal patches causes less skin irritation than conventional transdermal preparations, usage of the former leads to an increase in dihydro-testosterone (DHT) concentrations after 3 months of treatment [16]. Testosterone gels have also been developed, but care must be taken to prevent the transfer of testosterone to another person. Patients must wash their hands after applying the gel to a substantial surface area of the body and they must cover the application site with clothing once the gel has dried [17]. We have developed a more convenient method to deliver testosterone through the skin via a metered pump dispenser, using the TDS® delivery system (Transdermal Technologies Inc., Florida, USA). This is a proprietary technology, which has been developed for use in pharmaceutical, cosmetic and over-the-counter products. The system consists of a true solution of ethanol, propylene glycol, monolaurins, vitamins and pro-vitamins and cAMP energy donors. The safety of the TDS® system has been evaluated and confirmed by the Institute for In Vitro Sciences in Gaithersburg, Maryland USA, with respect to primary dermal irritation, skin sensitization and toxicity.
The aims of this study were to assess the ability of the TDS® preparation to deliver testosterone systemically and to characterize its pharmacokinetics in healthy males.
Methods
Study materials
TDS®-testosterone (batch number MBR-BFLIQ84), TDS®-placebo and Androgel® (batch number 20293 RC) were supplied by TransDermal Technologies, Inc., Florida, USA. TDS®-testosterone and TDS®-placebo were supplied as a liquid formulation, delivered by metered pump, with each spray containing 10 mg testosterone. Androgel® was supplied as a gel in unit-dose aluminium foil packets of 5 g, each containing 50 mg testosterone.
Study design and treatments
This was a single dose, randomized, three-way crossover study (with three treatments, three periods, and six sequences) with a minimum of 1 week washout period between each treatment. The three treatments were TDS®- testosterone 50 mg, TDS®-placebo, and Androgel® 1% (50 mg).
Subjects
Twelve healthy males successfully completed the protocol. Six subjects were Caucasian, and six were from other racial groups. The mean (SD) age of the subjects was 29.0 (6.2) years, and the mean (SD) BMI was 24.1 (3.2) kg m−2. The study was approved by the East London and The City Health Authority Research Ethics Committee and received a Doctors and Dentists Exemption Certificate (DDX) from MHRA (Medicines and Healthcare Products Regulatory Agency), UK. All the subjects gave written informed consent before taking part in the study, and each fulfilled all entry criteria based on physical examination, medical history, and clinical laboratory tests.
Study protocol
On the morning of each study day, blood pressure and heart rate were measured after subjects had rested for 10 min. A 20G cannula was placed in a large antecubital vein for blood collection. The drug formulation was then applied to the left arm and gently rubbed into the skin. Regular meals and beverages were provided throughout the study day. After dosing, subjects were permitted to engage in normal daily activities, but were excluded from significant physical exertion or activities likely to stimulate endogenous testosterone production.
Approximately 4 ml of blood was collected at −0.5 (30 min before dosing) and 0 h (immediately prior to dosing), to establish a baseline measurement of serum testosterone concentration. Subsequently, serial blood samples were collected at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 10, 12, and 24 h post dose. Samples were allowed to clot for approximately 20 min at room temperature (26 °C) and were then centrifuged at 1800 g for 10 min. The serum was transferred to labelled tubes and stored at −20 °C until analysis.
Testosterone analysis
Testosterone concentrations were measured in serum using an Enzyme Link Immunosorbent Assay (ELISA) method. The kit was obtained from DRG Instruments GmBH, Germany, lot no. 29K064. The three sets of testosterone control standards were obtained from Bio-Rad Laboratories, USA (lot no. 40631, 40632, and 40633 for set 1, 2, and 3, respectively). The plate reader used was a GENios, computer controlled fluorometer from TECAN Company, Austria. The kit was validated to demonstrate adequate sensitivity, specificity, recovery, accuracy and precision (within and between assays). The limit of quantification was 0.2 ng ml−1 for the assay. All the study samples were analyzed together with quality control samples containing three different concentrations. The coefficients of variation for imprecision and inaccuracy were all below 15%.
Data analysis
Pharmacokinetic parameters were determined and the statistical analysis was performed using Kinetica Version 4.2 software. Cmax and tmax were determined directly from the individual serum concentration-time curves, and the AUC was calculated using the linear trapezoidal method. The difference between treatments for AUC(0,12 h) and Cmax were analyzed after logarithmic transformation using analysis of variance (anova) for crossover studies which accounts for variation due to sequence, subject, formulation, and period.
Bioequivalence testing was based upon the 90% confidence interval (CI) for the ratio of population means between two treatments. This method is equivalent to the corresponding two one-sided test procedure, with the null hypothesis of bioequivalence at the 5% significance level. For formulations to be bioequivalent, the ratio (test : reference) must fall between the 0.8, 1.25 confidence interval [18].
Results
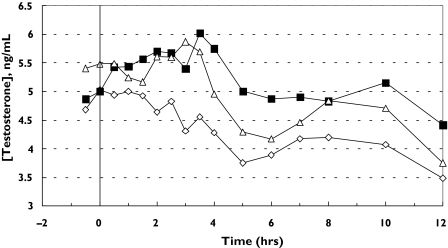
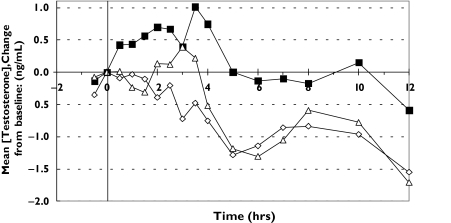
Figures 1 and 2 show the plots of mean serum concentration of testosterone vs. time and mean serum concentration changes from baseline. Testosterone concentrations fluctuated slightly in most of the subjects during all the treatments. Most of the subjects treated with Androgel® and TDS®-testosterone achieved higher concentrations compared with placebo at 24 h post dose.
Figure 1.
Plots of mean serum testosterone concentration (ng ml−1) vs. time (h) for each treatment. TDS®-placebo (⋄), TDS®-testosterone (▪), Androgel® (▵)
Figure 2.
Plots of mean serum testosterone concentration change from baseline (ng ml−1) vs. time (h) for each treatment. TDS®-placebo (♦), TDS®-testosterone (▪), Androgel® (▵)
The pharmacokinetic parameters AUC, Cmax, and tmax are listed in Table 1 for each treatment. The AUC and Cmax values were calculated for both 0–12 and 0–24 h. The mean AUC(0,12 h) was higher following application of TDS®-testosterone compared with Androgel® and TDS®-placebo. However, the mean AUC(0,24 h) for Androgel® was higher than TDS®-testosterone and TDS®-placebo. The mean Cmax (0–12 h) was similar for TDS®-testosterone and Androgel® and these values were higher than that obtained for TDS®-placebo. Owing to the higher concentrations of testosterone at 24 h in some subjects, the mean Cmax (0–24 h) value for Androgel® was higher than those for TDS®-testosterone and TDS®-placebo. Only the 0–12 h data were used for the determination of bioequivalence.
Table 1.
Geometric mean (CV percentage) values for Cmax, tmax and AUC for each treatment
| Cmax (ng ml−1) | tmax (h) | AUC (ng ml−1 h) | ||||
|---|---|---|---|---|---|---|
| 0–12 h | 0–24 h | 0–12 h | 0–24 h | 0,12 h | 0,24 h | |
| TDS®-testosterone | 6.64 (22.4) | 8.38 (49.7) | 2.42 (58.4) | 11.75 (92.3) | 61.85 (24.7) | 135.77 (34.1) |
| TDS®-placebo | 5.72 (21.1) | 5.95 (22.2) | 3.29 (111.5) | 9.00 (107.4) | 50.67 (23.6) | 101.67 (25.5) |
| Androgel® | 6.54 (24.1) | 13.17 (99.2) | 1.83 (52.4) | 16.79 (63.5) | 57.67 (19.3) | 157.41 (57.7) |
The 90% confidence intervals on the relative difference of the ratio for the AUC(0,12 h) and the Cmax (0–12 h) between TDS®-testosterone and Androgel®, were contained within the bioequivalence limit (80, 125%) (Cmax: 89.2, 112.3% and AUC(0,12 h): 93.5, 120.5%). Serum testosterone concentrations were lower following TDS®-placebo and were not bioequivalent either to the gel or the spray.
Discussion
No serious or unexpected adverse events were reported or observed during the study day. The drug formulations and protocol requirements were well tolerated by all subjects. Currently licensed transdermal delivery systems are available as patches, gels and buccal tabs. Patients report to us that patches may irritate the skin, gels can take time to dry and leave a residue, while the buccal tabs may be intrusive and have to be applied twice a day. The TDS preparation appears to provide a more convenient transdermal delivery of testosterone with rapid drying, and low to no skin residue or irritation compared with other transdermal systems. Based on these data we now plan a phase II study in hypogonadal males.
The TDS®-testosterone preparation was shown to deliver testosterone systemically to humans. The concentrations of hormone in the first 12 h following TDS® administration were found to be bioequivalent to an existing topical delivery gel.
Acknowledgments
This work was supported by a grant from the William Harvey Research Foundation, QMUL UK and The Langford Institute, Florida USA.
References
- 1.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–81. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 2.Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71:216–22. doi: 10.1210/jcem-71-1-216. [DOI] [PubMed] [Google Scholar]
- 3.Korenman SG, Viosca S, Garza D, Guralnik M, Place V, Campbell P, Davis SS. Androgen therapy of hypogonadal men with transscrotal testosterone systems. Am J Med. 1987;83:471–8. doi: 10.1016/0002-9343(87)90757-1. [DOI] [PubMed] [Google Scholar]
- 4.Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–78. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen SG, Bennett EP, Jensen VG. Therapeutic effectiveness of oral testosterone. Lancet. 1974;2:1473–5. doi: 10.1016/s0140-6736(74)90216-5. [DOI] [PubMed] [Google Scholar]
- 6.Stuenkel CA, Dudley RE, Yen SS. Sublingual administration of testosterone-hydroxypropyl-beta-cyclodextrin inclusion complex simulates episodic androgen release in hypogonadal men. J Clin Endocrinol Metab. 1991;72:1054–9. doi: 10.1210/jcem-72-5-1054. [DOI] [PubMed] [Google Scholar]
- 7.Jockenhovel F. Testosterone supplementation: what and how to give. Aging Male. 2003;6:200–6. [PubMed] [Google Scholar]
- 8.Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–10. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 9.Gooren LJ, Bunck MC. Transdermal testosterone delivery. testosterone patch and gel. World J Urol. 2003;21:316–9. doi: 10.1007/s00345-003-0368-6. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto AM. Hormonal therapy of male hypogonadism. Endocrinol Metab Clin North Am. 1994;23:857–75. [PubMed] [Google Scholar]
- 11.Snyder PJ. In: In Goodman and Gilman's The Pharmacological Basis of Therapeutics. Tenth edition. Hardman JG, Limbird LE, editors. New York: McGraw Hill; 2001. pp. 1635–1645. [Google Scholar]
- 12.Behre HM. Comparative pharmacokinetics of testosterone esters. In: Nieschlag E, Behre HM, editors. In TestosteroneAction, Deficiency, Substitution. 2. Berlin: Springer-Verlag; 1998. pp. 329–48. [Google Scholar]
- 13.Bird DR, Vowles KD. Liver damage from long-term methyltestosterone. Lancet. 1977;2:400–1. doi: 10.1016/s0140-6736(77)90323-3. [DOI] [PubMed] [Google Scholar]
- 14.Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977;2:262–3. [PubMed] [Google Scholar]
- 15.Lowdell CP, Murray-Lyon IM. Reversal of liver damage due to long term methyltestosterone and safety of non-17 alpha-alkylated androgens. BMJ. 1985;291:637. doi: 10.1136/bmj.291.6496.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behre HM, von Eckardstein S, Kliesch S, Nieschlag E. Long-term substitution therapy of hypogonadal men with transscrotal testosterone over 7–10 years. Clin Endocrinol (Oxf) 1999;50:629–35. doi: 10.1046/j.1365-2265.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Unimed Pharmaceutical Products. [4/4/2005];Androgel® Summary of Product Characteristics. 2002 http://www.drugs.com/PDR/AndroGel.html.
- 18.Pabst G, Jaeger H. Review of methods and criteria for the evaluation of bioequivalence studies. Eur J Clin Pharmacol. 1990;38:5–10. doi: 10.1007/BF00314794. [DOI] [PubMed] [Google Scholar]