Abstract
β-adrenoceptor blockers and thrombolytic agents are of established value in the pharmacological management of heart failure and ST-elevation myocardial infarction, respectively. However, there is uncertainty as to whether these therapeutic strategies can be safely and effectively adopted in elderly patients with comorbidities, particularly in old-old individuals. This review focuses on these trials and the age-related efficacy and safety of these drugs.
Keywords: congestive cardiac failure, elderly patients, myocardial infarction, randomized controlled trials, thrombolytic agents, β-adrenoceptor blockers
β-adrenoceptor blockers in heart failure
Based on several large clinical trials the current treatment guidelines for heart failure with left ventricular systolic dysfunction recommend the use of β-adrenoceptor blockers as routine treatment, provided that it is well tolerated [1–5]. Despite these recommendations, β-adrenoceptor blockers are underutilized, particularly in elderly patients [6]. This is probably due to concern about safety and tolerability as well as the paucity of published data regarding the beneficial effects of this class of drugs in this population. Most randomized controlled trials on the use of β-adrenoceptor blockers in heart failure have excluded elderly patients, in particulars subjects aged >80 years. A meta-analysis of 22 randomized trials of β-adrenoceptor blockers in heart failure showed that the mean age of patients studied ranged between 48 and 67 years [7]. Data regarding studies including patients >65 years are discussed.
The CIBIS (Cardiac Insufficiency Bisoprolol Study) trial investigated the effects of bisoprolol vs. placebo, in addition to background diuretic and vasodilator therapy, in patients with New York Heart Association (NYHA) class III-IV heart failure (Figure 1 and Table 1) [8]. Bisoprolol did not affect mortality rates but reduced hospitalization for cardiac decompensation (absolute risk reduction (ARR) 9.0%, P < 0.01) and improved NYHA class (P = 0.04). The lack of a significant effect of bisoprolol on mortality in this trial is likely to be secondary to the relatively small sample size and inadequate power as the ARR was 4.5%. There was no significant difference in withdrawal rates between bisoprolol and placebo (26%vs. 23%). Subgroup analysis in patients >65 years is not available.
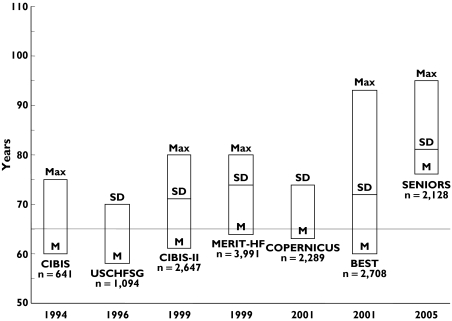
Figure 1.
Age distribution of trials on β-adrenoceptor blockers in elderly patients. M, mean age; SD, standard deviation; Max, maximum age
Table 1.
Clinical trials of β-adrenoceptor blockers in heart failure including elderly patients
| Result of primary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Agents | Follow-up | Elderly participants | Women | Inclusion criteria | Primary outcome measures | ARR P value | Incidence in active group | Incidence in placebo group |
| CIBIS [8] | Bisoprolol 1.25–5 mg vs. placebo | 1.9 years | NA | 17.5% | CHF NYHA III-IV | All-cause mortality | 16.6% | 20.9% | 4.3%P = 0.22 |
| CIBIS-II [1] | Bisoprolol 1.25–10 mg vs. placebo | 1.3 years | NA | 19.4% | CHF NYHA III-IV | All-cause mortality | 11.7% | 17.3% | 5.6%P < 0.0001 |
| US CHFSG [10] | Carvedilol 12.5–100 mg vs. placebo | 0.5 years | ≥ 59 years (50.1%) | 23.4% | CHF with EF ≤ 0.35 | All-cause mortality | 3.2% | 7.8% | 4.6%P < 0.001 |
| MERIT-HF [3] | Metoprolol CR/XL 200 mg vs. placebo | 1 year | 60–69 years (35.2%)≥ 70 years (31.2%) | 22.5% | CHF NYHA II-IV with EF ≤ 0.40 | All-cause mortality | 7.3% | 10.8% | 3.5%P = 0.00009 |
| COPERNICUS [2] | Carvedilol 50 mg vs. placebo | 0.9 years | NA | 20.5% | CHF NYHA IV with EF < 0.25 | All-cause mortality | 11.2% | 16.8% | 5.6%P = 0.0014 |
| BEST [12] | Bucindolol 200 mg vs. placebo | 2 years | >60 years 55.8% | 21.9% | CHF NYHA III-IV with EF ≤ 0.35 | All-cause mortality | 30.3% | 33.2% | 2.9%P = 0.13 |
| SENIORS [13] | Nevibolol 1.25–10 mg vs. placebo | 1.7 years | ≥70 years (100%) | 36.9% | History of CHF | All-cause mortality or CV hospitalization | 31.1% | 35.3% | 4.2%P = 0.039 |
CHF, congestive heart failure; NYHA, New York Heart Association; EF, ejection fraction; CV, cardiovascular; NA, not available; ARR, absolute risk reduction.
The CIBIS-II study, conducted on a larger study population, was stopped prematurely because bisoprolol showed a significant mortality benefit (Figure 1 and Table 1) [1]. Adverse events occurring more frequently with bisoprolol than placebo included dizziness (13.3 vs. 9.5%), bradycardia (15.2%vs. 4.5%), hypotension (11.4%vs. 7.3%) and fatigue (9.3%vs. 7.1%) [9].
The US Carvedilol Heart Failure Study Group investigated the effects of carvedilol vs. placebo in NYHA class II-IV heart failure patients already receiving diuretics and ACE inhibitors (Figure 1 and Table 1) [10]. All-cause mortality was significantly reduced with carvedilol. Treatment discontinuation occurred in 7.8% of patients receiving placebo and 5.7% of patients receiving carvedilol.
The MERIT-HF (Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure) study was a double-blind trial on patients with chronic heart failure, stabilized with standard therapy, receiving metoprolol CR/XL or placebo (Figure 1 and Table 1) [3]. Patients receiving metoprolol had reduced all-cause mortality (ARR 3.5%, P = 0.00009), sudden deaths (ARR 2.6%, P = 0.0002), and deaths from worsening heart failure (ARR 1.4%, P = 0.0023) [3]. In a recent subanalysis of MERIT-HF study, the total mortality in patients ≥65 years was reduced by 37% (ARR 4.7%, P = 0.0008) and death from worsening heart failure by 61% (ARR 2.9%, P = 0.0005) [11]. Hospitalizations for heart failure were reduced by 36% (ARR 9.1%, P = 0.0006). Elderly patients with heart failure NYHA class III-IV showed similar risk reductions [11]. Yearly discontinuation rates were 14.1% with placebo and 12.8% with metoprolol CR/XL (P = 0.2) in patients ≤65 years and 20.3% with placebo and 17.8% with metoprolol CR/XL (P = 0.2) in patients ≥65 years. In patients ≥65 years, adverse events occurred in 15.3% of the placebo group and 13.6% of the metoprolol CR/XL group [11].
In the COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival Study Group) study, patients with heart failure were randomized to treatment with carvedilol or placebo (Figure 1 and Table 1) [2]. In the patients treated with carvedilol there was a reduction in the risk of death [2]. The results were similar for patients ≥65 years [2]. Fewer patients in the carvedilol group required permanent discontinuation of treatment because of adverse effects or for reasons other than death [2].
In the BEST (Beta-Blocker Evaluation of Survival Trial Investigators) study, patients with heart failure predominantly in NYHA class III were randomized to bucindolol or placebo (Figure 1 and Table 1) [12]. There was no significant difference in mortality between the two groups although a trend in favour of bucindolol was observed [12]. The reasons for these negative findings might be related to the different patient populations (bucindolol had a favourable impact in nonblack patients) and/or pharmacological differences between the β-adrenoceptor blockers tested [12].
More recently, the results of the SENIORS (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure) study have been published [13]. Of 2135 elderly heart failure patients recruited, 2128 were randomized to nebivolol vs. placebo (Figure 1 and Table 1). Exclusion criteria were the following: new drug therapy for heart failure in the 6 weeks prior to randomization, any change in cardiovascular drug therapy in the 2 weeks prior to randomization, heart failure secondary to valvular heart disease, contraindication or previous intolerance to β-adrenoceptor blockers, current use of β-adrenoceptor blockers, significant hepatic or renal dysfunction, cerebrovascular events within the previous 3 months, and being on the waiting list for percutaneous coronary intervention or cardiac surgery or other major medical conditions that may have reduced survival during the period of the study. The primary outcome, a composite of all-cause mortality or cardiovascular hospital admission, occurred less frequently with nebivolol. Nebivolol was well tolerated except for an increased incidence of bradycardia.
Discussion
The available evidence suggests that the use of β-adrenoceptor blockers in elderly patients with heart failure is beneficial up to the age of 95 years (Figure 1). Importantly, however, the published randomized controlled trials have been conducted in patients with systolic heart failure, i.e. with left ventricular ejection fraction <35–40%. Although there is a high prevalence of diastolic heart failure in the elderly population, no study has specifically investigated the effects of β-adrenoceptor blockers on cardiovascular morbidity and mortality in this setting. Also, it is unknown whether β-adrenoceptor blockers provide similar benefits in frail, institutionalized elderly patients suffering from multiple comorbidities.
β-adrenoceptor blockers were generally well tolerated in elderly patients participating in controlled trials. In the subanalysis of the MERIT-HF study, adverse events such as atrio-ventricular block, depression, bronchospasm and aggravation of chronic obstructive pulmonary disease were remarkably similar in the metoprolol and placebo groups [11]. Slightly more patients in the metoprolol group discontinued the medication because of bradycardia, hypotension, dizziness, fatigue and dyspnoea. However, compared with placebo, the net difference in discontinuation for any of these reasons was less than one patient per 100 treated during 1 year [11].
Thrombolytics in acute myocardial infarction
Thrombolytic therapy represents a major advance in the treatment of patients with acute myocardial infarction [14]. A meta-analysis of the nine largest randomized controlled trials conducted between 1982 and 1992, involving 58 000 patients, showed that thrombolysis reduced 35-day mortality, particularly in patients <75 years treated within 12 h of symptom onset [15]. Among the 5754 patients ≥75 years, thrombolytic therapy was associated with an absolute reduction in mortality of 1%, which was not significant [15]. In contrast, the ARR in patients <55 years (1.2%, P = 0.0002), 55–64 years (1.7%, P < 0.00001) and 65–74 years (2.6%, P < 0.00001) were highly significant [15]. The major trials on the effects of thrombolysis including patients >65 years are discussed below.
In the GISSI-1 (Gruppo Italiano per lo Studio della Streptokinasi nell’Infarto Miocardico) study, patients with acute myocardial infarction were randomized to streptokinase or placebo (Figure 2 and Table 2) [14]. Mortality at 21 days postmyocardial infarction was significantly reduced in the group treated with streptokinase [14]. Statistically significant benefit, however, was observed only in patients ≤65 years (ARR 2.0%, P = 0.0005), whereas there was a nonsignificant benefit trend in patients >65–75 years (ARR 1.5%) and in patients >75 years (ARR 4.2%) [14]. There was no significant difference in the rate of cerebrovascular events between streptokinase (0.92%) and placebo (0.77%) [16].
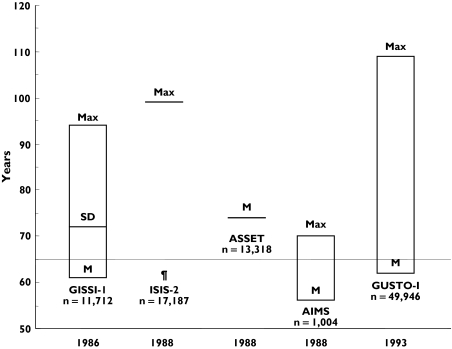
Figure 2.
Age distribution of trials on thrombolytics in elderly patients. M, mean age; SD, standard deviation; Max, maximum age; ¶, M and SD not available
Table 2.
Clinical trials of thrombolytic agents including elderly patients
| Result of primary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Agents and daily dose | Follow-up | Elderly participants | Women | Inclusion criteria | Primary outcome measures | Incidence in active group | Incidence in placebo group | ARR P value |
| GISSI [14] | Streptokinase 1.500.000 U vs. placebo | 21 days | 65–75 years (24.6%)>75 years (10.3%) | 19.7% | MI within 12 h of symptom onset | All-cause mortality | 10.7% | 13.0% | 2.3%P = 0.0002 |
| ISIS-2 [18] | Streptokinase 1.500.000 U vs. aspirin 160 mg vs. both | 5 weeks | 60–69 years (35.2%)≥70 years (19.8%) | 22.9% | MI within 24 h of symptom onset | Vascular mortality | 9.2% | 12.0% | 2.8%P < 0.00001 |
| ASSET [19] | rt-PA 100 mg + heparin vs. placebo + heparin | 1 month | >65 years (33.5%) | 23.0% | MI within 5 h of symptom onset | All-cause mortality | 7.2% | 9.8% | 2.6%P = 0.0011 |
| AIMS [17] | APSAC 30 U vs. placebo | 1 month | ≥60 years (39.9%) | NA | MI within 6 h of symptom onset | All-cause mortality | 6.4% | 12.2% | 5.8%P = 0.0016 |
| GUSTO-I [20] | Streptokinase 1.500.000 U + heparin s.c or streptokinase 1.500.000 U + heparin i.v. or accelerated TPA + heparin i.v. or streptokinase 1.500.000 U + accelerated TPA + heparin i.v. | 1 year | 65–74 years 27.3%75–85 years 11.3%>85% years 1.0% | 25.0% | MI within 6 h of symptom onset | All-cause mortality at 30 days and 1 year | 6.3% (t-PA) | 7.4% (streptokinase) | 1.1%P = 0.001 |
MI, myocardial infarction; NA, not available; ARR, absolute risk reduction.
The AIMS (APSAC Intervention Mortality Study) trial enrolled patients with acute myocardial infarction randomized to anisoylated plasminogen streptokinase activator complex (APSAC) or placebo and followed-up for 30 days (Figure 2 and Table 2) [17]. APSAC treatment was associated with a reduction in mortality (ARR 5.8%, P = 0.0016), the benefit being greater in patients 65–70 years (ARR 18.0%) than in patients <65 years (ARR 3.3%) [17]. However, the small number of patients in the first group (n = 176) limits data interpretation. Treatment with APSAC was associated with an increased risk of haematuria (P = 0.001) and haemoptysis (P = 0.07).
In the ISIS-2 (Second International Study of Infarct Survival) trial, patients with acute myocardial infarction were randomly allocated to treatment with streptokinase, aspirin, or both, and matched placebo (Figure 2 and Table 2) [18]. Five-week mortality in the streptokinase and aspirin group was reduced when compared with the single treatment groups [18]. Sub-analysis according to age did not show significant differences among patient groups. In the streptokinase and aspirin vs. placebo comparison the ARR was 2.5% in patients <60 years, 7.0% in patients 60–69 years, and 8.0% in patients ≥70 years [18]. There was a significant excess of cerebral haemorrhage with streptokinase during the first 2 days of treatment (absolute risk increase 0.1%, P = 0.02). However, after this early period, fewer strokes occurred in the streptokinase group (ARR 2.4%, P < 0.05).
The ASSET (Anglo-Scandinavian Study of Early Thrombolysis) study investigated the effects of recombinant tissue-type plasminogen activator (rt-PA) plus heparin vs. placebo plus heparin in patients with myocardial infarction within 5 h of symptom onset (Figure 2 and Table 2) [19]. After 1 month of follow-up, rt-PA treatment was associated with a reduction in mortality. ARR was 0.6% in patients ≤55 years, 1.4% in patients 56–65 years, and 5.6% in patients 66–75 years [19]. There was a slight increase in the incidence of stroke with rt-PA (absolute risk increase, ARI, 0.1%).
In a posthoc analysis of the GUSTO-I (Global Use of Strategies to Open Occluded Coronary Arteries) trial, clinical outcomes at 30 days and 1-year mortality were analysed according to different age ranges (<65, 65–74, 75–85, and >85 years) [20]. Death occurred less often with accelerated TPA in all but the oldest patients, who showed lower mortality with streptokinase plus subcutaneous unfractionated heparin [20]. The ARR was 0.7% in patients <65 years, 2.0% in patients 65–74 years, 1.0% in patients 75–85 years, and −2.0% in patients >85 years [20]. Similar results were obtained for 1-year mortality [20]. It must be emphasized, however, that patients >85 years represented only 1% of the study population and the results might be secondary to a chance effect [20].
Discussion
Given the strong evidence of benefit in patients 65–74 years and the potential benefit in patients ≥75 years, guidelines for the treatment of acute myocardial infarction have supported the use of thrombolysis for patients ≥75 years who present within 12 h of symptom onset [21]. Contrary to this perspective, an observational study showed that thrombolytic therapy is not beneficial and could actually be harmful in patients ≥75 years [22]. This study assessed the 30-day mortality among patients 65–86 years. Among the > 5000 patients aged 65–75 years, thrombolysis was associated with a 12% reduction in 30-day mortality [22]. However, among patients 76–86 years, thrombolytic therapy was associated with a statistically significant 38% relative increase in 30-day mortality and a particularly marked survival disadvantage among women [22]. The hazard ratio for thrombolytic therapy was 1.00 at age 74.3 years and increased by a factor of 1.056 per year (95% CI 1.030, 1.083, P < 0.001) [22]. The hazard ratio was 0.60 (95% CI 0.44, 0.82, P < 0.001) at age 65 years and 1.36 (95% CI 1.13, 1.64, P = 0.001) at age 80 years [22].
Another observational study has recently challenged these findings [23]. Data collection from the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions identified 6891 patients ≥75 years admitted with an acute ST-elevation myocardial infarction [23]. Fibrinolytic therapy was associated with a 13% adjusted relative reduction in the composite endpoint of mortality and cerebral bleeding after 1 year (95% CI 0.80, 0.94, P = 0.001) [23]. The results were similar if only patients 75–85 years were analysed (relative risk 0.86, 95% CI 0.78, 0.94, P = 0.01). However, the benefit was no longer present in patients >85 years (relative risk 0.94, 95% CI 0.81, 1.09, P = 0.4) [23]. These figures must be interpreted with caution though because of the retrospective nature of these two studies. Moreover, different endpoints were analysed (i.e. 30 day vs. 1 year mortality).
Possible mechanisms explaining the differences between patients 65–75 years and patients >75 years include progressive and accelerating apoptosis [24], increased susceptibility to postreperfusion injury [25], and reduced contractile recovery from ischaemia and hypoxia with advancing age [26].
Ideally, new randomized trials of reperfusion therapies should focus on patients >75 years with acute myocardial infarction and compare thrombolytic therapy with placebo treatment in hospitals that do not offer primary angioplasty and thrombolytic therapy with primary angioplasty in hospitals that do offer the latter procedure. One of these trials comparing thrombolysis with primary angioplasty has recently been published [27]. In this small study 130 consecutive patients aged ≥70 years were randomized to thrombolytic therapy with tissue-type plasminogen activator or primary angioplasty with stenting [27]. Thrombolysis was associated with worse clinical outcomes and a higher risk of bleeding complications [27]. These data must be interpreted with caution because of the small sample size. Moreover, primary angioplasty is not available at the hospitals where most elderly patients present with acute myocardial infarction.
The combination of reduced-dose thrombolytic therapy and platelet glycoprotein IIb/IIIa inhibition seems to enhance microvascular perfusion [28]. This regimen may be particularly favourable in elderly patients, in whom the propensity for reperfusion injury may be enhanced [28].
In summary, the available evidence supports the effectiveness of thrombolytic therapy in carefully selected patients with myocardial infarction <75 years but does not provide a definitive answer in patients ≥75 years. Research is urgently needed because this group now constitutes almost one-third of all patients with acute myocardial infarction, and this percentage is growing rapidly as the population ages.
In the absence of new randomized trials to resolve these questions, data from earlier trials and new observational studies can be used to define more clearly the predictors of positive and negative outcomes of reperfusion therapies for patients ≥75 years [29]. Some of these patients certainly benefit from thrombolytics but many face an increased risk of cerebral haemorrhage and other complications that can be disabling or fatal. Discerning physicians must recognize that age per se does not cause positive or negative outcomes of thrombolytic therapy, but rather that it is a marker for underlying pathophysiological factors and comorbidity that may influence treatment effects.
References
- 1.The Cardiac Insufficiency Bisoprolol Study II, (CIBIS-II) A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 2.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 3.Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Effect of metoprolol CR/XL in chronic heart failure. Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 4.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 5.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation. 2003;107:1570–5. doi: 10.1161/01.CIR.0000065187.80707.18. [DOI] [PubMed] [Google Scholar]
- 7.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134:550–60. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 8.The Cardiac Insufficiency Bisoprolol Study (CIBIS) A randomized trial of beta-blockade in heart failure. Circulation. 1994;90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 9.McGavin JK, Keating GM. Bisoprolol: a review of its use in chronic heart failure. Drugs. 2002;62:2677–96. doi: 10.2165/00003495-200262180-00017. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 11.Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC. Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. Eur Heart J. 2004;25:1300–9. doi: 10.1016/j.ehj.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 12.The BEST study. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 13.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 14.Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;1:397–402. [PubMed] [Google Scholar]
- 15.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction. Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311–22. [PubMed] [Google Scholar]
- 16.Maggioni AP, Franzosi MG, Farina ML, Santoro E, Celani MG, Ricci S, Tognoni G. Cerebrovascular events after myocardial infarction: analysis of the GISSI trial. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) BMJ. 1991;302:1428–31. doi: 10.1136/bmj.302.6790.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AIMS Trial Study Group. Effect of intravenous APSAC on mortality after acute myocardial infarction. Preliminary report of a placebo-controlled clinical trial. Lancet. 1988;1:545–9. [PubMed] [Google Scholar]
- 18.ISIS-(Second International Study on Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oralaspirin, both or neither among 17 187 cases of suspected acute myocardial infarction. Lancet. 1988;2:349–60. [PubMed] [Google Scholar]
- 19.Wilcox RGLG, Olsson CG, Jensen G, Skene AM, Hampton JR. Trial of tissue plasminogen activator for mortality reduction in acute myocardial infarction. Anglo-Scandinavian Study of Early Thrombolysis (ASSET) Lancet. 1988;2:525–30. doi: 10.1016/s0140-6736(88)92656-6. [DOI] [PubMed] [Google Scholar]
- 20.White HD, Barbash GI, Califf RM, Simes RJ, Granger CB, Weaver WD, Kleiman NS, Aylward PE, Gore JM, Vahanian A, Lee KL, Ross AM, Topol EJ. Age and outcome with contemporary thrombolytic therapy. Results from the GUSTO-I trial. Global Utilization of Streptokinase and TPA for occluded coronary arteries trial. Circulation. 1996;94:1826–33. doi: 10.1161/01.cir.94.8.1826. [DOI] [PubMed] [Google Scholar]
- 21.Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith EE, Jr, Weaver WD. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) J Am Coll Cardiol. 1996;28:1328–428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- 22.Thiemann DR, Coresh J, Schulman SP, Gerstenblith G, Oetgen WJ, Powe NR. Lack of benefit for intravenous thrombolysis in patients with myocardial infarction who are older than 75 years. Circulation. 2000;101:2239–46. doi: 10.1161/01.cir.101.19.2239. [DOI] [PubMed] [Google Scholar]
- 23.Stenestrand U, Wallentin L. Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med. 2003;163:965–71. doi: 10.1001/archinte.163.8.965. [DOI] [PubMed] [Google Scholar]
- 24.James TN. Normal and abnormal consequences of apoptosis in the human heart. Annu Rev Physiol. 1998;60:309–25. doi: 10.1146/annurev.physiol.60.1.309. [DOI] [PubMed] [Google Scholar]
- 25.Azhar G, Gao W, Liu L, Wei JY. Ischemia-reperfusion in the adult mouse heart: influence of age. Exp Gerontol. 1999;34:699–714. doi: 10.1016/s0531-5565(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 26.Headrick JP. Aging impairs functional, metabolic and ionic recovery from ischemia-reperfusion and hypoxia-reoxygenation. J Mol Cell Cardiol. 1998;30:1415–30. doi: 10.1006/jmcc.1998.0710. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg I, Matetzky S, Halkin A, Roth A, Di Segni E, Freimark D, Elian D, Agranat O, Har ZY, Guetta V, Hod H. Primary angioplasty with routine stenting compared with thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2003;145:862–7. doi: 10.1016/S0002-8703(02)94709-5. [DOI] [PubMed] [Google Scholar]
- 28.de Lemos JA, Antman EM, Gibson CM, McCabe CH, Giugliano RP, Murphy SA, Coulter SA, Anderson K, Scherer J, Frey MJ, Van der WR, Van de WF, Braunwald E. Abciximab improves both epicardial flow and myocardial reperfusion in ST-elevation myocardial infarction. Observations from the TIMI 14 trial. Circulation. 2000;101:239–43. doi: 10.1161/01.cir.101.3.239. [DOI] [PubMed] [Google Scholar]
- 29.Gurwitz JH, Goldberg RJ. Coronary thrombolysis for the elderly. Is clinical practice really lagging behind evidence of benefit? JAMA. 1997;277:1723–4. doi: 10.1001/jama.277.21.1723. [DOI] [PubMed] [Google Scholar]