Abstract
Aims
Spirometry, plethysmography and impulse oscillometry (IOS) measure different aspects of lung function. These methods have not been compared for their ability to assess long-and short-acting anticholinergic agents. We therefore performed a double-blind, placebo-controlled, four-way cross-over study in 30 healthy subjects.
Methods
Single doses of tiotropium bromide (Tio) 54 and 18 mcg, ipratropium bromide (IB) 40 mcg and placebo were administered. Specific conductance (sGaw), total lung capacity (TLC), inspiratory capacity (IC) and residual volume (RV) were measured using plethysmography, while IOS measured resistance (R5–25) and reactance (RF and X5). Pulmonary function was measured for 26 h post dose.
Results
Tio caused significant improvements in sGaw, forced expiratory voume in 1 s (FEV1), maximum mid-expiratory flow (MMEF) and R5–R25 at time points up to 26 h, with no clear differences between doses. IB improved the same parameters, but only up to 8 h. The weighted mean change (0–24 h) caused by Tio 54 mcg compared with placebo for FEV1 was 240 ml (95% confidence interval 180, 300), while for sGaw the ratio of geometric means (Tio compared with placebo) was 1.35 (1.28, 1.41). Neither drug caused consistent statistically significant changes in RF, forced vital capacity, TLC or IC over 26 h. RV was significantly improved from 8 to 24 h by Tio 54 mcg only.
Conclusions
In addition to spirometry, IOS resistance measurements and sGaw can distinguish between the effects of long-and shortacting anticholinergic effects in healthy subjects.
Keywords: healthy subject, impulse oscillometry, lung function
Introduction
Spirometry is the most commonly used method for the measurement of bronchodilation in clinical trials. However, there are potential advantages to the use of impulse oscillometry (IOS) and body plethysmography for the measurement of pharmacological effects. First, there is evidence that IOS and plethysmography are more sensitive than spirometry for detecting bronchodilator effects in patients with asthma and chronic obstructive pulmonary disease (COPD) [1–3]. Second, IOS and plethysmography assess physiological parameters not measured by spirometry, namely pulmonary resistance and reactance. Despite these potential advantages, IOS and plethysmography are not routinely used in double-blind, placebo-controlled clinical trials to assess the effects of bronchodilator drugs.
The initial clinical development of novel respiratory drugs usually involves studies in healthy volunteers (phase 1 studies). These are designed principally to assess pharmacokinetics and safety. The measurement of bronchodilator effects may also be useful, to assess whether the drug has the desired pharmacological activity. Clearly, it would be advantageous to use the most sensitive methods in such studies. It is known that IOS, plethysmography and spirometry can all detect bronchodilator effects in healthy volunteers [1, 2]. However, the sensitivity of these three methods in a placebo-controlled clinical trial in healthy volunteers has not been compared.
The measurement of bronchodilation in healthy volunteer phase 1 studies may provide important preliminary information regarding the onset, peak and duration of effect of the drug. This information may be used to aid the design of studies in patients with disease. While it is known that bronchodilators cause measurable effects in healthy subjects [1, 2], it is not known whether differences in the duration of effect of short-and long-acting bronchodilators can be robustly detected in healthy volunteers, and whether the duration of action is similar to that observed in patients. This important issue will determine whether results from healthy volunteer studies can be used to predict duration of action in patients.
Inhaled muscarinic receptor antagonists are used as bronchodilator agents in the treatment of asthma and COPD. These drugs inhibit central (M1) and postsynaptic (M3) receptor signalling [4]. The nonselective (M1, M2 and M3) antagonist ipratropium bromide (IB) has a relatively short duration of action (approximately 6 h) compared with tiotropium bromide (Tio) which has greater selectivity for the M1 and M3 receptor subtypes [4, 5]. In vitro studies have demonstrated that Tio is a competitive receptor antagonist with a slow dissociation half-life from the M3 receptor, contributing to a prolonged duration of action [6]. This prolonged duration of action has been observed in vivo; for example, Tio causes bronchoprotection against methacholine that lasts for at least 72 h in asthmatics [7], while bronchodilation in COPD patients is sustained for at least 24 h [5, 8].
The effects of Tio in healthy subjects have not previously been studied using IOS and plethysmography. We have conducted a placebo-controlled, double-blind, cross-over study in healthy volunteers using single doses of Tio (18 and 54 mcg) and IB (40 mcg) and measured pulmonary function using IOS, plethysmography and spirometry. The aims of this study were (i) to compare the ability of IOS, plethysmography and spirometry to assess anticholinergic effects in a clinical trial of healthy subjects and (ii) to assess whether the duration of effect of short-and long-acting bronchodilator drugs could be distinguished in a healthy volunteer study and whether these results were similar to the known data in patients. Our results have important implications for the future design of phase 1 studies of bronchodilator drugs.
Methods
Subjects
Thirty healthy male subjects participated (mean age 29 years). Screening lung function data are presented in Table 1. Subjects did not have any clinically significant disease and were either lifelong nonsmokers or ex-smokers with a history of less than 10 pack years. Exclusion criteria were a history of asthma or COPD, a respiratory tract infection in the previous 4 weeks, known hypersensitivity to IB, Tio bromide or atropine and forced expiratory voume in 1 s (FEV1) < 80% predicted or FEV1/forced vital capacity (FVC) < 70%. All participants were required to give written consent and the study was approved by the local research ethics committee.
Table 1.
Baseline lung function data for 30 healthy subjects. Mean (SD) presented
| FEV1 (l) | 4.8 (0.5) | R5 (kPa l−1 s) | 0.25 (0.04) |
| FVC (l) | 5.8 (0.5) | R15 (kPa l−1 s) | 0.22 (0.04) |
| MMEF (l s−1) | 5.0 (1.5) | R20 (kPa l−1 s) | 0.23 (0.04) |
| sGaw (s−1 kPa−1) | 1.6 (0.3) | R25 (kPa l−1 s) | 0.24 (0.04) |
| Raw (kPa l−1 s) | 0.17 (0.04) | RF (Hz) | 9.9 (2.0) |
| RV (l) | 2.2 (0.4) | X5 (kPa l−1 s) | −0.07 (0.03) |
| IC (l) | 3.9 (0.5) | ||
| TLC (l) | 7.9 (0.8) |
FEV1, Forced expiratory voume in 1 s; FVC, forced vital capacity; MMEF, maximum mid-expiratory flow; sGaw, specific conductance; Raw, airway resistance; RV, residual volume; IC, inspiratory capacity; TLC, total lung capacity.
Study design
This was a double-blind, randomized, placebo-controlled, four-way cross-over study performed at a single centre (PAREXEL Clinical Pharmacology Research Unit, Northwick Park Hospital). Subjects received a single dose of study treatment on each of four study days that were separated by 10 days to allow adequate washout. The randomized treatments were IB 40 mcg, Tio 18 mcg, Tio 54 mcg and placebo. All treatments were delivered using dry powder inhalers. Subjects were trained in the use of the inhaler devices. Subjects were given both of these inhaler devices on each study day, with at least one being a placebo. Study medication was administered at 08.00 h and pulmonary function was measured predose and at 2, 5, 8, 12, 16, 24 and 26 h postdose.
Pulmonary function measurements
For IOS (Masterscreen IOS; Erich Jaeger, Hoechberg, Germany) subjects supported their cheeks to reduce upper airway shunting while impulses were applied during tidal breathing for 30 s. R5, R10, R15, R20 and R25 (respiratory resistance 5–25 Hz), X5 (reactance at 5 Hz) and RF (resonant frequency) were recorded. Airway resistance (Raw), specific conductance (sGaw), functional residual capacity (FRC), vital capacity (VC) and inspiratory capacity (IC) were measured in a constant volume plethysmograph (Jaeger Masterscreen body). Total lung capacity (TLC) and residual volume (RV) were then calculated from these parameters. IOS and body box measurements were performed in triplicate and the mean used for further analysis. Maximum expiratory flow volume measurements [FEV1, FVC and MMEF] were performed using the Jaeger Masterscreen body. Readings were again performed in triplicate, with the highest FEV1 and FVC and the mean MMEF used in further analysis.
Statistical analysis
The sample size for this study was based on the primary endpoint of sGaw. Our previous data estimate the between-subject variability of sGaw to have a standard deviation of 0.22 kPa−1 s−1. Using 30 subjects provided 90% power to detect a 21% decrease in sGaw at 24 h. Pulmonary function data were analysed using a mixed model that included treatment, time, period, a treatment by time interaction and baseline (a continuous covariate) fitted as fixed effects and subject fitted as a random effect. Baseline was defined as the predose measurement for each treatment group. Individual time points were compared as well as weighted mean values over 24 h. Nonparametric data (sGaw, Raw and RF) were analysed following a natural logarithmic transformation and treatment ratios of all statistical comparisons calculated by taking the antilog of the difference between the least squared means. Effect sizes were determined by calculating the treatment differences (active vs. placebo) divided by the within-subject SD of repeated measurements. This allowed the magnitude of the change in the different measurements to be compared by standardizing the treatment effects by the variability of the measurement.
Results
Twenty-nine subjects completed the study. One subject withdrew consent after the first study day, at which IB was administered. The data for this subject are included in the statistical analysis.
Tio 54 mcg and 18 mcg caused statistically significant improvements in sGaw and Raw at all timepoints up to 26 h postdose compared with placebo (see Figure 1 for mean and 95% confidence intervals). However, this effect was observed only for IB up to 8 h postdose. Similar results for both Tio and IB were observed using the spirometry measurements FEV1 and MMEF (Figure 2) and the IOS resistance measurements R5, R15, R20 and R25 (Figure 3). IOS reactance measurements (RF and X5, see Figure 3) did not show any consistent pattern of change over 26 h. Lung volume measurements (FVC, TLC and IC, data not shown) did not change with any of the treatments. The only exception to this pattern was observed for RV; Tio (54 mcg dose) caused statistically significant decreases in RV from 8 to 24 h postdose (Figure 4).
Figure 1.
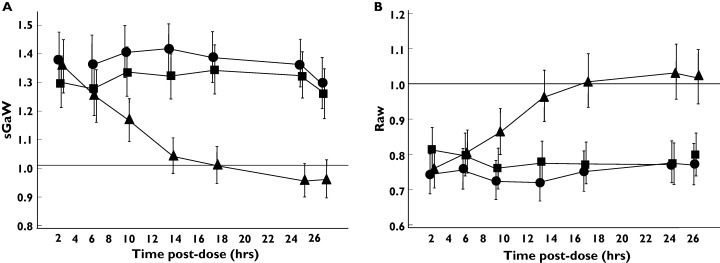
Effect of anticholinergic bronchodilators on body plethysmographic measurements in 30 healthy subjects. The data was log-transformed data and the ratio of the geometric means compared to placebo is presented. Error bars are 95% confidence intervals. •, Tio 54 mcg; ▪, Tio 18 mcg; ▴, IB 40 mcg
Figure 2.
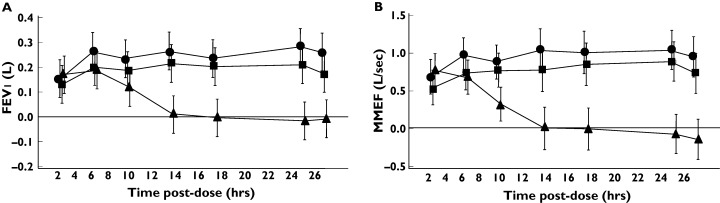
Effect of anticholinergic bronchodilators on spirometry measurements in 30 healthy subjects. The mean difference compared to placebo is presented. Error bars are 95% confidence intervals. •, Tio 54 mcg; ▪, Tio 18 mcg; ▴, IB 40 mcg
Figure 3.
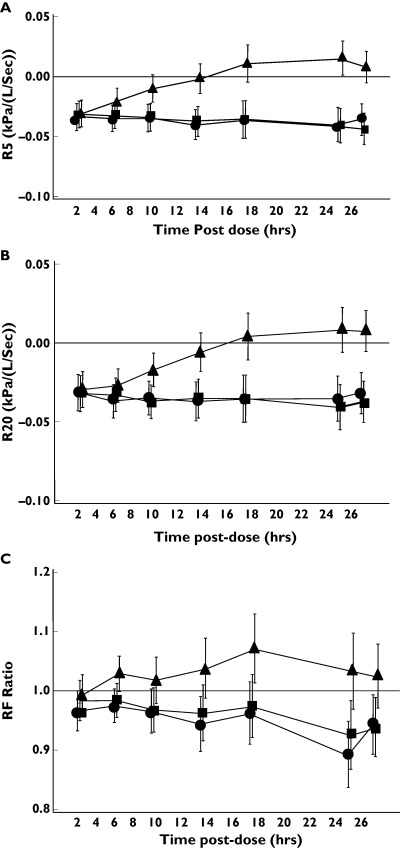
Effect of anticholinergic bronchodilators on IOS measurements in 30 healthy subjects. For IOS resistance measurements (R5 and R20), the mean difference compared to placebo is presented. R15 and R25 data (not shown) was similar to R5 and R20. RF data was log-transformed and the ratio of the geometric means compared to placebo is presented. X5 data (not shown) was similar to RF data. Error bars are 95% confidence intervals. •, Tio 54 mcg; ▪, Tio 18 mcg; ▴, IB 40 mcg
Figure 4.
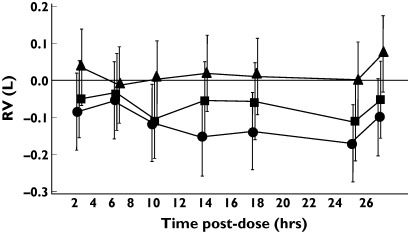
Effect of anticholinergic bronchodilators on RV measurements in 30 healthy subjects. The mean difference compared to placebo is presented. Error bars are 95% confidence intervals. •, Tio 54 mcg; ▪, Tio 18 mcg; ▴, IB 40 mcg
The weighted mean changes over 24 h in the pulmonary function measurements that showed improvements at individual time points after drug treatments (i.e. sGaw, Raw, FEV1, MMEF and IOS resistance measurements) are shown in Table 2. The 95% confidence intervals demonstrate statistically significant improvements in sGaw, Raw, FEV1, MMEF and IOS resistance measurements after both doses of Tio. As the duration of effect of IB was only 8 h, the weighted mean changes over 24 h were smaller. To compare the weighted mean changes obtained by different measurements, the weighted mean percentage changes (compared with predose) after the 54 mcg dose of Tio were calculated. The greatest percentage improvement compared with placebo was observed with sGaw (35%), with smaller changes for R5 (13.1%), R20 (13.6%), FEV1 (5.1%) and MMEF (19.4%). The relative changes in lung function were further compared by the use of effect sizes (Table 3); sGaw data demonstrated larger effect sizes compared with the other parameters.
Table 2.
Weighted mean change (0–24 h) in lung function measurements compared to placebo
| sGaw | Raw | FEV1 | MMEF | R5 | R20 | |
|---|---|---|---|---|---|---|
| Tio 54 mcg | 1.35 (1.28, 1.41) | 0.76 (0.72, 0.8) | 0.24 (0.18, 0.3) | 0.92 (0.74, 1.1) | −0.035 (−0.044 - −0.025) | −0.034 (−0.043 - −0.024) |
| Tio 18 mcg | 1.28 (1.22, 1.34) | 0.79 (0.75, 0.84) | 0.18 (0.12, 0.24) | 0.76 (0.58, 0.94) | −0.034 (−0.044 - −0.025) | −0.035 (−0.044 - −0.025) |
| IB | 1.08 (1.03, 1.14) | 0.93 (0.88, 0.99) | 0.06 (0.001, 0.12) | 0.2 (0.03, 0.39) | −0.002 (−0.012 - 0.008) | −0.008 (−0.017 - 0.002) |
Tio, tiotropium bromide; IB, Ipratropium bromide. Only parameters that showed statistical significance at individual time points are shown. R15 and R25 data not shown as similar to other IOS data. For log-transformed data [sGaw and Raw], the ratio compared to placebo is presented. For other endpoints, the difference compared to placebo is presented [units are FEV1: l; MMEF: l s−1; R5 and R20: kPa l−1 s). All data is mean (95% CI).
Table 3.
Effect size calculations for treatments compared with placebo
| Treatment | Time, h | sGaw | FEV1 | MMEF | R5 | R20 |
|---|---|---|---|---|---|---|
| IB 40 µg | 12 | 0.27 | 0.06 | 0.01 | −0.07 | −0.25 |
| Tio 18mcg | 12 | 2.31 | 1.46 | 1.43 | −1.57 | −1.49 |
| Tio 54mcg | 12 | 2.94 | 1.80 | 1.93 | −1.68 | −1.57 |
| IB 40mcg | 24 | −0.46 | −0.11 | −0.14 | 0.57 | 0.30 |
| Tio 18mcg | 24 | 2.30 | 1.43 | 1.79 | −1.49 | −1.49 |
| Tio 54mcg | 24 | 2.56 | 1.90 | 2.10 | −1.45 | −1.29 |
IB, Ipratropium bromide; Tio, tiotropium bromide; sGaw, specific conductance; FEV1, forced expiratory volume in 1 s; MMEF, maximum mid-expiratory flow. For sGaw, FEV1, MMEF and RV, improvements in lung function cause positive effect sizes. For R5 and R20, improvements in lung function cause negative effect sizes (as impulse oscillometry resistance parameters decrease as lung function improves).
Discussion
Our primary objectives were to compare the sensitivity of IOS, plethysmography and spirometry for measuring anticholinergic effects in a clinical trial of healthy subjects and to assess whether the duration of effect of short-and long-acting bronchodilator drugs could be distinguished in a healthy volunteer study. The key findings were of improvements in pulmonary resistance, whether measured by plethysmography or IOS (R5–R25) and spirometric parameters (FEV1 and MMEF). However, there were no consistent improvements in IOS reactance measurements and lung volume measurements (except for RV after the higher Tio dose). Pulmonary resistance, FEV1 and MMEF measurements were therefore the most sensitive for assessing the effects of anticholinergic bronchodilators in this healthy volunteer study. Importantly, these measurements clearly demonstrated differences in duration of action between Tio and IB, and may be of considerable use in defining the duration of action of novel bronchodilator agents in future healthy volunteer studies.
Previous studies comparing IOS, plethysmography and spirometry in healthy subjects were not placebo controlled or blinded and studied only the effects of short-acting bronchodilators [1, 2]. In contrast, the current study was double blinded and placebo controlled and involved both long-and short-acting agents. This study design enabled a robust comparison of the three different measurement techniques. It was clearly demonstrated that the magnitude of effect differs between techniques, e.g. the weighted mean change over 24 h after Tio 54 mcg compared to placebo was 35% for sGaw, but only 5.1% for FEV1. Interestingly, the percentage changes observed for body plethysmography, spirometry and IOS resistance parameters were very similar to the maximal effects reported in dose–response studies of IB [2] and salbutamol [1], i.e. in these previous reports, the maximal sGaw changes in healthy subjects were in the range 18–26%, FEV1 improvements were < 10%, while MMEF and IOS resistance parameters were between 10 and 20%. The current study, combined with these previous reports [1, 2], shows the degree of change that can be expected in these measurements in healthy subjects after bronchodilator treatments.
The sensitivity of a lung function method to detect a drug effect is determined by the magnitude of effect caused by the drug relative to the variability of the method. Indeed, it has been demonstrated that reducing the variability of sGaw in healthy subjects, by increasing the number of readings, reduces method variability and consequently increases sensitivity [2]. We used effect sizes to compare treatment effects with the variability of the measurement, and found that the largest effect size was observed with sGaw. This indicates that sGaw not only had the largest percentage improvements after drug treatment, but was also the most sensitive measurement in the current study. It should be noted that the variability of sGaw can be relatively high in healthy subjects [1, 2]. The current study, using a large sample size and placebo-controlled cross-over design, enabled this variability to be controlled so that sGaw could sensitively detect the effects of anticholinergic agents in healthy subjects.
IOS uses flow oscillations generated by a loud speaker that are superimposed on normal breathing. The resulting pressure and flow changes are analysed by Fourier analysis to determine respiratory resistance and reactance at multiple frequencies. The identical changes that we observed in resistance measurements across the range of frequencies between 5 and 25 Hz probably reflect physiological changes in large airways, without associated change in resistance in peripheral airways (9). In contrast, there was no change in IOS reactance measurements. This is entirely consistent with previous findings in healthy subjects using IB [2] or salbutamol [1]. This is probably because healthy subjects have normal respiratory reactance that does not change after bronchodilation. In contrast, patients with obstructive lung disease have abnormalities of reactance measurements, which can be improved by bronchodilation [1–3].
No changes in lung volume measurements at any time point except for RV after the higher Tio dose were observed. Tio is known to cause a reduction in hyperinflation in COPD patients [10, 11]. Obviously, healthy volunteers do not have hyperinflation, but nevertheless our data show that Tio does cause some changes in lung volumes in healthy subjects, albeit at a higher dose than used in clinical practice for COPD.
Tio is known to cause bronchodilation in COPD patients that lasts for at least 24 h [4, 5, 8]. Furthermore, this drug protects against methacholine challenge in asthmatics for up to 72 h [7]. In the current study, a single dose of Tio caused improvements in lung physiology in healthy subjects that lasted for at least 26 h. This indicates that healthy volunteer studies of bronchodilation caused by anticholinergic agents provide data on duration of action that are similar to results in patients. We encourage the future measurement of bronchodilation in healthy volunteer studies of novel anticholinergic drugs. The choice of measurement methods is usually guided by sensitivity, as well as practical issues. Although this study has shown that body plethysmography is a sensitive method, it is also the most complex and time consuming, requiring a greater degree of operator training. IOS is a practically simpler procedure for measuring respiratory resistance. It also provides measures of resistance across a range of frequencies to reflect separate indices of peripheral or more proximal airway effects [9].
In summary, we have shown that pulmonary anticholinergic effects in a healthy volunteer clinical trial can be measured using sGaw, IOS resistance measurements, FEV1 and MMEF. These measurements provide information regarding duration of action that is similar to published data in patients, and thus can be used to estimate the likely dosing frequency for future novel bronchodilator agents.
Competing interests: None declared.
References
- 1.Houghton CM, Woodcock AA, Singh D. A comparison of lung function methods for assessing dose–response effects of salbutamol. Br J Clin Pharmacol. 2004;58:134–41. doi: 10.1111/j.1365-2125.2004.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton CM, Woodcock AA, Singh D. A comparison of plethysmography, spirometry and oscillometry for assessing the pulmonary effects of inhaled ipratropium bromide in healthy subjects and patients with asthma. Br J Clin Pharmacol. 2005;59:152–9. doi: 10.1111/j.1365-2125.2004.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrill Z, Houghton C, Woodcock A, Vestbo J, Singh SD. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol. 2005;59:379–84. doi: 10.1111/j.1365-2125.2004.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med. 2004;117:24S–32S. doi: 10.1016/j.amjmed.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Gross NJ. Tiotropium bromide. Chest. 2004;126:1946–53. doi: 10.1378/chest.126.6.1946. [DOI] [PubMed] [Google Scholar]
- 6.Disse B, Reichl R, Speck G, Traunecker W, Ludwig Rominger KL, Hammer R. Ba 679 BR, a novel long-acting anticholinergic bronchodilator. Life Sci. 1993;52:537–44. doi: 10.1016/0024-3205(93)90312-q. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;154:876–80. doi: 10.1164/ajrccm.154.4.8887578. [DOI] [PubMed] [Google Scholar]
- 8.Maesen FP, Smeets JJ, Sledsens TJ, Wald FD, Cornelissen PJ. Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD) Eur Respir J. 1995;8:1506–13. [PubMed] [Google Scholar]
- 9.Goldman M, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Resp Physiol Neurobiol. 2005;148:179–94. doi: 10.1016/j.resp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–40. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 11.Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–8. doi: 10.1378/chest.124.5.1743. [DOI] [PubMed] [Google Scholar]


