Abstract
Aim
To assess the pharmacokinetic effect and tolerability of lamotrigine 200 mg day−1 and olanzapine 15 mg day−1 coadministration in healthy male volunteers.
Methods
Subjects were randomized to receive either lamotrigine titrated on days 1–42 with olanzapine added on days 43–56 (LTG + OLZ group; N = 16), lamotrigine titration with placebo added on days 43–56 (LTG group; N = 12), or placebo on days 1–42 with olanzapine added on days 43–56 (OLZ group; N = 16). Steady state (0–24 h) pharmacokinetic profiles were determined on day 56 in each group.
Results
The average (90% confidence interval) ratios of lamotrigine area under the concentration–time curve from 0 to 24 h and maximum observed concentration for the comparison of LTG + OLZ:LTG were 0.76 (0.65, 0.90) and 0.80 (0.71, 0.90), respectively. Olanzapine pharmacokinetics were essentially unaffected by lamotrigine. The most frequently reported adverse events (AEs) during combination therapy were fatigue, dizziness and mild transaminase elevations. These AEs occurred at similar frequencies in the LTG + OLZ and OLZ cohorts, while being less frequent or absent in the LTG group.
Conclusions
Lamotrigine and olanzapine coadministration in patients who may benefit from the combination was supported by this study. Lamotrigine dosing schedules are recommended to remain unchanged when combined with olanzapine therapy. However, the possibility exists that the lamotrigine dose for some patients may need adjustment to optimize treatment when olanzapine is added to or withdrawn from a regimen including lamotrigine.
Keywords: lamotrigine, olanzapine, pharmacokinetics, tolerability
Introduction
Combination therapy is frequently required in the management of the diverse and often fluctuating symptoms of bipolar disorder [1, 2]. The complementary efficacy profiles of lamotrigine and olanzapine may be beneficial in conferring broad-spectrum control of bipolar and other psychiatric symptoms. Olanzapine is primarily effective in the treatment of mania in bipolar I disorder, whereas lamotrigine appears to have mainly an antidepressant effect and is indicated for the maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes (mania, hypomania, depression and mixed symptoms) [3–7].
Lamotrigine is an anticonvulsant metabolized primarily by glucuronidation into an inactive conjugate. After oral dosing of 240 mg of 14C-lamotrigine (15 µCi) to six healthy subjects, 94% of the dose was recovered in the urine and 2% in the faeces. The urine components were unchanged lamotrigine (10%), the 2-N-glucuronide (76%), a 5-N-gulcuronide (10%), a 2-N-methyl metabolite (0.14%), and other unidentified minor metabolites (4%). Lamotrigine is not known to inhibit or induce other enzymes, including the cytochromes P450 isozymes [8, 9].
Olanzapine is an atypical antipsychotic metabolized primarily by glucuronidation and cytochrome P450-mediated oxidation into 10-N-glucuronide olanzapine and 2-hydroxymethylolanzapine, 4′-N-desmethyl olanzapine and 4′-N-oxide olanzapine [10–13].
Although inhibition of the cytochrome P450-mediated metabolism of one drug by the other is unlikely, a pharmacokinetic interaction between lamotrigine and olanzapine is possible, since both are eliminated by glucuronidation. This paper reports the results of a randomized, placebo-controlled study undertaken to assess the pharmacokinetics and tolerability of lamotrigine and olanzapine administered in combination to healthy subjects.
Methods
Subjects
Fifty-two healthy male subjects were randomized to treatment, with 43 completing the study. Nine subjects withdrew prematurely for personal reasons (n = 6), elevated transaminases (n = 1), a positive alcohol breath test (n = 1) or being lost to follow-up (n = 1). Subjects ranged from 18 to 42 years in age, with demographics and baseline characteristics being comparable across treatment groups (data not shown). Pharmacokinetic data were analysed from the 43 subjects who completed the study and one additional subject who prematurely withdrew but had evaluable data. Exclusion criteria included current tobacco use (or evidence thereof), alcohol use or drug abuse within 6 months of the study, concurrent medication, including vitamins or herbal remedies, that in the judgement of the investigator may have interfered with the study, a history of drug-induced rash, or the participation in a study of an investigational product within 84 days of the present study. Ibuprofen was the only concomitant medication permitted during the study.
Study design and treatments
This randomized, partially blind, placebo-controlled, parallel-group study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, at Richmond Pharmacology (Wimbledon, UK). All subjects provided written informed consent following Ethics Committee approval (Ravenscourt Ethics Committee, Ashford Hospital, London, UK).
The study consisted of a screening visit within 1 month of drug administration, a treatment period during which study medication was administered for 56 days (a titration phase on days 1–42 and an inpatient combination-therapy phase on days 43–56) and a follow-up visit occurring 7–10 days after the last treatment day. During the screening visit, study eligibility was determined by physical examination, standard clinical chemistry and haematology tests and 12-lead electrocardiograms.
Subjects were then randomized to receive lamotrigine during the titration phase with the addition of olanzapine during the combination-therapy phase (LTG + OLZ group, 17 subjects), lamotrigine during the titration phase with placebo during the combination-therapy phase (LTG group, 12 subjects), or placebo during the titration phase with olanzapine during the combination-therapy phase (OLZ group, 17 subjects). Both lamotrigine and olanzapine were administered as once-daily doses. Lamotrigine was given in the morning at 25 mg per day for 14 days, increased to 50 mg per day for 14 days, and then to 100 mg per day for 7 days, and finally to 200 mg per day for 7 days, and in double-blind fashion. Olanzapine or nonmatching placebo was given in single-blind fashion on day 43 at 5 mg per day and increased in 5 mg day−1 increments each evening for 3 days to a total dose of 15 mg per day. The full dose of 15 mg per day was administered in the mornings from the fifth day of coadministration onwards (i.e. days 47–56).
Inclusion of 15 subjects in the LTG + OLZ and the OLZ groups and 10 subjects in the LTG group was estimated to provide at least 90% power for detecting a 30% change in pharmacokinetic parameters. A change of this magnitude or greater was regarded as having statistical significance.
Pharmacokinetic assessments
Blood sample collection Blood samples (2 ml for lamotrigine and 10 ml for olanzapine analysis) were drawn before dosing and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h postdose on treatment day 56 following an overnight fast. Blood samples were also drawn before dosing on treatment days 54 and 55 to confirm that lamotrigine and olanzapine concentrations were at steady state.
Assays Blood samples for the analysis of lamotrigine were collected into serum separation vacutainers and allowed to clot for 20 min at room temperature, and then centrifuged within 60 min at 1500 g for 15 min at 4°C. The resultant serum was transferred to a screw-cap cryo/polypropylene tube, frozen immediately, stored at ≤ − 20 °C, and transferred to Advion Biosciences, Ithaca, New York, for analysis.
Lamotrigine and the internal standard, [13C215N5] lamotrigine, were extracted from 50-µl aliquots of human serum using a solid phase (Oasis HLB) extraction procedure. The extracts were reconstituted in 100 µl of 50 : 50 methanol:water and quantified by turbo ion spray liquid chromatography/tandem mass spectrometry (LC/MS/MS) in the positive ion mode. A MetaChem Inertsil ODS column (3 µm, 2 × 50 mm) with an isocratic mobile phase of 50 : 50 methanol:water containing 2 mm ammonium hydroxide (pH 8.5) at a flow rate of 200 µl min−1 was used. The mass spectrometer (Sciex API 365) operating conditions were established to monitor precursor to product ion transitions of m/z 256.0 to m/z 211.1 for lamotrigine and m/z 263.1 to m/z 215.1 for [13C215N5] lamotrigine (internal standard) with dwell times of 400 ms for each transition. The TurboIonSpray™ source temperature was maintained at 300 °C, and the ionspray voltage was set at 1500 V. All the gases (curtain, nebulizer, collision and TurboIonSpray™ auxiliary gas) were UHP nitrogen, with the TurboIonSpray™ auxiliary gas flow set at 8 l min−1, the declustering potential at 50 V and the collision energy at 33.5 V. The MS acquisition time was 3 min and the pause time 5 ms. Retention times were 1.8 min for both lamotrigine and [13C215N5] lamotrigine and the autosampler cycle time was 5 min. The assay had a lower limit of quantification of 4 ng ml−1 using 50 µl plasma and calibration lines were linear over the range 4–4000 ng ml−1. Quality control samples (QC), prepared at four different analyte concentrations and stored with study samples, were analysed with each batch of samples against separately prepared calibration standards. The applicable analytical runs met all predefined acceptance criteria. The mean precision/bias over the assay concentration range was better than 3.5%/3.7%, with maximum average precision/bias at any one concentration level better than 5.4%/5.1%.
Blood samples for the analysis of olanzapine were collected into plasma tubes containing EDTA as the anticoagulant and were stored at 4°C until centrifugation at 1500 g for 10 min at 4°C. Plasma was decanted into K3EDTA tubes containing 15 µl ascorbic acid (25 g per 100 ml), and were stored at − 70°C until transfer to Anapharm Inc., Quebec, Canada, for analysis.
Olanzapine and the internal standard, clozapine, were extracted from 100-µl aliquots of human plasma using solid-phase Oasis MCX® cartridges. The extracts were reconstituted in 800 µl of 60 : 30 : 10 methanol:water:isopropyl alcohol in 15 mm ammonium acetate (pH 3.8) and the drug was quantified by turbo ion spray liquid chromatography/tandem mass spectrometry (LC/MS/MS) in the positive ion mode. A Betasil CN column (5 µm, 4.6 × 100 mm) with an isocratic mobile phase of 70 : 30 methanol:water containing 15 mm ammonium hydroxide (pH 3.8) at a flow rate of 1 ml min−1 was used. The mass spectrometer (Sciex API 3000) operating conditions were established to monitor precursor to product ion transitions of m/z 313.2 to m/z 256.1 for olanzapine and m/z 372.2 to m/z 270.0 for clozapine (internal standard) with dwell times of 500 ms for each transition. The TurboIonSpray™ source temperature was maintained at 375 °C and the ionspray voltage was set at 2500 V. All the gases (curtain, nebulizer, collision and TurboIonSpray™ auxiliary gas) were UHP nitrogen, with the TurboIonSpray™ auxiliary gas flow set at 5 l min−1, the declustering potential at 50 V and the collision energy at 33 V. The MS acquisition time was 3.2 min and the pause time was 50 ms. The split ratio was 1–5 with a flow of 200 µl min−1 into the interface. Retention times were 1.7 min for olanzapine and 2.1 min for clozapine and the autosampler cycle time was 2.2 min. The assay had a lower limit of quantification of 0.5 ng ml−1 using 100-µl aliquot of plasma and calibration lines were linear over the range 0.5–50 ng ml−1. QC samples, prepared at three different analyte concentrations and stored with study samples, were analysed with each batch of samples against separately prepared calibration standards. The applicable analytical runs met all predefined acceptance criteria. Mean precision/bias over the assay concentration range was better than 4.8%/−1.4%, with maximum average precision/bias at any one concentration level better than 7.6%/−2.7%.
Tolerability assessment
Adverse events, defined as any untoward medical occurrences regardless of suspected cause, were recorded from the first administration of study medication through to the follow-up visit. All adverse events were reported spontaneously, or in response to an open question at clinic visits occurring on treatment days 8, 15, 22, 29, 36, and 43 to 56, or were recorded and summarized using descriptive statistics.
Data analyses
Serum lamotrigine and plasma olanzapine concentration–time data were analysed by noncompartmental pharmacokinetic methods using WinNonlin Professional Version 3.1 (Pharsight Corporation, Cary, NC, USA). The maximum observed concentration (Cmax), the time to reach Cmax (Tmax) and the area under the concentration–time curve from time zero (predose) to 24 h postdose (AUC(0−24)) were determined by a combination of linear and logarithmic trapezoidal methods [14].
The loge-transformed AUC(0−24) and Cmax of lamotrigine in the presence and absence of olanzapine and the loge-transformed AUC(0−24) and Cmax of olanzapine in the presence and absence of lamotrigine were analysed separately by analysis of variance (anova). To investigate the effects of lamotrigine on olanzapine pharmacokinetics, point estimates and 90% confidence intervals (CIs) were constructed, using the residual variance from the anova, for the differences in loge-transformed AUC(0−24) and Cmax of olanzapine in the presence and absence of lamotrigine. These values were back-transformed to obtain the estimates of the LTG + OLZ:OLZ ratio. The same methods were applied to determine the effects of olanzapine on lamotrigine pharmacokinetics, based on estimates of the LTG + OLZ:LTG ratio.
Results
Predose concentrations indicated that steady state was achieved for both lamotrigine and olanzapine in all groups by day 54 (data not shown).
The median serum concentration-vs.-time profile of lamotrigine administered with olanzapine or placebo is shown in Figure 1. Lamotrigine AUC(0−24) and Cmax were, on average, 24% and 20% lower, respectively, in the LTG + OLZ group compared with those of the LTG group (Table 1). The 90% CIs for the ratios indicated that the true differences lie between 10% and 35% lower for AUC(0−24) and between 10% and 29% lower for Cmax. Between-subject variability for lamotrigine AUC(0−24) and Cmax increased by approximately threefold when lamotrigine was administered with olanzapine compared with when lamotrigine was administered with placebo. Lamotrigine Tmax values were comparable in both groups.
Figure 1.
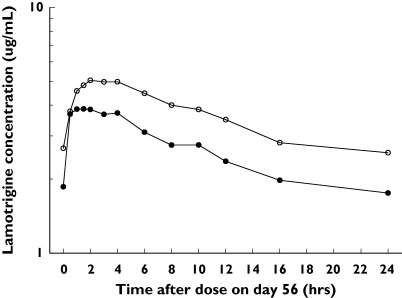
Median serum lamotrigine concentration–time profiles after oral administration with placebo or with olanzapine. LTG + OLZ (•), LTG (○)
Table 1.
Geometric mean (SD) pharmacokinetic parameters and statistical comparisons (ratios[90% CI]) after administration of lamotrigine with olanzapine (LTZ + OLZ), lamotrigine with placebo (LTG) or olanzapine with placebo (OLZ)
| Lamotrigine pharmacokinetic parameters | |||
|---|---|---|---|
| LTG + OLZ [N = 16] | LTG [N = 12] | Ratio LTG + OLZ:LTG [90% CIs] | |
| Cmax (ug ml−1) | 4.29 (1.04) | 5.36 (0.43) | 0.80 [0.71, 0.90] |
| AUC(0−24) (ug h ml−1) | 66.2 (24.2) | 87.2 (11.5) | 0.76 [0.65, 0.90)] |
| Median (range) Tmax (h) | 2.00 (0.50–4.00) | 2.00 (1.00–4.00) | NA |
| Olanzapine pharmacokinetic parameters | |||
| LTG + OLZ [N = 16] | OLZ [N = 16] | Ratio LTG + OLZ:OLZ [90% CIs] | |
| Cmax (ng ml−1) | 45.9 (10.7) | 44.4 (11.6) | 1.03 [0.90, 1.19] |
| AUC(0−24) (ng h ml−1) | 817 (174) | 786 (223) | 1.04 [0.90, 1.20] |
| Median (range) Tmax (h) | 3.06 (2.00–8.02) | 4.00 (1.50–8.00) | NA |
The median plasma concentration-vs.-time profile of olanzapine administered with lamotrigine or placebo is shown in Figure 2. Olanzapine AUC(0−24), Cmax, and Tmax were essentially unchanged in the LTG + OLZ group relative to the OLZ group (Table 1).
Figure 2.
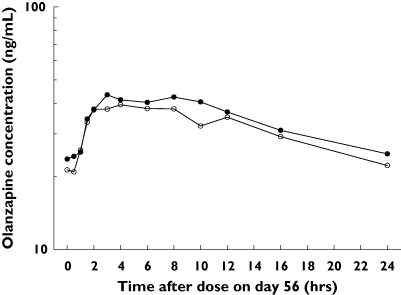
Median plasma olanzapine concentration–time profiles after oral administration with placebo or with lamotrigine. LTG + OLZ (•), OLZ (○)
The most common and only frequent adverse event (AE) during the lamotrigine dose titration phase was headache occurring at similar frequency in the cohorts receiving lamotrigine and placebo (Table 2).
Table 2.
Summary (number subjects (% of total in each group)) of adverse events reported
| Days 1–42: Treatment with lamotrigine or placebo | |||
|---|---|---|---|
| Study group Drug exposure | LTG + OLZ (n = 19) Lamotrigine | LTG (n = 13) Lamotrigine | OLZ (n = 20) Placebo |
| Headache | 3 (15%) | 3 (23%) | 5 (25%) |
| Dizziness | 0 | 1 (8%) | 0 |
| Fatigue | 0 | 0 | 1 (5%) |
| Abnormal transaminases | 0 | 0 | 0 |
| Days 43–56: Treatment with lamotrigine and olanzapine, lamotrigine and placebo, or placebo and olanzapine | |||
| Study group Drug exposure | LTG + OLZ (n = 16) Lamotrigine and olanzapine | LTG (n = 12) Lamotrigine | OLZ (n = 18) Olanzapine |
| Headache | 2 (13%) | 4 (31%) | 1 (6%) |
| Dizziness | 4 (25%) | 0 | 6 (33%) |
| Fatigue | 7 (44%) | 3 (23%) | 9 (50%) |
| Abnormal transaminases | 8 (50%) | 0 | 8 (44%) |
The most common AEs during the combination therapy phase of the study were fatigue, dizziness and abnormal transaminases. The frequency of these AEs was similar in the LTG + OLZ and OLZ cohorts and lower for the LTG cohort (Table 2). None of the aspartate transaminase elevations exceeded three times the upper limit of normal, and the highest alanine transaminase elevation was 262 IU l−1 (reference range 8–45 IU l−1). None of the transaminase elevations was accompanied by significant increases in bilirubin concentrations. One subject, having been treated with placebo, had an unexplained elevation of transaminases on day 43 when he was due to have OLZ added to his placebo therapy, and was withdrawn from the study.
Discussion
Information on the pharmacokinetics of coadministered lamotrigine and olanzapine is necessary for healthcare providers in deciding whether and how to prescribe the combination to patients with bipolar disorder. This study in healthy subjects was conducted to investigate the pharmacokinetics and tolerability of lamotrigine and olanzapine administered in combination at clinically relevant doses and at steady-state concentrations. The target dose of lamotrigine 200 mg day−1 was chosen because it is the recommended dose for maintenance therapy of bipolar disorder. The target dose of olanzapine 15 mg day−1 was chosen because it lies within the recommended range (5–20 mg), and is associated with the greatest efficacy in bipolar disorder.
The results of this study have demonstrated that lamotrigine does not affect the pharmacokinetics of olanzapine when the drugs are administered in combination at the recommended dose and using the recommended dose-escalation schedule. Moreover, these findings suggest that the previously reported inhibition of olanzapine glucuronidation by lamotrigine observed in vitro[13] may not be relevant clinically.
Exposure to lamotrigine was moderately decreased (24% in the AUC(0−24) and 20% in the Cmax) in the presence of olanzapine. Although this decrease in lamotrigine concentrations is not expected to be clinically important, it does not preclude the possibility that the lamotrigine dose may need adjustment to optimize treatment when olanzapine is added to or withdrawn from the regimen. Between-subject variability for lamotrigine AUC(0−24) and Cmax increased by approximately threefold when it was administered with olanzapine compared with when lamotrigine was administered with placebo. Whether this difference in variabilities is due to the effect of olanzapine on lamotrigine disposition or to the parallel group nature of the study design remains to be determined. However, because of this variability, some patients may not be subject to a pharmacokinetic interaction, whereas others may experience a change in lamotrigine concentrations that may approach clinical significance.
Lamotrigine is primarily metabolized by the liver and eliminated by glucuronidation. The finding of a modest decrease in exposure to lamotrigine during coadministration with olanzapine suggests a degree of induction by olanzapine of the glucuronidation pathway(s) involved in elimination of the former drug. Olanzapine has not previously been reported as an inducer of hepatic metabolism. Because the pharmacokinetics sampling regimen in this study did not permit the determination of the terminal elimination half-lives of lamotrigine, it was not possible to deduce whether or not there was a change in the systemic elimination of the drug on coadministration with olanzapine. However, given that lamotrigine is completely absorbed after oral dosing with negligible first-pass metabolism [8], it is unlikely that the observed differences resulted from changes to the presystemic clearance of lamotrigine.
The tolerability of lamotrigine and olanzapine administered singly or in combination in this study was consistent with documented tolerability profiles of the drugs given separately [3–7]. During the lamotrigine drug titration phase, the most common adverse event was headache, with a similar incidence in the lamotrigine and placebo cohorts. During combination therapy, the most common clinical adverse events were fatigue and dizziness, which were reported far more frequently among subjects receiving the olanzapine-containing regimens compared with subjects receiving lamotrigine and placebo. Elevations in transaminase concentrations were also frequently reported among subjects receiving the olanzapine-containing regimens, but not among subjects receiving lamotrigine and placebo. Dose-dependent subclincial elevations in transaminase concentrations comparable to the ones reported in this study are known to occur with olanzapine [15]. The incidence of these changes in liver enzymes was similar between the LTG + OLZ and OLZ groups.
In conclusion, the combination of lamotrigine and olanzapine was well tolerated in this healthy subject population. When these drugs are coadministered in a manner consistent with their individual use in clinical practice, the pharmacokinetics of olanzapine is not affected by lamotrigine. Although olanzapine moderately decreased exposure to lamotrigine, this effect is not expected to be clinically important in most patients. However, the possibility exists that for some patients the lamotrigine dose may need adjustment to optimize treatment when olanzapine is added to or withdrawn from a regimen including lamotrigine.
Competing interests: None declared.
References
- 1.Nemeroff CB. An ever-increasing pharmacopoeia for the management of patients with bipolar disorder. J Clin Psychiat. 2000;61(Suppl 13):19–25. [PubMed] [Google Scholar]
- 2.Freeman MP, Stoll AL. Mood stabilizer combinations: a review of safety and efficacy. Am J Psychiat. 1998;155:12–21. doi: 10.1176/ajp.155.1.12. [DOI] [PubMed] [Google Scholar]
- 3.Bowden CL, Calabrese JR, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J. A placebo-controlled, 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch General Psychiat. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese J, Bowden C, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J. A placebo-controlled, 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiat. 2003;64:1013–24. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- 5.Baldessarini RJ, Hennen J, Wilson M, Calabrese J, Chengappa R, Keck PE, McElroy SL, Sachs G, Vieta E, Welge JA, Yatham LN, Zarate CA, Baker RW, Tohen M. Olanzapine versus placebo in acute mania: treatment responses in subgroups. J Clin Psychopharm. 2003;23:370–6. doi: 10.1097/01.jcp.0000085410.08426.9a. [DOI] [PubMed] [Google Scholar]
- 6.Tohen M, Goldberg JF, Gonzalez-Pinto AAM, Azorin JM, Vieta E, Hardy-Bayle MC, Lawson WB, Emsley RA, Zhang F, Baker RW, Risser RC, Namjoshi MA, Evans AR, Breier A. A 12-week, double-blind comparison of olanzapine vs haloperidol in the treatment of acute mania. Arch General Psychiat. 2003;60:1218–26. doi: 10.1001/archpsyc.60.12.1218. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin GM, Bowden CL, Calabrese JR, Grunze H, Kasper S, White R, Greene P, Leadbetter R. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiat. 2004;65:432–41. doi: 10.4088/jcp.v65n0321. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey RE, Pellock JM, Garnett WR, Sanchez RM, Valakas AM, Wargin WA, Lai AA, Hubbell J, Chern WH, Allsup T. Pharmacokinetic and safety of lamotrigine (Lamictal) in patients with epilepsy. Epilepsy Res. 1991;10:191–200. doi: 10.1016/0920-1211(91)90012-5. [DOI] [PubMed] [Google Scholar]
- 9.Magdalou J, Herber R, Bidault R, Siest G. In vitro N-glucuronidation of a novel antiepileptic drug, lamotrigine, by human liver microsomes. J Pharmacol Exp Ther. 1992;260:1166–73. [PubMed] [Google Scholar]
- 10.Kassahun K, Mattiuz EL, Nyhart E, Obermeyer B, Gillespie T, Murphy A, Goodwin RM, Tupper D, Callaghan JT, Lemberger L. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25:81–93. [PubMed] [Google Scholar]
- 11.Ring BJ, Catlow J, Lindsay TJ, Gillespie T, Roskos LK, Cerimele BJ, Swanson SP, Hamman MA, Wrighton SA. Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J Pharmacol Exp Ther. 1996;276:658–66. [PubMed] [Google Scholar]
- 12.Keck PE, McElroy SL. Clinical pharmacodynamics and pharmacokinetics of antimanic and mood-stabilizing medications. J Clin Psychiat. 2002;63(Suppl 4):3–11. [PubMed] [Google Scholar]
- 13.Linnet K. Glucuronidation of olanzapine by cDNA-expressed human UDP-glucuronosyltransferases and human liver microsomes. Hum Psychopharmacol. 2002;17:233–8. doi: 10.1002/hup.403. [DOI] [PubMed] [Google Scholar]
- 14.Chinchilli VM. The assessment of individual and population bioequivalence. J Biopharmaceut Stat. 1996;6:1–14. doi: 10.1080/10543409608835118. [DOI] [PubMed] [Google Scholar]
- 15.Beasley CM, Jr, Tollefson GD, Tran PV. Safety of olanzapine. J Clin Psychiat. 1997;58(Suppl 10):13–17. [PubMed] [Google Scholar]


