Abstract
Although elderly patients represent a rapidly growing population often requiring multiple drug treatment, the evidence of effectiveness is limited for many interventions and therapies in this age group. Only during the last 30 years has a requirement to incorporate evidence into the treatment of older subjects become part of the pre- and postmarketing regulatory process in Europe and the United States. Recently, elderly patients have been shown to benefit comparably from several treatments. These studies have supported the validity of an increasingly interventional approach to disorders common in late life. However, an important issue is the applicability of the growing body of clinical trials to ‘real life’ patients. This is particularly true in very old (i.e. >80 years) patients and those with significant comorbidities. We review the current evidence and controversies related to the effectiveness and safety of several therapeutic strategies in cardiovascular disease (i.e. statins, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β-adrenoceptor blockers, and thrombolytic agents) and bone health (i.e. vitamin D and bisphosphonates).
Keywords: cardiovascular risk, elderly patients, primary prevention, randomized controlled trials, secondary prevention, statins
Introduction
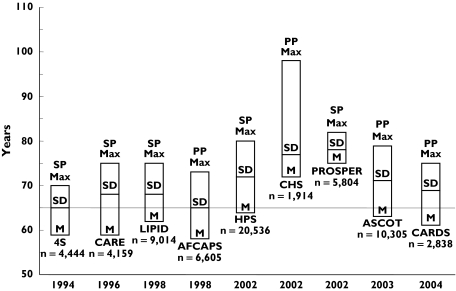
Several large clinical trials have demonstrated that lipid lowering treatment with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins) reduces cardiovascular risk by at least one-third in patients with or without cardiovascular disease [1–5]. An extremely important question, however, is whether statins are effective in reducing cardiovascular risk in elderly subjects given that cardiovascular morbidity and mortality occurs mainly in patients >65 years [6]. Even when the clinical manifestations of ischaemic heart disease occur before the age of 65 years, the majority of affected people survive the initial event and live to an older age. These subjects are candidates for secondary prevention measures including statin therapy even though the association between plasma cholesterol concentrations and cardiovascular risk diminishes with increasing age [7–9]. The results of the primary and secondary prevention trials investigating the use of statins in study groups including elderly subjects are discussed in Figure 1 and Table 1.
Figure 1.
Age distribution of trials on statins in elderly patients. M, mean age; SD, standard deviation; Max, maximum age; PP, primary prevention; SP, secondary prevention
Table 1.
Primary prevention trials of statins including elderly patients
| Result of primary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Agents | Follow-up | Elderly participants | Women | Inclusion criteria | Primary outcome measures | Incidence in active group | Incidence in placebo group | P-value |
| AFCAPS/TEXCAPS [5] | Lovastatin 20–40 mg vs. placebo | 5.2 years | ≥ 65 years (21.5%) | 15.0% | No previous CVD + LDL-cholesterol 3.36–4.91 mmol l−1 | Rate of first fatal or nonfatal MI, UA or SCD | 3.5% | 5.5% | 2.0%P < 0.001 |
| ASCOT-LLA [11] | Atorvastatin 10 mg vs. placebo | 3.3 years | >60 years (63.8%) | 18.8% | HT + total cholesterol ≤ 6.5 mmol l−1 | Non-fatal MI and fatal CHD | 1.9% | 3.0% | 1.1%P = 0.005 |
| CARDS [12] | Atorvastatin 10 mg vs. placebo | 3.9 years | >60 years (62.6%) | 32.0% | T2D and LDL-cholesterol ≤ 4.14 mmol l−1 plus one of the following: HT, retinopathy, microalbuminuria, smoking | Time to first occurrence of acute CHD events, coronary revascularization or stroke | 5.8% | 9.0% | 3.2%P = 0.001 |
| CHS [10] (retrospective cohort study) | Statin use vs. nonstatin use | 7.2 years | >65 years (100%) | 66.2% | No previous CVD | Combined endpoint of MI, stroke, CHD death | 16.7% | 20.4% | 3.7%P = 0.001 |
CVD cardiovascular disease; MI myocardial infarction; UA unstable angina; SCD sudden cardiac death; CHD coronary heart disease; HT hypertension; T2D type 2 diabetes; ARR absolute risk reduction.
Primary prevention
In the AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study) study, subjects were randomized to lovastatin or placebo (Figure 1 and Table 1) [5]. Lovastatin reduced the incidence of first coronary events [5]. The results were similar in men >57 years and women >62 years, although the absolute risk reduction (ARR) in these subgroups has not been published [5]. The effect of lovastatin on the rate of first acute major coronary events was greater in women than in men (46%vs. 37% reduction in relative risk); however, the actual number of women who had a primary endpoint event was small (20 of 997), and there were no statistical differences in treatment effects between sexes. The percentage of participants with adverse effects leading to discontinuation was 13.6% in the lovastatin group and 13.8% in the placebo group. Significant elevations in liver enzymes and creatinine kinase occurred in 0.6% and 0.7% of patients receiving lovastatin and in 0.3% and 0.6% of patients receiving placebo, respectively [5].
In a smaller nonrandomized prospective study from the Cardiovascular Health Study, statin use significantly reduced the incidence of cardiovascular events and all-cause mortality (Figure 1 and Table 1) [10]. Risk estimates were similar in patients <74 years (hazard ratio 0.46, 95% CI 0.26, 0.81) and patients ≥74 years (hazard ratio 0.42, 95% CI 0.15, 1.14), and in men and women (data not published) [10]. This study, however, was not a controlled clinical trial and confounding factors might have affected the results. No information is available regarding safety and tolerability in statin vs. nonstatin users from this study.
In the ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm) study, subjects with total cholesterol ≤6.5 mmol l−1 were randomized to atorvastatin or placebo (Figure 1 and Table 1) [11]. Atorvastatin significantly reduced the incidence of nonfatal myocardial infarction and fatal coronary heart disease. Subgroup analysis revealed no apparent benefit in women. However, there was no significant interaction between sex and the impact of statin treatment on the primary endpoint. The effects of atorvastatin in patients >60 years were similar to younger patients [11]. The incidence of adverse events and abnormalities of liver enzymes did not differ between the atorvastatin and placebo groups [11].
More recently, the results of the CARDS (Collaborative Atorvastatin Diabetes Study) trial have been published [12]. In this study, type 2 diabetic patients without cardiovascular disease and with LDL-cholesterol <4.14 mmol l−1 were randomized to atorvastatin or placebo (Figure 1 and Table 1) [12]. Patients treated with atorvastatin had significantly less cardiovascular events. Adjustment for baseline age and sex did not affect the estimate of the treatment effect (36% risk reduction with atorvastatin, P = 0.002) [12]. Discontinuation rates and incidence of myopathy and abnormal liver enzymes were similar in the two groups [12].
Secondary prevention
The 4S (Scandinavian Simvastatin Survival Study) study investigated the effects of simvastatin vs. placebo in patients with ischaemic heart disease (Figure 1 and Table 2) [1]. A significant reduction in all-cause mortality was observed with simvastatin [1]. Results were similar in patients ≥60 years (incidence in active group 11.0%; incidence in placebo group 14.8%; ARR 3.8%, P < 0.01) [1], for both the primary and secondary endpoints. There were no significant interactions between treatment and either sex or age. Six per cent of patients in both groups discontinued the study because of adverse events. Significant elevations in liver enzymes occurred in 1% of patients in both groups [1].
Table 2.
Secondary prevention trials of statins including elderly patients
| Result of primary outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Agents | Follow-up | Elderly participants | Women | Inclusion criteria | Primary outcome measures | Incidence in active group | Incidence in placebo group | ARRP value |
| 4S [1] | Simvastatin 20 mg vs. placebo | 5.4 years | ≥60 years (51.3%) | 18.6% | Previous CHD | All-cause mortality | 8.2% | 11.5% | 3.3%P = 0.0003 |
| CARE [2] | Pravastatin 40 mg vs. placebo | 5 years | ≥60 years (51.1%) | 14.0% | Previous MI | CHD death and nonfatal MI | 10.2% | 13.2% | 3.0%P = 0.003 |
| LIPID [3] | Pravastatin 40 mg vs. placebo | 6.1 years | 65–69 years (24.0%) ≥70 years (15.0%) | 17.0% | Previous MI or hospitalization for UA | CHD death | 12.3% | 15.9% | 3.6%P < 0.001 |
| HPS [13] | Simvastatin 40 mg vs. placebo | 5 years | 65–69 years (25.0%) 70–74 yearS (22.0%) >74 yearS (6.0%) | 24.7% | CVD, DM, or treated HT | Cardiovascular and all- cause mortality | 12.9% | 14.7% | 1.8%P = 0.0003 |
| PROSPER [14] | Pravastatin 40 mg vs. placebo | 3.2 years | >65 years (100%) | 48.3% | Subjects with CVD or at high CVD risk | Combined endpoint of CHD death, MI, and fatal and nonfatal stroke | 14.1% | 16.2% | 2.1%P = 0.014 |
CVD cardiovascular disease; MI myocardial infarction; UA unstable angina; CHD coronary heart disease; HT hypertension; DM diabetes mellitus; ARR absolute risk reduction.
The CARE (Cholesterol And Recurrent Events) study involved patients with a previous myocardial infarction randomized to pravastatin or placebo (Figure 1 and Table 2) [2]. Patients treated with pravastatin had a significant reduction in the incidence of fatal coronary heart disease or nonfatal myocardial infarction. The effects of pravastatin were greater in patients ≥60 years (incidence in active group 20%; incidence in the placebo group 27%; ARR 7.0%, P < 0.001) [2]. As compared with patients treated with placebo, both men and women treated with pravastatin had significantly lower rates of major coronary events (46% lower for women, P = 0.001, and 20% lower for men, P = 0.001). The effects of pravastatin were greater among women than among men (P = 0.05 for the interaction between sex and treatment). Discontinuation rates were 3.6% in the placebo group and 2.2% in the pravastatin group (P = 0.007), respectively [2]. The incidence of abnormal liver function, elevated creatinine kinase and myositis was similar in the two groups. Despite a similar incidence of newly diagnosed cancer (7.7% with placebo group and 8.3% with pravastatin), organ-specific analysis revealed that pravastatin treatment was associated with an increased risk of breast cancer [2].
In the LIPID (Long-term Intervention with Pravastatin in Ischaemic Disease) study, patients with previous myocardial infarction or hospitalization for unstable angina were randomized to pravastatin or placebo (Figure 1 and Table 2) [3]. Pravastatin reduced death from coronary heart disease. Sub-analysis in patients aged 65–69 years (incidence in active group 14.0%; incidence in placebo group 18.7%; ARR 4.7%) and ≥70 years (incidence in active group 18.0%; incidence in placebo group 21.3%; ARR 3.3%) yielded similar results [3]. The effects of pravastatin were greater in men than in women (ARR 3.9%vs. 1.8%). There were no significant differences between the two groups in the incidence of adverse effects, abnormal liver function and myopathy [3]. Newly diagnosed cancers occurred in 8.4% of patients in the pravastatin group and 8.9% of patients in the placebo group. Organ-specific analysis yielded similar results [3].
The HPS (Heart Protection Study) included subjects at high cardiovascular risk up to age 80 years randomized to simvastatin or placebo (Figure 1 and Table 2) [13]. The large patient numbers made subgroup analysis for the elderly cohort more robust. Elderly patients achieved similar relative benefits from simvastatin, i.e. incidence of first major vascular event, as did other subgroups (patients <65 years, incidence in active group 16.9%, incidence in placebo group 22.1%, ARR 5.2%; patients >65 years and <70 years, incidence in active group 20.9%, incidence in placebo group 27.2%, ARR 6.3%; patients ≥70 years, incidence in active group 23.6%, incidence in placebo group 28.7, ARR 5.1%) [13]. The effects of simvastatin were not significantly different in men and women (ARR 6.0%vs. 3.3%, P ≥ 0.05). Both the simvastatin and the placebo groups had similar rates of newly diagnosed cancer, liver abnormalities and myopathy [13].
At the end of 2002, the results of the first randomized controlled trial on the effects of statin treatment specifically targeting elderly patients were published [14]. In the PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) study, patients with a history of, or risk factors for, vascular disease, were randomized to pravastatin or placebo (Figure 1 and Table 2). Pravastatin significantly reduced a composite endpoint of coronary death, nonfatal myocardial infarction, and fatal or nonfatal stroke [14]. Risk reduction was more pronounced in men than in women (ARR 3.9%vs. 0.5%). However, testing for interaction did not reveal significant differences between these subgroups. The pravastatin and placebo groups had similar rates of serious adverse events, myopathy and liver abnormalities. However, a higher incidence of gastrointestinal cancers was reported in the pravastatin group (hazard ratio 1.25, 95% CI 1.04, 1.51, P = 0.02) [14].
Discussion
There is good evidence that statins reduce cardiovascular risk in elderly patients ≥80 years. The maximum age in studies published after the year 2000 is higher than in the previous decade (82.8 vs. 73.2 years, Figure 1). Primary prevention trials show efficacy up to the age of 79 years and, according to the CHS study, it is possible that subjects up to 98 years may benefit from treatment. However, the CHS study was not a randomized controlled trial and the results must be interpreted with caution. Secondary prevention trials demonstrate efficacy up to the age of 82 years. Statin therapy does not seem to impact negatively on quality of life and is well tolerated, although there is no specific safety data analysis in elderly subgroups in any of the published trials. Moreover, there is uncertainty as to whether the available evidence is fully applicable to female patients, often poorly represented in these trials, as well as in frail elderly subjects. A retrospective cohort study on frail elderly subjects living in nursing homes has demonstrated that 1-year mortality was significantly reduced (ARR 12.1%) in statin users vs. non-users [15]. However, more research in this area is needed.
It was previously thought that reducing serum cholesterol would not reduce cardiovascular risk in elderly patients as prospective epidemiological studies showed that the cardiovascular risk imparted by cholesterol declines with age [6–9, 16–19]. However, in this context it is important to distinguish between relative and absolute benefit of therapy. Elderly subjects are clearly at greater absolute risk for cardiovascular morbidity and mortality, mainly because of more advanced atherosclerosis [20]. Beyond this, however, cardiovascular events in elderly patients could have a different aetiology than in middle age and thus be less dependent on cholesterol concentrtions. If so, LDL-cholesterol lowering may target less of the totality of cardiovascular disease causation in elderly than in middle-aged patients. Even so, the absolute (attributable) benefit of LDL-cholesterol lowering could be as great or even greater in elderly patients even if the relative risk reduction is lower [21].
In a meta-analysis of five major randomized controlled trials to estimate the risk reduction of coronary heart disease and total mortality associated with statins, the risk reduction was statistically significant in all four trials among patients ≥65 years and in four of five trials among patients <65 years [22]. The overall proportional risk reduction was similar for patients ≥65 years (32%; 95% CI 23% to 39%) and patients <65 years (31%, 95% CI 24%, 36%) [22]. The ARR, however, was slightly higher in patients ≥65 years (44 per 1000; 95% CI 30, 58 per 1000) compared with patients <65 years (32 per 1000; 95% CI 24, 40 per 1000) [22]. The consistency of these findings leaves little doubt that treatment with statins lowers the cardiovascular risk up to age 80 years. The recent US National Cholesterol Education Program third Adult Treatment Panel (ATP III) recommends the same management paradigm for elderly subjects as for middle-aged adults [23].
Of note, the HPS study showed benefit of statin therapy regardless of patients’ baseline LDL-cholesterol [13]. This benefit extends to patients with diabetes, especially elderly patients with multiple metabolic risk factors. Thus, in light of the HPS study, elderly patients should be given statin therapy regardless of their LDL-cholesterol concentrations.
The ATP III introduced the concept of coronary heart disease equivalent, defined as a risk factor that carries the same risk for major coronary events as does established coronary heart disease (i.e. >20% 10-year coronary heart disease event risk, as defined by Framingham risk scoring) [23]. Although this recommendation extends to the older population, it must be noted that the accuracy of Framingham risk predictions declines with advancing age. According to the Framingham algorithm, advancing age becomes the predominant risk factor affecting risk prediction. However, age is a surrogate marker for coronary plaque burden, which is the true risk predictor. The fact that plaque burden varies greatly among elderly subjects accounts for the decline in reliability of Framingham scoring for risk assessment with advancing age.
A possible solution in elderly patients is to perform accurate measures of plaque burden. Carotid artery thickness measured by B-mode sonography has been shown to correlate with coronary plaque burden [24]. A more accurate estimate of plaque burden can be obtained by measurement of coronary calcium by computed tomography [20, 25]. Some investigators have proposed a technique to substitute coronary plaque burden for age as a risk factor in Framingham risk scoring [20, 21]. This approach might allow a better risk stratification in elderly subjects. Statin therapy could then be targeted more specifically to higher-risk patients.
Finally, a distinction between younger elderly and older elderly subjects may be useful. The former includes subjects <80 years, who represent the group most frequently studied in trials. In patients ≥80 years, the evidence supporting the use of statins, particularly in primary prevention, is lacking. In this setting, statins should be used cautiously as these patients often have risk factors for statin-induced myopathy such as impaired drug metabolism, polypharmacy, multisystem disease, more female patients of low body weight, and more frequent surgical procedures.
Statin therapy was safe and well tolerated. An increased risk of cancer was observed in the CARE and PROSPER studies [2, 14]. However, a meta-analysis of studies using pravastatin or other statins for >3 years did not confirm this finding [14].
In summary, there is good evidence that statins effectively reduce cardiovascular risk in elderly patients ≤80 years. Current guidelines recommend intensive cholesterol-lowering therapy in elderly patients with established ischaemic heart disease [26]. The ATP III extends this approach to elderly subjects with coronary heart disease risk equivalents, especially noncoronary forms of atherosclerosis and type 2 diabetes [23]. Although ATP III recommends management of patients according to Framingham risk scoring, the limitations of this scoring highlight the need for better methods of risk assessment in this age group.
References
- 1.The Scandinavian Simvastatin Survival Study (4S) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 2.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 6.Denke MA, Grundy SM. Hypercholesterolemia in elderly persons: resolving the treatment dilemma. Ann Intern Med. 1990;112:780–92. doi: 10.7326/0003-4819-112-10-780. [DOI] [PubMed] [Google Scholar]
- 7.Shipley MJ, Pocock SJ, Marmot MG. Does plasma cholesterol concentration predict mortality from coronary heart disease in elderly people? 18 year follow up in Whitehall study. BMJ. 1991;303:89–92. doi: 10.1136/bmj.303.6794.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–23. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 9.Schatz IJ, Masaki K, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358:351–5. doi: 10.1016/S0140-6736(01)05553-2. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre RN, Psaty BM, Heckbert SR, Kronmal RA, Newman AB, Burke GL. Therapy with hydroxymethylglutaryl coenzyme A reductase inhibitors (statins) and associated risk of incident cardiovascular events in older adults: evidence from the Cardiovascular Health Study. Arch Intern Med. 2002;162:1395–400. doi: 10.1001/archinte.162.12.1395. [DOI] [PubMed] [Google Scholar]
- 11.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 12.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 13.MRC/BHF. Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, MacFarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 15.Eaton CB, Lapane KL, Murphy JB, Hume AL. Effect of statin (HMG-Co-A-Reductase Inhibitor) use on 1-year mortality and hospitalization rates in older patients with cardiovascular disease living in nursing homes. J Am Geriatr Soc. 2002;50:1389–95. doi: 10.1046/j.1532-5415.2002.50360.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins M, Keller JB. Cholesterol, coronary heart disease, and total mortality in middle-aged and elderly men and women in Tecumseh. Ann Epidemiol. 1992;2:69–76. doi: 10.1016/1047-2797(92)90039-s. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E. Hypercholesterolemia predicts early death from coronary heart disease in elderly men but not women. The Rancho Bernardo Study. Ann Epidemiol. 1992;2:77–83. doi: 10.1016/1047-2797(92)90040-w. [DOI] [PubMed] [Google Scholar]
- 18.Welin L, Eriksson H, Larsson B, Svardsudd K, Tibblin G, Wilhelmsen L. Triglycerides and blood glucose are the major coronary risk factors in elderly Swedish men. The study of men born in 1913. Ann Epidemiol. 1992;2:113–9. doi: 10.1016/1047-2797(92)90045-r. [DOI] [PubMed] [Google Scholar]
- 19.Simons LA, Simons J, Friedlander Y, McCallum J. Cholesterol and other lipids predict coronary heart disease and ischaemic stroke in the elderly, but only in those below 70 years. Atherosclerosis. 2001;159:201–8. doi: 10.1016/s0021-9150(01)00495-6. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM. Age as a risk factor: you are as old as your arteries. Am J Cardiol. 1999;83:1455–7. doi: 10.1016/s0002-9149(99)00125-3. A7. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Rifkind BM, Kuller LH. Cholesterol lowering in the elderly population. Coordinating Committee of the Nationall Cholesterol Education Program. Arch Intern Med. 1999;159:1670–8. doi: 10.1001/archinte.159.15.1670. [DOI] [PubMed] [Google Scholar]
- 22.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Smith SC, Jr, Greenland P, Grundy SM. American Heart Association Conference Proceedings. Prevention Conference V. Beyond secondary prevention: identifying the high-risk patient for primary prevention: executive summary. Circulation. 2000;101:111–6. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR, III, Friedman L, Fuster V, Herrington DM, Kuller LH, Ridker PM, Roberts WC, Stanford W, Stone N, Swan HJ, Taubert KA, Wexler L. Prevention Conference V. Beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden. Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 26.British Cardiac Society, British Hyperlipidaemia Association, British Hypertension Society, British Diabetic Association. Joint British recommendations on prevention of coronary heart disease in clinical practice. Summary. BMJ. 2000;320:705–8. doi: 10.1136/bmj.320.7236.705. [DOI] [PMC free article] [PubMed] [Google Scholar]