Abstract
Aims
To characterize determinants of the elimination of methotrexate (MTX) and 7-hydroxy-methotrexate (7-OH-MTX) in patients receiving high-dose MTX therapy (HDMTX).
Methods
24 and 48-h blood samples from 76 patients receiving HDMTX (dose range 300 mg m−2 to 12 g m−2) were analysed, and concentration-time data were subjected to population pharmacokinetic and covariate analysis using nonlinear mixed-effect modelling (NONMEM).
Results
Treatment-related mortality was 1.3% (one patient with renal failure). Values for MTX clearance (CLMTX) and 7-OH-MTX clearance (CL7-OH-MTX) were estimated at 8.85 and 2 L−1, respectively. Baseline creatinine clearance correlated with CLMTX and CL7-OH-MTX. Concurrent administration of benzimidazoles led to a 27% decrease in CLMTX and a 39% decrease in CL7-OH-MTX. Prior administration of nonsteroidal anti-inflammatory drugs (NSAIDs) resulted in a 16% decrease in CLMTX and a 38% decrease in CL7-OH-MTX. Plasma MTX concentrations were significantly higher in patients also receiving benzimidazoles at 24 h (2.01 µmol L−1 vs. 0.66 µmol L−1, P < 10−4) and at 48 h (0.25 µmol L−1 vs. 0.12 µmol L−1, P < 10−4). 7-OH-MTX plasma concentrations were also significantly higher in patients with concurrent benzimidazoles as compared with patients without benzimidazoles at 24 h (4.47 µmol L−1 vs. 2.52 µmol L−1, P = 0.0009) and at 48 h (1.11 µmol L−1 vs. 0.72 µmol L−1, P = 0.031).
Conclusions
In patients receiving HDMTX, concurrent administration of benzimidazoles was associated with a significant decrease of CLMTX and CL7-OH-MTX, resulting in significantly higher plasma concentrations of MTX and 7-OH-MTX. The data suggest that benzimidazole treatment should be seen as a relative contraindication for HDMTX.
Keywords: methotrexate, 7-hydroxy-methotrexate, NONMEM, benzimidazoles, drug interactions
Introduction
Methotrexate (MTX) is a widely used antifolate drug. Antiproliferative activity is achieved by blocking thymidylate synthase, dihydrofolate reductase and de novo purine synthesis [1]. 7-Hydroxy-methotrexate (7-OH-MTX) is the main metabolite in serum following high-dose MTX (HDMTX) [1], and it contributes to the activity [2] and toxicity [3, 4] of the drug. 7-OH-MTX concentrations exceed those of the parent compound in plasma shortly after MTX infusion [5]. MTX and 7-OH-MTX both exhibit first-order pharmacokinetics [1, 3, 5–8]. MTX enters the cell through the reduced folate carrier system, and by additional diffusion at higher plasma concentrations (>20 µmol L−1) [3]. Finally, MTX undergoes intracellular activation by polyglutamation [3], which is increased at higher MTX doses and results in enhanced drug activity. MTX is eliminated by renal excretion involving passive glomerular filtration and active tubular reabsorption and secretion. 7-OH-MTX is also renally cleared but more slowly than MTX [5]. The elimination of MTX is prolonged in patients with renal impairment or third space fluid collections, due to a slow redistribution from these extravascular fluid accumulations [1, 3]. MTX is particularly prone to drug—drug interactions. Non-steroidal anti-inflammatory drugs (NSAIDs), salicylates [9, 10], sulphonamides [11], penicillin [12], benzimidazoles [13] and probenecid [14] can increase exposure to MTX, and may result in increased drug toxicity. Concurrent administration of NSAIDs in particular has been associated with increased MTX toxicity and combination is contraindicated [10]. Various compounds, including sulphonamides [15], leucovorin, vincristine [16], l-asparaginase [17] and corticosteroids [18] interact with MTX by altering its cellular uptake.
Intravenous HDMTX is used to treat high-grade lymphoma, osteogenic sarcoma and acute leukaemia. It is typically administered at doses of 500 mg m−2 or higher over 6–24 h [19]. Intravenous HDMTX requires pharmacokinetic monitoring to identify patients at high risk for developing significant toxicity, especially those with renal dysfunction [20]. In general, plasma drug concentrations >0.1 µmol L−1 at 48 h after administration, and/or any plasma concentration >10 µmol L−1 require intensive leucovorin rescue [21, 22]. A number of nomograms have been developed for monitoring MTX, using varying plasma drug concentration-time data [21, 23]. The development of HDMTX-induced acute renal dysfunction, which is mediated by the precipitation of MTX and 7-OH-MTX in the kidney tubules, is a potentially life-threatening complication and occurs in 1.8% of patients receiving HDMTX. Elderly patients and those on concurrent nephrotoxic agents are at particular risk of HDMTX-induced renal failure [24]. The introduction of aggressive hydration, urine alkalinization and pharmacokinetically guided leucovorin rescue has been shown to decrease the morbidity rate in patients receiving HDMTX [25], but severe morbidity and mortality secondary to HDMTX-induced renal dysfunction are still major concerns [26].
The aims of this study were to (1) develop a population pharmacokinetic model of MTX and 7-OH-MTX (2), analyse the influence of various anthropometric and biochemical covariates as well as comedication on MTX elimination, and (3) provide guidance on how to increase the safety of HDMTX schedules.
Methods
Patient population and study protocol
Patients with solid tumours receiving intravenous HDMTX either as single agent or in combination with other cytotoxic drugs treated at the Netherlands Cancer Institute were included in the analysis. Some patients participated in a Phase II study that used fixed-dose MTX (3000 mg over a 3-h infusion) as single agent or in combination with fixed-dose intravenous doxorubicin (40 mg every 2 weeks) for the treatment of malignant pleural mesothelioma. The other patients received MTX within standard schedules for the treatment of NHL, acute lymphocytic leukaemia, head and neck cancer and osteosarcoma. The dose of MTX given for head and neck cancer (400 mg m−2 MTX) and choriocarcinoma (300 mg m−2 MTX) was slightly below usual HDMTX schedules as defined previously [19]. Those patients were retained in the study group for data analysis. For HDMTX treatment, patients had to have sufficient blood cell counts (leucocytes = 4.0 × 109 L−1, granulocytes = 2.0 × 109 L−1, platelets = 100 × 109 L−1), normal renal (serum creatinine < 120 µmol L−1) and liver function (serum bilirubin < 25 µmol L−1) and a performance status of 0–2 according to WHO criteria. All patients within the pleural mesothelioma study and those with extended pharmacokinetic sampling (see below) were treated within an institutional review board (The Netherlands Cancer Institute) approved study and provided written informed consent.
Supportive therapy was identical within the group studied and included aggressive prehydration and urine alkalinization with oral and intravenous sodium bicarbonate. MTX was only given after the urine was alkalinized to a pH of =7. MTX was dissolved in 1 L sodium chloride solution (0.9%). After MTX was given, a continuous infusion of sodium chloride (0.45%) and glucose (2.5%) was maintained over 22 h. Hydration and alkalinization were continued for three days with 1000 mg oral sodium bicarbonate being given every 6 h. Oral leucovorin rescue (15 mg) every 6 h was begun 24 h after the start of MTX. Routine 24- and 48-h blood samples were collected in all patients. If plasma MTX concentrations at 48 h were ≤ 0.04 µmol L−1, leucovorin was discontinued, but if they were >0.04 and <0.1 µmol L−1, leucovorin was continued for another 24 h. If plasma MTX concentrations were ≥ 0.1 µmol L−1 48 h after starting MTX, leucovorin rescue was intensified (continuous intravenous infusion of 1200 mg every 24 h) until concentrations were below 0.1 µmol L−1. Twenty-one patients underwent additional blood sampling (one cycle in eight patients and two cycles in 13 patients) at the following time-points: at the end of the MTX infusion and 3, 6 and 8 h later. Laboratory assessment, including haemoglobin, leucocyte, absolute neutrophil and blood platelet counts, liver function tests and serum creatinine, was usually repeated daily until day 3, and weekly thereafter. Clinical assessment was performed on a daily basis.
Blood samples were collected in glass tubes containing heparin or EDTA and plasma was obtained by centrifugation (2000 g) immediately after sampling. Ascorbic acid (1 mg mL−1) was added and samples were stored at −20 °C until analysis. MTX and 7-OH-MTX plasma concentrations were measured using a validated reversed-phase high-pressure liquid chromatography (HPLC) method as published previously [27]. The lower detection limit of the HPLC assay was 0.04 µmol L−1 for both MTX and 7-OH-MTX, and the within and between-day coefficients of variation were ≤ 7.0%.
Population pharmacokinetic analysis
Population pharmacokinetic analysis was performed using the nonlinear mixed-effect modelling program (NONMEM) version V (double precision, level 1.1) [28]. NONMEM uses a maximum likelihood criterion to simultaneously estimate population values of fixed-effects parameters (e.g. drug clearance (CL) and anthropometric or biochemical covariates that modify these values in the population) and values of the random-effects parameters (e.g. interindividual, interoccasion and residual variability). Log-transformed plasma drug concentrations were used together with the first-order (FO) estimation method throughout. Standard errors for all parameters were calculated using the COVARIANCE option of NONMEM and individual Bayesian pharmacokinetic parameters were obtained with the POSTHOC option [28]. The S-plus (MathSoft Inc, Seattle, USA) based model building aid Xpose 3.0 was used for graphical processing [29]. In the first step, a basic pharmacokinetic model was developed for MTX and 7-OH-MTX concentration-time data using the NONMEM subroutine ADVAN5. In the second step, an extended covariate analysis was performed on the basic population pharmacokinetic model to study the associations between anthropometric, biochemical covariates as well as comedication on the pharmacokinetic parameters for MTX and 7-OH-MTX. In general, model selection was based on the minimum value of objective function (OFV), as calculated by NONMEM, the precision of parameter estimates (according to the standard error values of the parameter estimates), and the fit of the model to the data.
Interindividual and interoccasion variability were estimated using a proportional error model. For example, interindividual and interoccasion variability in MTX clearance (CLMTX) was defined as follows:
Where CLMTX i represents the clearance of MTX of the ith individual, CLMTX POP is the typical population value of CLMTX, ηiCLMTX is the interindividual random effect with mean zero and variance ω2, and κiCLMTX is the interoccasion random effect with mean zero and variance π2. Residual variability was modelled as log(Cij) = log(Cij) + ɛij, in which Cij and Cij are the jth measured and model predicted drug concentration of the ith individual, respectively, and ɛij is the residual random error with mean zero and variance σ2.
The following covariates were subsequently tested with respect to their correlation with PK parameters such as CLMTX and 7-OH-MTX clearance (CL7-OH-MTX): Creatinine clearance (CLCREA according to the Cockroft-Gault formula, and with values >140 mL min−1 truncated at this value) before the start of each HDMTX cycle, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (AP) as surrogate markers for liver function, patient age, gender, weight and body-surface area (BSA), third-space fluid collections, comedication with benzimidazoles, NSAIDs, corticosteroids, vinca alkaloids, 5-fluorouracil and l-asparaginase. Additionally, patient weight, age and BSA were tested as potential covariates on the volume of distribution of MTX and 7-OH-MTX. Covariates were entered individually into the basic population pharmacokinetic model by forward inclusion. Continuous covariates such as patient weight were centred to their median values. For example, the relationship between CLMTX and body weight was described as follows:
Where θ1 represents CLMTX of a (median) patient with a body weight of 70 kg, and θ2 is the increase or decrease in CLMTX per kg difference in body weight. Dichotomous covariates such as patient gender were modelled as follows:
Where θ1 represents the CLMTX value in females (GEN = 0), and θ2 is the change in CLMTX in males (GEN = 1). Forward selection and backward elimination were used for the purpose of covariate testing, with OFV as the main discriminator between different models. The OFV is equal to minus twice the log likelihood of the data and the difference in OFV between hierarchical models approximates to a chi-squared distribution with one degree of freedom. The difference in OFV was evaluated after the introduction of a covariate into the model (forward inclusion), and the significance level was set at P < 0.005, which corresponds to a decrease of OFV of >7.83. All significant covariates were included in an intermediate multivariate model followed by a stepwise backward elimination procedure. Covariates remained in the model when elimination of the covariate caused an OFV increase of >10.8 that is associated with a significance level of P < 0.001.
Finally, plasma concentrations of MTX and 7-OH-MTX at 24 and 48 h were compared in patient subgroups who were or were not taking benzimidazoles or prior NSAIDs. To properly account for multiple treatment cycles per patient, repeated measurement analysis of variance (RM-anova) was used [30]. To improve normality of the residuals and constancy of the variation, the analysis was based on the logarithmically transformed concentrations. The correlation between concentrations in the same patient was modelled by assuming a random baseline concentration and first-order autoregressive correlations (i.e. declining according to a power law with increasing distance between cycles). This choice was based on a preliminary analysis of the MTX concentrations at 24 h. RM-anova also allows adjustment of the benzimidazole effect for confounding by the NSAID effect and vice versa. Both effects were additionally adjusted for confounding by a possible cycle effect. The level of significance was set at 0.05.
Results
In total, 76 patients who received 304 cylces of HDMTX were included in the study. The dataset consisted of two subpopulations, one group with intensive blood sampling (n = 21; 34 courses) and one group with routine 24- and 48- h concentration-time data (n = 55; 270 courses). Clinical and laboratory parameters of the study group are further detailed in Table 1. The MTX dose ranged from 300 mg m−2 to 12 g m−2 given over an infusion period of 1–24 h. Sixty-one of the 76 patients (80%) received MTX at doses between 1000 and 5000 mg m−2, and the duration of MTX infusion was between 1–6 h in 61 patients (80%). Twenty-nine patients suffered from malignant pleural mesothelioma, 20 from gastro-oesophageal cancer, 12 from non-Hodgkin lymphoma (NHL), 10 from head and neck cancer, two from choriocarcinoma, and one patient each from acute lymphocytic leukaemia, trophoblastic tumour and osteosarcoma. Foregoing administration of nephrotoxic anticancer treatment included cisplatin in two patients with choriocarcinoma and ifosfamide in one patients with osteosarcoma. All patients with NHL were also being treated with corticosteroids. HDMTX-associated grade 1 creatinine elevations (>ULN to 1.5×ULN) occurred in 22 of 304 treatment cycles (7.2%), grade 2 (>1.5×ULN to 3×ULN) in five courses (1.6%) and grade 3 (>3×ULN to 6×ULN) in nine courses (3%). Benzimidazoles were administered to 13 patients (10 receiving omeprazole 20–40 mg daily and three lansoprazole 30 mg daily). Six patients were being treated with NSAIDs (diclofenac 75–300 mg daily in five patients, ibuprofen 100 mg three times daily in one patient). NSAIDs were discontinued in all patients on the day of HDMTX administration.
Table 1. Patient characteristics (total number of patients = 76).
| Parameter | n | Normal range | Median value | Range |
|---|---|---|---|---|
| Age (years) | 51.1 | 17.1–77.0 | ||
| Males/females | 62/14 | |||
| WHO performance status | ||||
| 0 | 10 | |||
| 1 | 58 | |||
| 2 | 8 | |||
| Body surface area (m2) | 1.94 | 1.56–2.45 | ||
| Serum albumin (g L−1) | 35–50 | 40.0 | 16–71 | |
| Aspartate aminotransferase (U L−1) | ≤40 | 17.0 | 4–234 | |
| Alanine aminotransferase (U L−1) | ≤45 | 20.0 | 3–368 | |
| Total bilirubin (µmol L−1) | <16 | 6.0 | 2–60 | |
| Alkaline phosphatase (U L−1) | 40–120 | 92.0 | 38–519 | |
| Gammaglutamyl transpeptidase (U L−1) | ≤50 | 43.0 | 7–675 | |
| Lactic dehydrogenase (U L−1) | ≤450 | 273.0 | 51–3370 | |
| Serum creatinine (µmol L−1) | 50–105 | 77.0 | 32–433 | |
| Creatinine clearance (Cockroft-Gault) (mL min−1) | 60–140 | 87.5 | 40–140 |
n = number of patients.
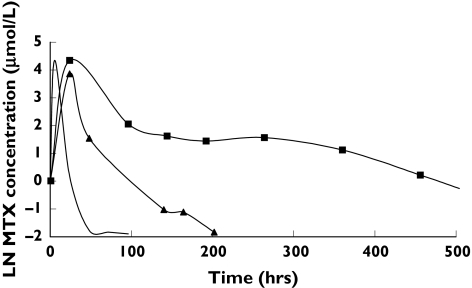
Visual inspection of the concentration-time data showed two outliers with markedly increased drug exposure (Figure 1). The patient with the highest MTX concentrations (ID-63) was a 29-year-old male being treated for relapsed malignant pleural mesothelioma with MTX (3 g as a 3 h infusion) combined with intravenous doxorubicin (40 mg). On day two, the patient experienced anuric renal failure CTC grade 3, requiring immediate dialysis. The patient also experienced neutropenia CTC 4, thrombopenia CTC 3 and infectious complications with gram-positive staphylococcal spp. bacteraemia. Basic measures such as urine alkalinization, flushing, drug monitoring and leucovorin rescue were implemented according to guidelines. The patient died 20 days after having received HDMTX, despite immediate continuous veno-venous haemofiltration, antibiotic treatment and intensified intravenous leucovorin and thymidine-rescue as described previously [31]. Haemorrhaghic pericarditis was suspected to be the most probable cause of death. Postmortem examination was not performed.
Figure 1.
Mean MTX plasma concentration time profiles and those of the two most extreme outliers (patients 36 and 63), both of whom received 3 mg MTX as a 3 h infusion. ID-36 (▴); ID-63 (▪); mean curve (—)
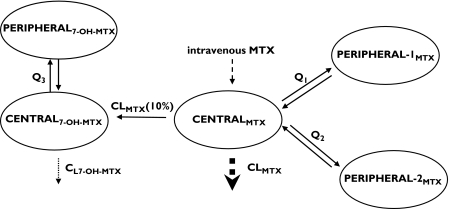
MTX concentration-time data were best described by a linear three-compartment model with first-order elimination from the central compartment. Those for 7-OH-MTX were best described by a linear two-compartment model (one central and one peripheral compartment) with first-order elimination of the metabolite from the central compartment (Figure 2). Because an independent calculation of the metabolic fraction of MTX to 7-OH-MTX was not possible, we assumed that 10% of MTX was metabolized to 7-OH-MTX, in accordance with literature data [32]. Fixing the metabolic fraction to higher (up to 50%) or lower (down to 2%) values resulted in a decreased fit with an increased OFV. The combined five-compartment model generated pharmacokinetic parameter estimates as outlined in Table 2. Interindividual variability for the central compartment of MTX (CENTRALMTX), the first peripheral compartment of MTX (PERIPHERAL-1MTX) and the intercompartmental clearance between CENTRALMTX and PERIPHERAL-2MTX (Q2) were not included in the final model as they did not improve the fit. Interoccasion variability for CL7-OH-MTX was also not included as it was estimated at <5%.
Figure 2.
Five compartment model for MTX and 7-OH-MTX pharmacokinetics; CL = clearance, Q = intercompartmental clearance
Table 2. Population pharmacokinetic data.
| Pharmacokinetic parameter | Full data setEstimate | RSE |
|---|---|---|
| CLMTX (L h−1) | 8.85 (see equation 1) | 1.96 |
| CL7-OH-MTX (L h−1) | 2 (see equation 2) | 0.417 |
| Volume of CENTRALMTX (L) | 23.0 | 4.04 |
| Volume of CENTRAL7-OH-MTX (L) | 21.6 | 3.16 |
| Volume of PERIPHERAL-1MTX (L) | 185 | 25.5 |
| Volume of PERIPHERAL-2MTX (L) | 5.34 | 2.01 |
| Volume of PERIPHERAL7-OH-MTX (L) | 27.7 | 2.93 |
| Q1 (L h−1) | 0.444 | 8.78 × 10−2 |
| Q2 (L h−1) | 0.716 | 0.371 |
| Q3 (L h−1) | 0.429 | 4.79 × 10−2 |
| Interindividual variability | ||
| CLMTX (%) | 19.6 | 6.66 |
| CL7-OH-MTX (%) | 31.0 | 9.77 |
| Volume of CENTRAL7-OH-MTX (%) | 8.22 | 3.80 |
| Volume of PERIPHERAL-2MTX (%) | 31.0 | 16.7 |
| Volume of PERIPHERAL7-OH-MTX (%) | 41.4 | 10.2 |
| Q1 (%) | 32.0 | 21.1 |
| Q3 (%) | 27.1 | 18.5 |
| Interoccasion variability | ||
| CLMTX (%) | 13.3 | 4.28 |
| Residual variability | ||
| MTX plasma concentration (%) | 52.3 | 27.8 |
| 7-OH-MTX plasma concentration (%) | 57.1 | 27.3 |
RSE, relative standard error; Q1, intercompartmental clearance between CENTRALMTX and PERIPHERAL-1MTX; Q2, intercompartmental clearance between CENTRALMTX and PERIPHERAL-2MTX; Q3, intercompartmental clearance between CENTRAL7-OH-MTX and PERIPHERAL7-OH-MTX.
The two patients with noticeably increased exposure to MTX and 7-OH-MTX (ID-63 and ID-36 in Figure 1) had a markedly decreased CLMTX (Bayesian estimates of 1.70 L−1 and 4.11 L−1, respectively). The CL7-OH-MTX was also significantly impaired in ID-63 (1.15 L−1), but was almost normal in ID-36 (1.85 L−1). The two patients administered prior cisplatin had moderately decreased CLMTX (7.37 and 5.03 L−1) and an approximately normal CL7-OH-MTX (3.36 and 1.91 L h−1). The patient who had been given ifosfamide prior to MTX showed no decrease in CLMTX (9.15 L h−1) or CL7-OH-MTX (2.49 L h−1). The addition of cisplatin or ifosfamide as covariates to the population model did not improve the fit. However, there were insufficient data to estimate the influence of these parameters on MTX pharmacokinetics.
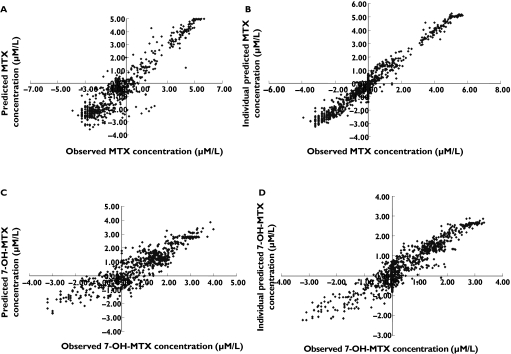
Subsequent covariate testing was performed with and without the inclusion of the individuals with markedly decreased CLMTX and CL7-OH-MTX to allow the detection of a covariate relationship highly driven by these outliers. The following covariates were identified to be significantly correlated with CLMTX and CL7-OH-MTX: Creatinine clearance (CLCREA), prior administration of NSAIDs, and concurrent administration of benzimidazoles. The inclusion of all three covariates on both CLMTX and CL7-OH-MTX led to a decrease in OFV (174.5 points, P < 10−4), in the interindividual variability in CLMTX (from 35.2% to 19.6%) and CL7-OH-MTX (from 32.5% to 31.0%), and in the interoccasion variability in CLMTX (from 18.6% to 13.3%). Furthermore, an improved fit was observed on inspection of the graphical plots (Figure 3). The concurrent administration of benzimidazoles led to a 27% and 39% decrease in CLMTX and CL7-OH-MTX, respectively, and prior administration of NSAIDs led to a 16% and 38% decrease in CLMTX and CL7-OH-MTX, respectively. These relationships were consistent in the datasets containing or not containing the two individuals with the highest exposure to MTX. No difference in the effect on CLMTX or CL7-OH-MTX could be demonstrated for the two benzimidazoles (omeprazole and lansoprazole) or the two NSAIDs (diclofenac and ibuprofen). Furthermore, inclusion of the dosage of the benzimidazoles and NSAIDs as covariates on CLMTX and CL7-OH-MTX did not improve the fit. Third-space fluid collections, comedication with corticosteroids or anticancer drugs, anthropometric parameters and surrogate markers of liver function (AST, ALT, AP, total bilirubin) had no influence on the pharmacokinetics of MTX and 7-OH-MTX.
Figure 3.
Goodness-of-fit plots from the final population pharmacokinetic model (all data are log-transformed). (A) Observed vs. predicted MTX concentrations. (B) Observed vs. individual Bayesian predicted MTX concentrations. (C) Observed vs. the model predicted 7-OH-MTX concentrations. (D) Observed vs. individual Bayesian predicted of 7-OH-MTX concentrations.
Equations 1 and 2 describe CLMTX and CL7-OH-MTX as a function of CLCREA (median value = 87 mL min−1) and comedication (benzimidazoles (PPI) and NSAID is 1 with concurrent comedication and 0 without comedication):
| (1) |
| (2) |
As a consequence, plasma MTX concentrations at 24 h were significantly higher in patients taking benzimidazoles (geometric mean 2.01 µmol L−1, 95% CI 1.44–2.81 µmol L−1) compared with patients not taking benzimidazoles (0.66 µmol L−1, 0.52–0.84 µmol L−1, P < 10−4), as were plasma MTX concentrations at 48 h in patients taking benzimidazoles (0.25 µmol L−1, 0.17–0.35 µmol L−1) compared with patients not taking benzimidazoles (0.12 µmol L−1, 0.09–0.15, P < 10−4). Plasma 7-OH-MTX concentrations at 24 h were also significantly higher in patients taking benzimidazoles (4.47 µmol L−1, 3.17–6.31 µmol L−1) compared with patients not taking concurrent benzimidazoles (2.52 µmol L−1, 1.99–3.20 µmol L−1, P = 0.0009), as were plasma 7-OH-MTX concentrations at 48 h in patients taking benzimidazoles (1.11 µmol L−1, 0.73–1.68 µmol L−1) compared with patients not taking concurrent benzimidazoles (0.72 µmol L−1, 0.53–0.98 µmol L−1, P = 0.031). For patients who had been administered NSAIDs, the differences were not statistically significant except for plasma 7-OH-MTX concentrations at 48 h which were higher in patients given NSAIDs compared with patients not given NSAIDs (1.61 µmol L−1vs. 0.73 µmol L−1, P = 0.0023) (Table 3). Plasma MTX concentrations ≥ 0.1 µmol L−1 at 48 h were found in 78.1% of patients taking benzimidazoles, in 71.4% of patients who had been given NSAIDs, but in only 33.5% of patients not taking comedication. Compared with patients without the respective comedication, median 24- h plasma MTX concentrations were not different in patients receiving 5-fluorouracil (0.59 µmol L−1vs. 1.09 µmol L−1, P = 0.063), vinca alkaloids (1.79 µmol L−1vs. 0.66 µmol L−1, P = 0.38), l-asparaginase (4.27 µmol L−1vs. 0.68 µmol L−1, P = 0.12), corticosteroids (1.30 µmol L−1vs. 0.66 µmol L−1, P = 0.96) or in patients treated for third-space fluids (0.51 µmol L−1vs. 0.70, P = 0.51).
Table 3. MTX- and 7-OH-MTX plasma concentrations at 24 and 48 h in patients also taking benzimidazoles or who had been treated with NSAIDS prior to MTX.
| Geom. mean (95% CI) (µmol L−1) | |||||
|---|---|---|---|---|---|
| 24-h plasma conc. | 48-h plasma conc | ||||
| Patient subgroup | n | MTX | 7-OH-MTX | MTX | 7-OH-MTX |
| +Benzimidazoles | 13 | 2.01 (1.44–2.81) | 4.47 (3.17–6.31) | 0.25 (0.17–0.35) | 1.11 (0.73–1.68) |
| No benzimidazoles | 63 | 0.66 (0.52–0.84) | 2.52 (1.99–3.20) | 0.12 (0.09–0.15) | 0.72 (0.53–0.98) |
| Ratio | 3.03 (2.19–4.19) | 1.77 (1.27–2.48) | 2.10 (1.50–2.95) | 1.53 (1.04–2.26) | |
| P-value | <10−4 | 0.0009 | <10−4 | 0.031 | |
| Prior NSAIDs | 6 | 0.98 (0.63–1.51) | 1.74 (1.10–2.76) | 0.17 (0.11–0.28) | 1.61 (0.93–2.80) |
| No NSAIDs | 70 | 0.77 (0.62–0.97) | 2.83 (2.25–3.55) | 0.13 (0.10–0.16) | 0.73 (0.55–0.99) |
| Ratio | 1.26 (0.84–1.91) | 0.61 (0.40–0.95) | 1.34 (0.84–2.13) | 2.20 (1.33–3.65) | |
| P-value | 0.26 | 0.27 | 0.21 | 0.0023 | |
Discussion
HDMTX-schedules are associated with an incidence of nephrotoxicity of 1.8% and a fatality rate of almost 0.1%, despite routine drug monitoring and supportive therapy [24]. Additionally, HDMTX-induced renal impairment exacerbates overall drug toxicity by delaying MTX elimination. Because concurrent administration of NSAIDs has been shown to increase MTX toxicity [10, 33], it is recommended that the two drugs should not be administered together. However, the interaction between benzimidazoles and MTX is less well known, and has been described in only a few case reports [13, 34, 35]. Previously, there have been no published data on the effects of NSAIDs and benzimidazoles on the elimination of MTX and 7-OH-MTX using a Bayesian approach.
Estimates for CLMTX and CL7-OH-MTX were in accordance with literature data [7, 36]. CLCREA correlated with model-predicted CLMTX and CL7-OH-MTX, internally validating the presented population model. Concurrent administration of benzimidazoles and prior administration of NSAIDs, even if discontinued on the day of HDMTX administration, significantly impaired the elimination of both MTX and 7-OH-MTX. Decreased clearance of MTX has been described in cancer patients on concurrent HDMTX and ketoprofen, with fatalities in three out of 36 patients [10]. Indomethacin was similarly reported to affect the pharmacokinetics of MTX with resulting severe renal failure [33]. No clinically significant interactions were found between ketoprofen, piroxicam and flurbiprofen and MTX if the latter was administered to patients with rheumatoid arthritis at low oral doses [37]. Interactions with NSAIDs result from inhibition of renal prostaglandin synthesis [10], by displacement of 7-OH-MTX from protein binding [38], and by competition between NSAIDs and MTX/7-OH-MTX for renal excretion [10], possibly mediated by human organic anion transporters [14]. We found six patients in whom NSAIDs were only stopped shortly before HDMTX administration, for unknown reasons.
Two case reports indicated that MTX elimination is impaired with concurrent administration of omeprazole, resulting in sustained, highly toxic plasma MTX concentrations [13, 34]. Additionally, impaired MTX elimination has been reported during treatment with pantoprazole in a patient with cutaneous T-cell lymphoma receiving low-dose intramuscular MTX [35]. Benzimidazoles are potent inhibitors of MTX transport by the drug-transporter ABCG2 in vitro[39], which could explain the present clinical findings. It remains unclear if interactions between benzimidazoles and MTX are a class-effect or restricted to individual drugs.
Anthropometrics, biochemical markers, comedication with anticancer drugs or corticosteroids and third-space effusions were not found to affect the elimination of MTX or 7-OH-MTX. The third-space effusions in 15 out of the 76 patients studied were treated before the administration of HDMTX. Whereas corticosteroids, vinca alkaloids and l-asparaginase have been shown to interact with MTX at the cellular level in vitro[16–18], these covariates had no influence on the elimination of MTX or 7-OH-MTX in the present work. However, patient subgroups were small except for those taking benzimidazoles and corticosteroids, and there was a considerable lack of homogeneity with respect to MTX dosage and duration of infusion.
The patient who died had significantly decreased CLMTX (0.69 L−1) and CL7-OH-MTX (0.77 L−1). The reasons for the marked impairment of drug elimination remain unclear, as supportive therapy and intensive leucovorin rescue were performed, comedication was absent and baseline CLCREA was 92 mL min−1. According to a recent meta-analysis, HDMTX-induced nephrotoxicity CTC grade ≥2 occured in 1.8% of patients receiving HDMTX for osteosarcoma, with an overall fatality rate of 0.08%, despite supportive therapy [24]. The latter study did not include data on comedication. A specific mutation within the drug transporter ABCC2 (Arg412Gly) has recently been found in a patient with impaired MTX elimination and nephrotoxicity, who was treated with HDMTX for large B-cell lymphoma [40]. MTX was shown to be a substrate for ABCG2, another drug transporter, in vitro[41], and variant alleles of the gene ABCG2 may also account for altered MTX elimination.
In conclusion, concurrent administration of benzimidazoles and HDMTX causes a significant decrease in CLMTX and CL7-OH-MTX, resulting in significantly higher plasma concentrations of MTX and 7-OH-MTX. The data suggest that benzimidazoles should not be administered with HDMTX. Assessment of patient self-medication of over-the-counter drugs before HDMTX is administered, would seem prudent to prevent toxicity.
M. Joerger is supported by a fellowship grant funded by the European Society of Medical Oncology and by a research grant from the Swiss National Science Foundation (PBBSB-102331).
References
- 1.Schornagel JH, McVie JG. The clinical pharmacology of methotrexate. Cancer Treat Rev. 1983;10:53–75. doi: 10.1016/s0305-7372(83)80032-2. [DOI] [PubMed] [Google Scholar]
- 2.Lankelma JKE, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980;9:133–42. doi: 10.1016/0304-3835(80)90117-2. [DOI] [PubMed] [Google Scholar]
- 3.Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 4.Fuskevag OM, Kristiansen C, Lindal S, Aarbakke J. Maximum tolerated doses of methotrexate and 7-hydroxy-methotrexate in a model of acute toxicity in rats. Cancer Chemother Pharmacol. 2000;46:69–73. doi: 10.1007/s002800000111. [DOI] [PubMed] [Google Scholar]
- 5.Erttmann R, Bielack S, Landbeck G. Kinetics of 7-hydroxy-methotrexate after high-dose methotrexate therapy. Cancer Chemother Pharmacol. 1985;15:101–4. doi: 10.1007/BF00257517. [DOI] [PubMed] [Google Scholar]
- 6.Batey MA, Wright JG, Azzabi A, Newell DR, Lind MJ, Calvert AH, Boddy AV. Population pharmacokinetics of adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF) Eur J Cancer. 2002;38:1081–9. doi: 10.1016/s0959-8049(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 7.Bore P, Bruno R, Lena N, Favre R, Cano JP. Methotrexate and 7-hydroxy-methotrexate pharmacokinetics following intravenous bolus administration and high-dose infusion of methotrexate. Eur J Cancer Clin Oncol. 1987;23:1385–90. doi: 10.1016/0277-5379(87)90124-6. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AL, Margison JM, Wilkinson PM, Lucas SB. The pharmacokinetics of 7-hydroxymethotrexate following medium-dose methotrexate therapy. Cancer Chemother Pharmacol. 1985;14:165–7. doi: 10.1007/BF00434358. [DOI] [PubMed] [Google Scholar]
- 9.Evans WE, Christensen ML. Drug interactions with methotrexate. J Rheumatol. 1985;12(Suppl 12):15–20. [PubMed] [Google Scholar]
- 10.Thyss A, Milano G, Kubar J, Namer M, Schneider M. Clinical and pharmacokinetic evidence of a life—threatening interaction between methotrexate and ketoprofen. Lancet. 1986;1:256–8. doi: 10.1016/s0140-6736(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrazzini G, Klein J, Sulh H, Chung D, Griesbrecht E, Koren G. Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. J Pediatr. 1990;117:823–6. doi: 10.1016/s0022-3476(05)83351-7. [DOI] [PubMed] [Google Scholar]
- 12.Ronchera CL, Hernandez T, Peris JE, Torres F, Granero L, Jimenez NV, Pla JM. Pharmacokinetic interaction between high-dose methotrexate and amoxycillin. Ther Drug Monit. 1993;15:375–9. doi: 10.1097/00007691-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Reid T, Yuen A, Catolico M, Carlson RW. Impact of omeprazole on the plasma clearance of methotrexate. Cancer Chemother Pharmacol. 1993;33:82–4. doi: 10.1007/BF00686028. [DOI] [PubMed] [Google Scholar]
- 14.Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302:666–71. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 15.Jansen GHJ, Oerlemans R, Lems WF, Ifergan I, Scheper RJ, Assaraf YG, Dijkmans BA. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum. 2004;50:2130–9. doi: 10.1002/art.20375. [DOI] [PubMed] [Google Scholar]
- 16.Smeland E, Bremnes RM, Bessesen A, Jaeger R, Aarbakke J. Interactions of vinblastine and vincristine with methotrexate transport in isolated rat hepatocytes. Cancer Chemother Pharmacol. 1993;32:209–14. doi: 10.1007/BF00685837. [DOI] [PubMed] [Google Scholar]
- 17.Capizzi RL. Asparaginase-methotrexate in combination chemotherapy: schedule-dependent differential effects on normal versus neoplastic cells. Cancer Treat Rep. 1981;65(Suppl 4):115–21. [PubMed] [Google Scholar]
- 18.Bruckner HW, Schreiber C, Waxman S. Interaction of chemotherapeutic agents with methotrexate and 5-fluorouracil and its effect on de novo DNA synthesis. Cancer Res. 1975;35:801–6. [PubMed] [Google Scholar]
- 19.de Vita VT, Hellman S, Rosenberg SA, editors. Cancer — Principles and Practice of Oncology. Philadelphia, PA: Lippincott. Williams & Wilkins; 2005. Pharmacology of Cancer Chemotherapy. 19.5: Antimetabolites — Methotrexate: Schedules of Administration. Chapter 19. [Google Scholar]
- 20.van den Bongard HJ, Mathot RA, Beijnen JH, Schellens JH. Pharmacokinetically guided administration of chemotherapeutic agents. Clin Pharmacokinet. 2000;39:345–67. doi: 10.2165/00003088-200039050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Favre R, Monjanel S, Alfonsi M, Pradoura JP, Bagarry-Liegey D, Clement S, Imbert AM, Lena N, Colonna DJ, Cano JP, Carcassonne Y. High-dose methotrexate: a clinical and pharmacokinetic evaluation. Treatment of advanced squamous cell carcinoma of the head and neck using a prospective mathematical model and pharmacokinetic surveillance. Cancer Chemother Pharmacol. 1982;9:156–60. doi: 10.1007/BF00257744. [DOI] [PubMed] [Google Scholar]
- 22.Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, Evans WE. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–72. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 23.Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42:1322–9. [PubMed] [Google Scholar]
- 24.Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100:2222–32. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 25.Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–4. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 26.Takami M, Kuniyoshi Y, Oomukai T, Ishida T, Yamano Y. Severe complications after high-dose methotrexate treatment. Acta Oncol. 1995;34:611–2. doi: 10.3109/02841869509094036. [DOI] [PubMed] [Google Scholar]
- 27.van Tellingen O, van der Woude HR, Beijnen JH, van Beers CJ, Nooyen WJ. Stable and sensitive method for the simultaneous determination of N5-methyltetrahydrofolate, leucovorin, methotrexate and 7-hydroxymethotrexate in biological fluids. J Chromatogr. 1989;488:379–88. doi: 10.1016/s0378-4347(00)82962-x. [DOI] [PubMed] [Google Scholar]
- 28.Beal SL, Sheiner LB. San Francisco: NONMEM Project Group, University of California at San Francisco; 1998. User's Guide. [Google Scholar]
- 29.Jonsson EN, Karlsson MO. Xpose — an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 30.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–20. [PubMed] [Google Scholar]
- 31.van den Bongard HJGD, Mathot RA, Boogerd W, Schornagel JH, Soesan M, Schellens JH, Beijnen JH. Successful rescue with leucovorin and thymidine in a patient with high-dose methotrexate induced acute renal failure. Cancer Chemother Pharmacol. 2001;47:537–40. doi: 10.1007/s002800000269. [DOI] [PubMed] [Google Scholar]
- 32.Breithaupt H, Kuenzlen E. Pharmacokinetics of methotrexate and 7-hydroxymethotrexate following infusions of high-dose methotrexate. Cancer Treat Rep. 1982;66:1733–41. [PubMed] [Google Scholar]
- 33.Maiche AG. Acute renal failure due to concomitant action of methotrexate and indomethacin. Lancet. 1986;1:1390. doi: 10.1016/s0140-6736(86)91706-x. [DOI] [PubMed] [Google Scholar]
- 34.Beorlegui B, Aldaz A, Ortega A, Aquerreta I, Sierrasesumega L, Giraldez J. Potential interaction between methotrexate and omeprazole. Ann Pharmacother. 2000;34:1024–7. doi: 10.1345/aph.19094. [DOI] [PubMed] [Google Scholar]
- 35.Troger U, Stotzel B, Martens-Lobenhoffer J, Gollnick H, Meyer FP. Drug points: Severe myalgia from an interaction between treatments with pantoprazole and methotrexate. BMJ. 2002;324:1497. doi: 10.1136/bmj.324.7352.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slordal L, Kolmannskog S, Prytz PS, Moe PJ, Aarbakke J. Pharmacokinetics of methotrexate and 7-hydroxy-methotrexate after high-dose (33.6 g/m2) methotrexate therapy. Pediatr Hematol Oncol. 1986;3:127–34. doi: 10.3109/08880018609031208. [DOI] [PubMed] [Google Scholar]
- 37.Tracy TS, Worster T, Bradley JD, Greene PK, Brater DC. Methotrexate disposition following concomitant administration of ketoprofen, piroxicam and flurbiprofen in patients with rheumatoid arthritis. Br J Clin Pharmacol. 1994;37:453–6. doi: 10.1111/j.1365-2125.1994.tb05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slordal L, Sager G, Aarbakke J. Pharmacokinetic interactions with methotrexate: is 7-hydroxy-methotrexate the culprit? Lancet. 1988;1:591–2. doi: 10.1016/s0140-6736(88)91387-6. [DOI] [PubMed] [Google Scholar]
- 39.Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, Schinkel AH, van Tellingen O, Borst P, Schellens JH. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug—drug interactions. Cancer Res. 2004;64:5804–11. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 40.Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, William-Faltaos D, Fernandez C, Hatem S, Deray G, Komajda M, Leblond V, Lechat P. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15:277–85. doi: 10.1097/01213011-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63:5538–43. [PubMed] [Google Scholar]