Abstract
Aims
To characterize the population pharmacokinetics of tipifarnib.
Methods
A total of 1083 subjects treated orally with a solution, capsule or tablet formulations of tipifarnib, given as a single dose or as multiple twice-daily doses (range 25–1300 mg) were combined with data from 1, 2 and 24 h intravenous infusions. A total of 3445 concentrations in the index data set were fitted by an open three-compartment linear disposition model with sequential zero-order input into the depot compartment, followed by a first-order absorption process, and lag time, using NONMEM V. The effect of patient covariates on tipifarnib pharmacokinetics was explored. The model was evaluated using goodness of fit plots and relative error measurements for 3894 concentrations in the test data set. Computer simulations were undertaken to evaluate the effect of covariates on tipifarnib pharmacokinetics.
Results
Tipifarnib oral bioavailability (26.7%) did not differ between the formulations. The absorption rate from the solution was faster than from the solid forms. Whereas the absorption rate and systemic clearance were more rapid in healthy subjects, the extent of absorption and the steady-state volume of distribution were comparable in cancer patients and healthy subjects. Systemic clearance in cancer patients (21.9 l h−1) exhibited a statistically significant relationship with total bilirubin. The typical volume of the central compartment in cancer patients (54.6 l 70 kg−1) was directly proportional to body weight. The clinical relevance of these covariates in cancer patients is questionable as there was a substantial overlap in simulated concentration-time profiles across a wide range of covariate values.
Conclusions
A population PK approach has been used to integrate data gathered during clinical development and to characterize the pharmacokinetics of tipifarnib. Individualization of dose based on body weight or total bilirubin concentration in adult cancer patients is not warranted.
Keywords: anticancer drugs, farnesyltransferase inhibitor, population pharmacokinetics, tipifarnib
Introduction
Tipifarnib (R115777, Zarnestra®) is a potent, selective and competitive inhibitor of the enzyme farnesyltransferase (FTase) [1–3]. This enzyme is important in the processing and activation of signalling molecules linked to cell proliferation and malignant transformation, such as Ras, Rho-B, Rac, and lamin proteins [1]. Inhibition of FTase by tipifarnib induces antileukaemic and antitumoral activity, which has been demonstrated in both in vitro and in vivo animal models [2]. The nature of the cellular and tumour tissue responses elicited by tipifarnib treatment in vivo is consistent with the hypothesis that the antitumour effects are being derived from disruption of multiple effectors downstream of FTase inhibition.
Several phase 1 dose-escalation studies in patients with advanced solid tumours have been performed with oral tipifarnib as a single agent, using twice-daily schedules ranging from 5 days every 2 weeks, to continuous dosing [3–6]. These studies were designed to determine the maximum tolerated dose and to characterize the safety and the pharmacokinetics of tipifarnib. Those studies demonstrated that the pharmacokinetics of tipifarnib are linear in doses of up to 600 mg twice daily and allowed the measurement of the oral bioavailability of the solution, capsule and tablet formulation in cancer patients. Little or no evidence of time-dependent pharmacokinetics was observed after repeated administration [4, 6].
Tipifarnib is rapidly absorbed after oral intake, with a peak plasma concentration being reached within 2–4 h [3, 6]. Plasma tipifarnib concentrations decline in a bi-exponential manner, and the half-life (t1/2) associated with the first disposition phase is 2–5 h. Its terminal half-life is 16–20 h and exposure to the drug over this period constituted only a small portion of the overall area under the plasma concentration vs. time curve. Minimal plasma accumulation is seen upon twice-daily administration, indicating that the first disposition phase dominates the plasma concentration-time profile of tipifarnib [3].
The drug is almost completely bound (99.38%) to plasma protein and binding is independent of the plasma drug concentrations (range 0.1–5 µg ml−1). In human plasma, tipifarnib is mostly bound to albumin and to a lesser extent to α1—acid glycoprotein [5].
Tipifarnib is extensively metabolized in humans. The drug could not be detected in urine and less than 6% of the initial dose was recovered in faeces as the parent compound. In vitro and in vivo studies demonstrated that phase II metabolism, particularly, hepatic N-glucuronidation, followed by urinary excretion of the product, is an important route for tipifarnib elimination. In addition, oxidative N-deamination, oxidative N-demethylation, and loss of the methyl-imidazole moiety are the major phase 1 pathways involved in tipifarnib metabolism In vitro studies have shown that cytochrome P450 (CYP) 3A4 is the predominant enzyme involved in the metabolism of this compound and that CYP2C19, CYP2A6, CYP2D6 and CYP2C8/9/10 might play a lesser role in the biotransformation of tipifarnib (data on file, Johnson & Johnson Pharmaceutical Research & Development, J & JPRD).
In the present paper, data from 15 clinical studies conducted in healthy subjects or cancer patients were pooled to examine the pharmacokinetic behaviour of tipifarnib. The objectives of this population pharmacokinetic analysis were three-fold: i) to model tipifarnib pharmacokinetics after intravenous and oral administration of solution, capsule and tablet formulations, ii) to obtain estimates of population pharmacokinetic parameters in healthy and cancer subjects, and iii) to evaluate the influence of demographic characteristics and other covariates on tipifarnib pharmacokinetics.
Methods
Patient eligibility criteria and study design
Data from eight phase 1 studies (two in healthy subjects and six in adult cancer patients) with extensive blood sampling and seven phase 2/3 studies with sparse sampling from subjects with advanced cancer were pooled (Table 1) [3, 4, 6, 7–13 ]. All these studies were conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki (1983) and were approved by the Human Investigational Review Board of each study centre. Informed consent was obtained from each subject after being told the potential risks and benefits, as well as the purpose of the study.
Table 1. Designs of the different studies from which plasma tififarnib data were obtained.
| Study typea | Data set (n) | Indication | Study type | Dose range (mg) | Dosing regimen | Formulation | Duration of dosing regimen | Sampling schedule |
|---|---|---|---|---|---|---|---|---|
| Phase 1 | ||||||||
| 1 [3] | Index (27) | Advanced cancer | MTD | 25–325 | b.d. | Solution | 5 consecutive days separated by a minimum of 7 days of rest period | Intensive sampling on days 0 and 5, plus 1 isolated trough sample |
| 500–1300 | s.d. | Capsule | ||||||
| 200–300 | Capsule | |||||||
| 2 [4, 15] | Index (28) | Advanced cancer | MTD | 200–300 | s.d. | Tablet | Continuous treatment | Intensive sampling on days 1, 2 and 28, plus 3–4 isolated trough samples |
| 50–500 | b.d. | Capsule | ||||||
| 3 | Index (34) | Advanced cancer | MTD | 100–850 | b.d. | Capsule | 21 consecutive days separated by a minimum of 7 days of rest period | Intensive sampling on days 0 and 21, plus 2 isolated trough samples |
| 100–850 | Tablet | |||||||
| 4 [16] | Index (25) | Advanced cancer | MTD | 100–300 | b.d. | Capsule | 21 consecutive days separated by a minimum of 7 days of rest period | Intensive sampling on days 1 and 8 |
| 100–300* | Tablet | |||||||
| 5 | Index (12) | Healthy subjects | Absolute bioavailability | 50 | s.d. | Tablet | – | Intensive sampling on two occasions |
| 25 | 1 h i.v. inf | |||||||
| 7 [6] | Index (9) | Advanced cancer | MTD | 200–500 | b.d. | Capsule | 28 consecutive days | Intensive sampling on days 1 and 28, plus 4 isolated trough samples |
| 15 | Index (31) | Advanced cancer | Bioequivalence | 200 | b.d. | Tablet | 4 consecutive days each treatment without washout period (Sequence of treatment randomized) | Intensive sampling on day 4 of each treatment |
| 240 | Continuous | i.v. Inf | ||||||
| 60–120 | b.d. | 2 h i.v. inf | ||||||
| 44 | Test (36) | Healthy subjects | Bioequivalence | 50 | s.d. | Tablet | – | Intensive sampling on two occasions |
| Phase 2 | ||||||||
| 6 [17] | Test (64) | Advanced breast cancer | Safety, efficacy | 300–400 | b.d. | Tablet | 21 consecutive days separated by a minimum of 7 days of rest period or continuously | Sparse isolated samples (week 2, 4, 8 and 12), 4–5 per subject |
| 300–400 | Capsule | |||||||
| 8 [18] | Test (19) | Small-cell lung cancer | Safety, efficacy | 400 | b.d. | Capsule | 14 consecutive days separated by a minimum of 7 days | Sparse sample, 2 samples on days 1 and 8 |
| 10 [19] | Test (28) | Advanced urothelial tract transitional cell cancer | Safety, efficacy | 300 | b.d. | Tablet | 21 consecutive days separated by a minimum of 7 days | Sparse isolated samples (days 1, 8, 15, 22, 15 (month 1), 1 (month 2)): 6 per subject |
| 12 | Test (8) | Superficial bladder cancer | Safety, efficacy | 300 | b.d. | Capsule | 6 weeks | Sparse samples: 7 samples over days 15, 29 and 42 |
| 17 | Test (242) | Acute myeloid leukaemia | Safety, efficacy | 600 | b.d. | Tablet | 21 consecutive days separated by a minimum of 7 days | Sparse isolated samples: 4 samples over days 1, 8 and 15. Intensive sampling on day 1 for a subset of 20 subjects |
| Phase 3 | ||||||||
| 9 [20] | Test (212) | Advanced colorectal cancer | Survival | 300 | bd | Tablet | 21 consecutive days separated by a minimum of 7 days of rest period | Sparse isolated samples (Days 15, 22, 1 (month 2)), 4 per subject |
| 11 [21] | Test (308) | Advanced pancreatic cancer | Efficacy | 200 | b.d. | Tablet | Continuous (1 year) | Sparse isolated samples, 4 samples over days 1, 8 and 15 |
b.d, twice a day; s.d., single dose; i.v., intravenous.
Reference number.
In combination with gemcitabine.
In the clinical studies conducted in cancer patients, subjects were eligible if they had histological or cytological confirmation of a malignant tumour not amenable to established forms of effective therapy. Other eligibility criteria included a World Health Organization performance status of 0–2, anticipated life expectancy of at least 3 months, and age >18 years. Previous anticancer radiation therapy and/or chemotherapy, if given, had to have finished at least 4 weeks before entry into the study, or 6 weeks in case of pretreatment with nitrosoureas or mitomycin C. Patients had to have adequate unassisted oral or adequate enteral intake to maintain a reasonable state of nutrition, a negative pregnancy test (only for female patients with reproductive potential), and normal hepatic and renal function, defined as bilirubin ≤1.5 times the normal upper limit, AST and ALT ≤2.5 times the normal upper limit (≤5 times the normal upper limit when hepatic metastases were present), and serum creatinine ≤1.5 times the normal upper limit. All patients had to have had acceptable bone marrow function, defined as white blood cells >3,500 µl−1, absolute neutrophil count >1,500 µl−1 and platelets >100,000 µl−1, except in patients with a diagnosis of acute myeloid leukaemia. Subjects with one or more of the following treatments were not selected: prior administration of an FTase inhibitor, prior extensive radiation therapy (>25% of bone marrow reserve), prior bone marrow transplantation or high dose chemotherapy with marrow or stem cell rescue, concurrent radiation therapy, chemotherapy, hormonal therapy or immunotherapy. Participation in a clinical trial involving an investigational drug in the past 30 days, concurrent enrolment in any other investigational trial and any coexisting medical condition that was likely to interfere with the study procedures and/or results were additional reasons for exclusion. Patients randomized to placebo treatment groups were not included in the population pharmacokinetic analysis. A summary of patient characteristics at baseline is presented in Table 2.
Table 2. Summary of subject characteristics at baseline.
| Subject characteristics | Index data set1 | Test data set2 | Combined data set | Missing covariates3 |
|---|---|---|---|---|
| Age (years) | 56.0 (22.0–81.0) | 62.0 (18.0–89.0) | 60.0 (18.0–89.0) | 0.0 |
| Body weight (kg) | 71.8 (44.0–124) | 69.8 (34.0–145) | 70.0 (34.0–145) | 1.3 |
| Body surface area (m2) | 1.85 (1.38–2.57) | 1.80 (1.18–2.68) | 1.81 (1.18–2.68) | 3.5 |
| Sex (number) | 0.0 | |||
| Male | 93 | 487 | 603 | |
| Female | 73 | 394 | 480 | |
| Race (number) | 0.0 | |||
| Caucasian | 156 | 858 | 1014 | |
| African-American | 6 | 14 | 20 | |
| Others | 4 | 45 | 49 | |
| Subject status (number) | 0.0 | |||
| Healthy | 12 | 36 | 48 | |
| Cancer | 154 | 881 | 1035 | |
| ALT (IU l–1) | 25.5 (3.0–142) | 26.0 (4.0–400) | 25.5 (3.0–400) | 2.1 |
| AST (IU l–1) | 25.0 (6.0–125) | 26.0 (4.0–249) | 26.0 (4.0–249) | 2.5 |
| Alkaline phosphatase (IU l–1) | 103 (40–1030) | 148 (23–3180) | 133 (23–3180) | 1.2 |
| Lactate dehydrogenase (IU l–1) | 384 (141–3320) | 370 (22–7900) | 387 (22–7900) | 8.8 |
| Total bilirubin (µmol l–1) | 9.0 (3.0–41.0) | 10.0 (2.0–116) | 10.0 (2.0–116) | 1.8 |
| Serum albumin (g l–1) | 41.0 (25.0–57.0) | 38.0 (13.0–60.0) | 38.0 (13.0–60.0) | 19.7 |
| Total protein (g l–1) | 70.5 (48.0–86.0) | 70.0 (24.0–102) | 70.0 (24.0–102) | 7.8 |
| Creatinine clearance4 (ml min–1) | 98.0 (33.0–150) | 88.0 (20.0–150) | 90.5 (20.0–150) | 2.1 |
Continuous variables are expressed as median (range), whereas categorical variables are expressed as counts.
Range of observations: 1.0–4932 ng ml−1
Range of observations: 2.3–6266 ng ml−1
Missing covariates expressed as percentage of subjects in the combined data set with missing values.
Creatinine clearance was calculated using Cockroft-Gault formula and values higher than 150 ml min−1 were truncated to 150 ml min−1
Subjects were treated orally and/or intravenously with tipifarnib under fed conditions. Three different oral formulations containing between 25 and 1300 mg of drug (solution, capsule and tablet) were administered as a single dose or as multiple doses twice daily. The data set consisted of 1083 subjects, including 1035 cancer patients and 48 healthy subjects, who contributed 7339 plasma concentrations.
An index and a test data set were prepared and used to develop and evaluate, respectively, the population pharmacokinetic model. The index data set was obtained from seven data-rich phase 1 studies available at the time of model development. It had sufficient data spanning of the whole plasma concentration-time profile of tipifarnib to develop a reference population pharmacokinetic model. The index data set was obtained from 154 cancer patients and 12 healthy subjects, and included 3447 plasma concentrations. Subsequently, two outlying data points were excluded from the analysis after structural model selection. The test data set included sparsely sampled data from 881 cancer patients participating in five phase 2 studies and two phase 3 studies, and 36 healthy subjects included in a phase 1 study that finished after model development. Of 3896 plasma concentrations, two with very high values with respect to the time since the last dose were identified and excluded from the test data set. Index and test data sets were merged, and the final model was fitted to the combined data set to obtain final estimates of parameters and their asymptotic standard errors.
Drug analysis
All venous blood samples were collected in heparinized tubes and centrifuged, and separated plasma was stored at −20 °C and transported to J & JPRD (Beerse, Belgium) for analysis. Plasma concentrations of tipifarnib were measured using either high performance liquid chromatography with ultraviolet detection (HPLC-UV) or liquid chromatography with tandem mass spectrometry (LC-MS/MS). Four phase 2/3 clinical trials [studies 9–11, 17] utilized the LC-MS/MS method and the remaining 11 studies utilized the HPLC-UV method. A successful cross-validation study for both techniques was performed (unpublished observations, J & JPRD. The lower limit of quantification for the HPLC-UV and LC-MS/MS methods was 1.00 and 0.50 ng ml−1, respectively. The mean overall coefficient of variation was less than 7.1% across the validated range of concentrations. More detailed information about the HPLC-UV method has been published elsewhere [3].
In the LC-MS/MS method, plasma samples were analysed as follows. To 0.1 ml aliquots of human plasma, 10 ng of a stable isotope labelled internal standard (R121550) was added. After adding 1 ml of NaOH (0.1 m), the samples were extracted with 5 ml of heptane containing 10% of isoamylalcohol. The organic layer was evaporated under nitrogen at 65 °C and the residue dissolved in 200 µl of methanol, and 50 µl of ammonium formate 0.002 m (pH 4) was added. Extracts (4 µl) were injected on to an API 3000 (Applied Biosystems) LC-MS/MS with a TurboIonspray interface, operated in the positive-ion mode. Separation was on a 5 cm × 4.6 mm chromatographic column, packed with 3 µm C18 BDS-Hypersil (Alltech). The mobile phase was 0.002 m ammonium formate : acetonitrile (40 : 60) delivered at a flow rate of 1.5 ml min−1. The total run time was 2.5 min. The mass transitions monitored were m/z 489.1 to m/z 407.1 and m/z 492.1 to m/z 407.1 for tipifarnib and the internal standard, respectively.
Pharmacokinetic model development
Software
Nonlinear mixed-effects modelling by extended least squares regression using the first order (FO) approximation method was implemented using the NONMEM V level 1.1 software package (GloboMax, Hanover, MD, USA) [14]. Compilations were achieved using Digital Visual Fortran version 6.6b. Graphical and all other statistical analyses, including evaluation of NONMEM outputs, were performed using S-PLUS 2000 release 3 for Windows (Insightful, Seattle, WA, USA).
Structural model selection
Based on the exploratory graphical analyses, open two- and three-compartment disposition models with linear elimination and first-order oral absorption were fitted to the index data set. The following features were subsequently evaluated for improvement of the model: an absorption lag time, zero- and first-order input to the depot compartment followed by first-order input from the depot to the central compartment, and combinations of these features. Typical values of all model parameters were allowed to differ between healthy and cancer subjects. Also, absorption parameters were allowed to differ between oral formulations (solution, capsule and tablet).
The interindividual (IIV, between subject) and interoccasion (IOV, within subject) [15] variabilities in the pharmacokinetic parameters were assumed to follow the lognormal distribution according to the equation:
where Pjk is an individual pharmacokinetic parameter for the jth individual and kth occasion, P* is the typical value of the pharmacokinetic parameter, ηpj is a normally distributed random variable with zero-mean and variance ωp2 and τpk is a normally distributed interoccasion random variable with zero-mean and variance πp2. The magnitudes of IIV and IOV were expressed as coefficients of variations (CV). Four occasions were distinguishable at maximum: three for the full pharmacokinetic profiles within a subject, and one for the isolated measurements, defined as any sample drawn at least 24 h after or before any other sample collected for the same subject. Residual variability was evaluated using an additive error model after natural logarithmic transformation of the measured plasma concentrations and model predictions. Two random effects were included to account for the residual variability for full pharmacokinetic profiles as well as isolated measurements of tipifarnib, according to the equation:
where Cobs is the observed plasma concentration of tipifarnib, Cpred is the corresponding model predicted concentration, ISM is an indicator variable and takes the value 1 for isolated measurements and 0 for plasma samples collected in a full pharmacokinetic profile, and ɛ1 and ɛ2 are independent normally distributed random variables with zero mean and variances, σ12 and σ22, respectively. Different residual terms for the two bioanalytical methods were not tested, as a cross-validation study between the methods confirmed their interchangeability.
The improvement in the fit obtained for each model was assessed in several ways. First, the resulting NONMEM-generated minimum value of the objective function (MVOF) after fitting the models evaluated was used to perform the likelihood ratio test (LRT). This test is based on the change in the minimum value of the objective function (ΔMVOF), which is equal (up to a constant) to minus twice the log-likelihood of the data and is asymptotically distributed like χ2 with the degrees of freedom equal to the number of parameters added to the model. For hierarchical models, a ΔMVOF of ≥ 6.63 is required to reach statistical significance (P = 0.01) for the addition of 1 fixed effect. In addition, the improvement in the fit was assessed by the examination of diagnostic plots such as scatter plots of observed vs. predicted tipifarnib concentrations, scatter plots of weighted residuals vs. predicted tipifarnib concentrations and time since last dose. This process allowed selecting the reference model.
Covariate analyses
Covariates explored as possible sources of IIV in tipifarnib pharmacokinetics are listed in Table 2. As the drug is extensively metabolized by the liver, the following measures of hepatic function were evaluated as potential pharmacokinetic descriptors: alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase and total bilirubin concentration. Although the renal elimination of unchanged tipifarnib is a minor route of elimination, the potential influence of creatinine clearance, calculated from serum creatinine concentrations, was evaluated as a potential pharmacokinetic descriptor. As the protein binding of tipifarnib is > 99%, albumin and total protein were evaluated as potential sources of pharmacokinetic variability. Lactate dehydrogenase, monitored for general tissue toxicity, was also assessed as a potential pharmacokinetic descriptor. Derived body size variables were not tested as independent covariates because of their tight correlation with body weight. If body weight was to be identified as a significant covariate, then body surface area, lean body mass and ideal body weight were to be evaluated in the combined data set to determine whether they improved the fit relative to body weight. Missing values for the quantitative covariates were imputed using the median value in each data set, with the exception of body weight, which was imputed using the median value for subjects of the same gender in the data set.
Once the reference model was identified, empirical Bayes estimates of the interindividual random effects were computed. The covariate screening was guided by graphical assessment and stepwise linear regression of the relationships between the Bayesian estimates of interindividual random effects and the covariates. Those covariates identified by the screening analysis as having a potential influence on a particular parameter were statistically tested one by one for inclusion in the population pharmacokinetic model (forward inclusion). Continuous covariates were evaluated using power equations after centring on the median described by the equation:
where P* is a typical value of a pharmacokinetic parameter P, and θx and θy are fixed-effects parameters. Categorical covariates were analysed as index variables.
Covariates with statistically significant effects on pharmacokinetic parameters were incorporated into the model simultaneously, and subsequently, the covariate screening process was repeated. A full model was identified when no further covariate additions were possible. The relative contribution of each covariate to the goodness of fit of the full model to the data set was then evaluated one at a time by deleting it from the model (backward elimination) [16]. At this stage, differences in pharmacokinetics attributed to disease status (healthy vs. cancer subjects) and formulation (tablet, capsule or solution) were also evaluated. All nonsignificant effects on pharmacokinetic parameters were removed from the model and the intermediate model was obtained.
The resulting MVOF after fitting the reference model to the data was considered as the starting value to test the statistical significance of the covariates by using the LRT. ΔMVOFs of ≥6.63 and 7.88 were required to reach statistical significance at P ≤ 0.01 and P≤ 0.005, respectively, for the inclusion or elimination of one fixed effect. These stringent statistical criteria were used to avoid the inclusion of weak and clinically nonrelevant effects due to the multiple comparisons inherent in the forward inclusion and the backward elimination procedures. In addition, the improvement of the fit obtained by adding a fixed effect to the model was evaluated from the diagnostic plots and the change in the IIV and residual variability.
Model refinement
The distribution of the interindividual random effects and the correlation between them were examined graphically to evaluate the normality and the independence assumption, respectively. A model including all the nondiagonal elements of the random effects matrix was fitted to the data. The random effects with the highest correlation were tested by including the corresponding nondiagonal elements in the matrix of random effects. If implementing a correlation significantly improved the fit (ΔMVOF ≥ 7.88), the off-diagonal element of the random effects matrix was kept in the model and the process was repeated until no further improvement of the fit could be achieved.
Pharmacokinetic model qualification and final model development
The model developed using the index data set was evaluated based on its predictive performance on the test data set. Population predictions and empirical Bayesian (individual) predictions for all concentrations in the test data set were obtained and the diagnostic plots were examined for bias and scatter. Model qualification was done by comparing the mean and the variance of the 10% trimmed relative error obtained from the index and test data set. In the absence of bias, defined as the inclusion of 0 within the 90% confidence interval of the 10% trimmed relative error, the model was considered qualified. In case qualification failed, modification of the population pharmacokinetic model was implemented using the combined data set.
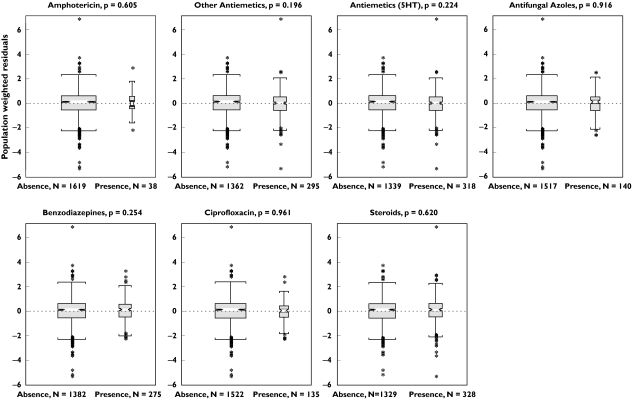
The qualified model was refitted to the combined data set in order to obtain the final estimates of tipifarnib pharmacokinetic parameters. Then, empirical Bayes estimates of the individual pharmacokinetic parameters were obtained and the effect of the covariates on the interindividual random effects was again graphically evaluated to ensure that no covariates with significant effects were left out of the model. In addition, the effect of concomitant medication including steroids, antiemetics (5HT3-inhibitors, metoclopramide and domperidone), azole antifungals, benzodiazepines, ciprofloxacin, and amphotericin B, on the population weighted residual (WRES) was evaluated. Then, the final model was identified and final parameters and their standard errors were estimated. Model diagnostics were evaluated to determine the goodness of fit of the model to the combined data set.
Model-based pharmacokinetic simulations
The objective of the model-based simulations was three-fold: i) to compare the plasma tipifarnib concentration vs. time profiles in healthy and cancer subjects receiving a solid formulation, ii) to evaluate the effect of solid and liquid formulations on tipifarnib pharmacokinetic profiles in cancer subjects, and iii) to assess the potential clinical relevance of identified covariate effects (such as body weight) on tipifarnib pharmacokinetics in cancer patients receiving the solid formulation after food.
Based on the final estimates of the model parameters obtained from the combined data set, the tipifarnib pharmacokinetic profiles after multiple oral doses of 600 mg twice daily were simulated for healthy subjects (n = 3000) and cancer patients receiving solid (n = 3000) and liquid (n = 3000) formulations after food. For each data set, the covariates of interest were obtained by resampling from the subject covariates available in the combined data set.
To evaluate the results of the simulation, the population median and 80% prediction interval of the simulated plasma tipifarnib concentration vs. time profiles after multiple doses were plotted together.
Results
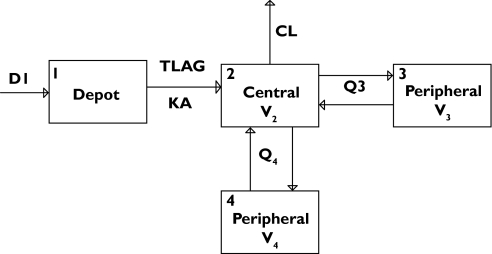
Two- and three-compartment disposition models with linear elimination from central compartments and absorption models of varying complexities were tested sequentially. A three-compartment disposition model (MVOF = −769.383) provided a substantially better fit to the index data set than a two-compartment model (MVOF = −114.998). The goodness of fit was further improved by inclusion of zero-order input into the depot compartment followed by first-order absorption from the depot compartment to the systemic circulation (MVOF = −966.211). Substantial further improvement of the fit was achieved by including an absorption lag time (MVOF = −1855.191). Model selection criteria were also in agreement with Akaike Information Criteria (AIC). A schematic representation of the model is presented in Figure 1. Diagnostic plots showed random, uniform scatter around the line of identity and indicate an absence of bias, whereas histograms of individual random effects on parameters showed approximately normal distribution.
Figure 1.
Schematics of the compartmental model used to describe plasma tipifarnib concentration-time profiles
The first iteration of covariate testing indicated a statistically significant effect of AST on systemic clearance (ΔMVOF = −9.611, d.f. = 1, P = 0.0019), of body weight (ΔMVOF = −36.006, d.f. = 1, P < 0.0001) and sex (ΔMVOF = −11.878, d.f. = 1, P = 0.0006) on central volume of distribution, and of body weight on absolute bioavailability (ΔMVOF = −8.766, d.f. = 1, P = 0.0031). The inclusion of these four covariates in the model decreased MVOF by 50.744 (d.f. = 4, P < 0.0001) relative to the reference model. The second iteration of covariate testing indicated an effect of total bilirubin on systemic clearance (ΔMVOF = −9.844, d.f. = 1, P = 0.0017) and of creatinine clearance on lag time (ΔMVOF = −7.811, d.f. = 1, P = 0.0052). The full model included all six covariate effects described and significantly improved the fit as compared with the reference model (ΔMVOF = −68.065, d.f. = 6, P < 0.0001).
Table 3 shows the results of the backward elimination process for the covariates retained in the model. The effect of body weight on central volume of distribution and total bilirubin concentration on systemic clearance were found to be significant and these covariates retained in the model. The power coefficient quantifying the effect of body weight on the central volume of distribution was not statistically different from 1 (ΔMVOF = −1.748, d.f. = 1, P = 0.186), and was therefore set to this value. In addition, the differences between healthy subjects and cancer patients were confirmed for all structural model parameters, except Q3, V3, tlag and F. Whereas there was no difference in the absorption of tipifarnib from either of the solid formulations, absorption was faster from the solution (associated with an increased duration of the zero-order input, D1 and KA) with a shorter lag time (tlag). The extent of absorption (F1) was similar for all formulations.
Table 3. Summary of covariate analysis: backward elimination.
| Model | Covariate effect | ΔMVOF | Degrees of freedom | P value |
|---|---|---|---|---|
| 10 | Healthy status on CL | −17.410 | 1 | – |
| 11 | Healthy status on V2 | −23.415 | 1 | <0.00001 |
| 12 | Healthy status on Q4 | −57.431 | 1 | <0.00001 |
| 13 | Healthy status on V4 | −50.314 | 1 | <0.00001 |
| 14 | Healthy status on D1 | −11.745 | 1 | 0.00061 |
| 15 | Healthy status on KA | −14.901 | 1 | 0.00011 |
| 16 | Liquid formulation on ALAG1 | −82.642 | 1 | <0.00001 |
| 17 | Liquid formulation on D1 | −42.831 | 1 | <0.00001 |
| 18 | Liquid formulation on KA | −36.299 | 1 | <0.00001 |
| 19 | WGT on V22 | −30.176 | 1 | <0.00001 |
| 20 | TBIL on CL | −9.894 | 1 | 0.00166 |
1Apart from the disease status and the formulation effect, the full model included the following covariate effects: AST and TBIL on CL, WGT and SEX on V2, WGT on F1 and CLCR on ALAG1
The power coefficient quantifying the effect of WGT on V2 was not statistically different from 1 (Δ MVOF = –1.748, d.f. = 1, P = 0.186). Δ MVOF Change in the minimum value of the objective function.
Absorption of tipifarnib was highly variable, with observed lag times ranging from negligible to greater than 2 h. To account for this, the subject population was assumed to consist of two subpopulations with different typical values of lag time. This difference was implemented using the mixture model, where each subject is assigned to one of the subpopulations and the estimated probabilities associated with each subpopulation are estimated. Individual values of the lag time were constrained to less than 6 h using the logit transformation as follows:
where Ttlag is the typical value of tlag, and the IIV and IOV within each subpopulation were assumed to be the same. Inclusion of this feature in the model resulted in a significant improvement in the MVOF (ΔMVOF =−46.344).
In order to prevent individual estimates of the absolute bioavailability being greater than 100%, the logit transformation was also applied to the absolute bioavailability, with a constraint ranging from 0 to 1. No major differences in parameter estimates were observed.
The implementation of a full variance-covariance matrix resulted in further improvement in the MVOF (ΔMVOF = −66.835). However, only the correlation between the interindividual random effects for CL and Q4, and between those for KA and tlag were found to be significant. Further hypothesis testing confirmed that both correlation coefficients were not different from 1, and therefore both were set to this value.
Diagnostic plots revealed good concordance between observed and predicted plasma concentrations of tipifarnib, and failed to detect any trend in cancer patients. The 10% trimmed relative errors for the 3445 observations in the index data set follow approximately a normal distribution with a mean (SD) of −0.52% (25.51%) and a range of −32.81% to +29.93%. The 10% trimmed relative errors for the 3849 observations in the test data set also follow approximately a normal distribution with a mean (SD) of −1.07% (25.59%) and a range of −30.77% to +27.35%. Overall, the model appeared to characterize adequately the pharmacokinetics of tipifarnib in a variety of different dosing conditions and populations.
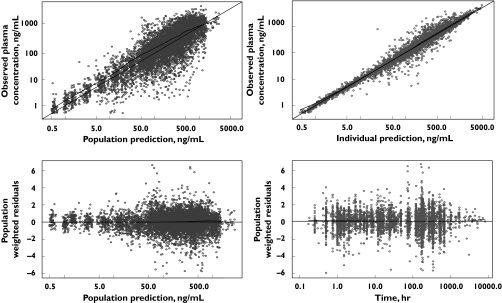
The qualified population pharmacokinetic model was fitted to the combined data set and two minor refinements were implemented to obtain the final model. First, the inclusion of the phase 2/3 data resulted in an increase in the magnitude of the residual error component for isolated measurements. This is a reasonable outcome considering that there is generally greater uncertainty about compliance and the accuracy of the timing of blood samples and drug administration in outpatient settings typical of phase 2/3 studies compared with the more controlled settings for phase 1 studies [17]. Therefore, an additional random effect parameter accounting for the ISM in phase 2/3 studies was included in the residual error model. Second, the zero-order input into the depot compartment was found to be similar between cancer patients and healthy subjects. As a consequence, a common parameter was estimated for both populations. Diagnostic plots showed tight uniform scatter around the line of identity and indicated an absence of bias (Figure 2) and histograms of random effects on structural parameters exhibited normal distribution. The final parameter estimates obtained for the combined data set are shown in Table 4.
Figure 2.
Diagnostic plots for the final model fit to the combined data set. The lines of identity and regression line (LOESS smoother)(bold) are presented
Table 4. Population pharmacokinetic model parameters for the tipifarnib: combined data set.
| Pharmacokinetic | Typical value | Ratio healthy subjects : | Between subjects | Variability(h) Within | |
|---|---|---|---|---|---|
| parameter | Cancer subjects* | Healthy subjects | cancer subjects* | (IIV,%)* | subjects (IOV,%)* |
| CL (l h−1)(a) | 21.9 (4.11) | 26.5 | 1.21 (6.06) | 24.9 (20.2) | 11.9 (35.3) |
| V2 (l 70 kg−1) | 54.9 (7.61) | 30.0 | 0.55 (31.8) | 20.3 (78.5) | – |
| Q3 (l h−1) | 4.11 (8.37) | 4.11 | – | 74.0 (30.7) | 51.0 (27.6) |
| V3 (l) | 92.4 (9.69) | 92.4 | – | 81.4 c | – |
| Q4 (l h−1) | 14.8 (18.6) | 131 | 8.83 (35.7) | 35.9d | – |
| V4 (l) | 21.4 (13.6) | 56.9 | 2.66 (20.7) | 24.3 e | – |
| D1 (h) | 1.20(i) (3.00) | 1.20 | – | 52.7 (30.1) | 72.7 (18.3) |
| KA (h−1) | 0.71j (7.41) | 1.63 | 2.31 (16.1) | 86.1 (24.4) | 71.8 (21.3) |
| Fabs (%) | 26.7 (4.23) | 26.7 | – | 0.74 (9.78)g | 0.32 (26.2)g |
| tlag (h)(b,k) | 1.24f,g | 2.32 (40.7)g | |||
| Subpopulation 1 | 0.11 (16.7) | 0.11 | – | ||
| Subpopulation 2 | 0.24 (14.1) | 0.24 | |||
Results expressed as parameter (RSE: relative standard error of parameter estimate,%).
Clearance normalized for a bilirubin of 9 µmol l−1. The normalization coefficient is equal to (TBIL/9)θ1, where TBIL is bilirubin (expressed as µmol l−1), and θ1 is −0.103 (RSE = 27.0%).
Proportion of patients in subpopulation 1 is 71.7 (RSE = 36.3%).
Correlation between IIV of Q3 and V3 set to 1. Expansion factor of V3 is 1.21 (RSE = 8.93%).
Correlation between IIV of CL and Q4 set to 1. Expansion factor of Q4 is 2.08 (RSE = 30.6%).
Correlation between IIV of CL and V4 set to 1. Expansion factor of V4 is 0.95 (RSE = 24.4%).
Correlation between IIV of KA and tlag set to 1. Expansion factor of tlag is 2.06 (RSE = 27.7%).
Expressed as standard deviation of the logit domain.
Residual variability, expressed as percentage: Full PK profiles: 24.5 (RSE = 9.52%). Isolated measurements of phase 1 studies: 43.8 (RSE = 18.5%). Isolated measurements of phase 2/3 studies: 72.3 (RSE = 11.3%).
D1 for solid formulation. 0.418 h for liquid formulation. Ratio liquid : solid 0.348 (RSE = 6.01%).
KA for solid formulation. 01.46 h−1for liquid formulation. Ratio liquid : solid 2.07 (RSE = 13.5%).
tlag for solid formulation. 0.019 h (Subpopulation 1) and 0.044 (Subpopulation 2) for liquid formulation. Ratio liquid : solid 0.183 (RSE = 25.8%). IIV = interindividual variability, IOV = interoccasion variability.
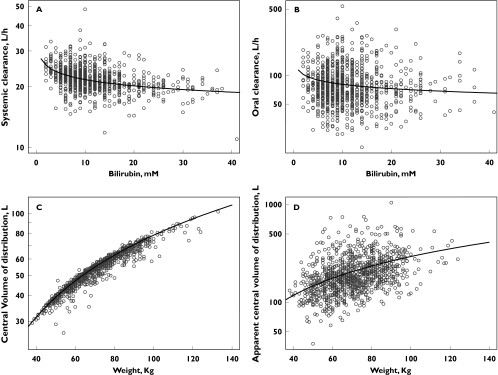
Empirical Bayes estimates of the individual pharmacokinetic parameters were obtained and the additional exploration of the parameter-covariate relationships did not reveal any additional significant associations. As body weight was a significant covariate on V2, other body size indices (lean body mass, ideal body weight and body surface area) were evaluated. None of these indices improved the fit and therefore body weight was retained in the final population pharmacokinetic model. The effects of body weight on the central volume of distribution and the apparent central volume of distribution are displayed in Figure 3, together with the effect of total serum bilirubin concentration on the systemic and oral clearance. No effect of comedication on tipifarnib pharmacokinetics was identified (Figure 4).
Figure 3.
Effect of total serum bilirubin on the systemic (A) and oral (B) clearance and effect of body weight on the central volume of distribution (C) and the apparent volume of distribution (D). The regression line represents the model prediction
Figure 4.
Box plots and statistical significance of the comparisons between weighted residuals (WRES) in the presence and absence of concomitant medications
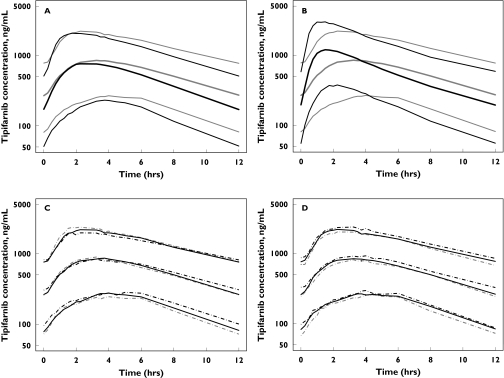
Simulated plasma concentration-time profiles after twice daily dosing with tipifarnib using the solid dosage formulation showed that cancer patients are expected to have slightly greater systemic exposure to tipifarnib relative to healthy subjects (Figure 5A). The simulations also showed that absorption of the solid dosage formulation is slower relative to the liquid form (Figure 5B). Plasma tipifarnib concentration vs. time profiles after the multiple dose regimen for subjects with body weights of <60 kg, 60–80 kg and >80 kg were similar (Figure 5C), as were profiles for subjects having bilirubin concentrations of <7.5 mm, 7.5–15 mm and >15 mm (Figure 5D).
Figure 5.
Simulated steady-state plasma concentration vs. time profiles of tipifarnib 600 mg twice daily in healthy ( ) and cancer (
) and cancer ( ) subjects receiving a solid formulation (A), in cancer subjects receiving solid (
) subjects receiving a solid formulation (A), in cancer subjects receiving solid ( ) and liquid formulations (
) and liquid formulations ( ) (B), and the effect of body weight <60 kg (
) (B), and the effect of body weight <60 kg ( ), 60–80 kg (
), 60–80 kg ( ), >80 kg (
), >80 kg ( ) (C) and bilirubin <7.5 mM (
) (C) and bilirubin <7.5 mM ( ), 7.5–15 mM (
), 7.5–15 mM ( ), >15 mM (
), >15 mM ( ) concentrations (D) in cancer subjects receiving a solid formulation. Lines represent 10 (lower), 50 (middle) and 90 (upper) quantiles of simulated plasma concentration
) concentrations (D) in cancer subjects receiving a solid formulation. Lines represent 10 (lower), 50 (middle) and 90 (upper) quantiles of simulated plasma concentration
Discussion
An open, three-compartment disposition model with linear elimination from the central compartment was used to describe the plasma pharmacokinetics of tipifarnib after intravenous administration. The initial rapid distribution half-life was about 36 min, followed by a dominant elimination half-life of about 2.4 h and a slower terminal half-life of about 19 h, with the latter phase constituting only a small portion of the overall area under the plasma concentration vs. time curve.
In adult cancer patients, the typical value of the estimated systemic clearance of tipifarnib was 21.9 l h−1, with low between and within subject variabilities of 24.9% and 11.9%, respectively. Total bilirubin concentrations in serum exhibited a statistically significant relationship with tipifarnib systemic clearance. A 6.9% decrease in tipifarnib systemic clearance was associated with a two-fold increase in total bilirubin concentration at baseline (Figure 3). The mechanism behind this relationship has not been identified but bilirubin concentrations at baseline may be an indirect biomarker of glucuronidation; as 14% of a dose of tipifarnib is excreted as its glucuronate metabolite, high bilirubin concentrations at baseline would reflect a decrease in the glucuronidation activity, and therefore, a decreased clearance. However, owing to the extent of the glucuronidation and the variability in oral bioavailability, a large overlap in simulated tipifarnib plasma concentrations-time profiles was observed for subpopulations representing a wide range of total bilirubin concentrations.
The relationship between the area under the concentration-time curve (AUC(0,24 h)) of tipifarnib and the incidence of neutropenia grade 3–4 has been described previously with a linear logistic regression [9]. According to this report, the probability of neutropenia grade ≥ 3 occurring in patients with solid tumours receiving tipifarnib 300 mg twice daily for 21 days of a 28-day cycle was predicted to be 14.5% for the median value of tipifarnib AUC 3.82 mg l−1 h, in subjects with bilirubin values between 7.5 and 15 mm. This result is consistent with the observed incidence of neutropenia grade ≥ 3 reported in the phase II study used to develop the present model [9]. As a comparison, simulations showed that patients with solid tumours receiving the same tipifarnib treatment and having bilirubin concentrations at baseline below 7.5 mm and above 15 mm would have a median tipifarnib AUC of 3.60 mg l−1 h and 4.15 mg l−1 h, which would be associated with a predicted probability of neutropenia grade ≥ 3 of 13.1% and 16.8%, respectively. Relative to patients with bilirubin baseline values between 7.5 and 15 mm, a 15% dose reduction would be needed for the 44 patients with bilirubin values at baseline higher than 15 mm to prevent one additional episode of neutropenia grade ≥ 3. In summary, total bilirubin concentration at baseline is a statistically significant determinant of tipifarnib systemic clearance, but this effect is expected to be of minimal clinical relevance in adult cancer patients. Therefore, dosage adjustments for tipifarnib on the basis of total bilirubin concentration at baseline are not warranted.
Systemic clearance was greater in healthy subjects (26.5 l h−1) relative to cancer patients (21.9 l h−1). This finding may be explained by differences in the binding of tipifarnib to α1-acid glycoprotein. Concentrations of this protein are reported to be higher in cancer patients than in healthy subjects [18]. Hence, the former would have less free drug in their plasma available for elimination. Similarly, increased α1-acid glycoprotein bound drug in cancer patients would account for the lower volume of distribution observed in this group.
The typical volume of the central compartment (between subject variability) in cancer subjects was estimated to be 54.9 l 70 kg−1 (20.3%), which is similar to the volume of total body water. As body weight is related to the amount of total body water, the volume of the central compartment would be expected to be directly proportional to body weight, as has been shown. A marked overlap in simulated plasma tipifarnib concentration-time profiles was observed in subpopulations representing a wide range of body weights. Simulations also showed that the effect of body weight on tipifarnib AUC is lower than that of total bilirubin concentration. Therefore, the effect of body weight on the central volume of distribution of tipifarnib would be expected to be of minimal clinical relevance.
In adult cancer patients, the volume of distribution at steady state (169 l) was three-fold higher than the central volume of distribution. A potential explanation for this observation is that tipifarnib accumulates in bone marrow [5] and distributes to other peripheral tissues. Differences in the volumes of various compartments were also observed between cancer patients and healthy subjects. However, the clinical relevance of this latter finding is questionable given the similarity in steady-state volume of distribution (179 l in healthy subjects vs. 169 l in cancer patients) and the substantial overlap observed in simulated plasma concentration-time profiles in healthy subjects and cancer patients. No definitive explanation for the difference in steady state volume of distribution could be found, although it may be due to variations in the sampling schemes across trials. Thus, during the first 30 min after the end of the infusion, samples were collected at 5, 10, 20 and 30 min from the healthy subjects (study 5), whereas only one sample at 15 min after the end of infusion was collected from the cancer patients (study 15).
Tipifarnib absorption was best described by a sequential zero-order input into the depot compartment, followed by first-order absorption from the depot compartment to the systemic circulation, after a lag time. Tipifarnib oral bioavailability did not differ between formulations. As expected, the absorption rate from the solution was faster than from the solid forms, as shown by the differences in KA, D1 and tlag. While the rate of oral absorption was more rapid in healthy subjects (1.63 h−1) as compared with cancer patients (0.71 h−1), no differences were apparent in the extent of absorption. Between- and within-subject variabilities in all absorption parameters were moderately large. The population pharmacokinetic parameters estimated in the current analysis for cancer patients receiving a solid formulation were very similar to those from a previously reported population pharmacokinetic analysis of phase 1 studies [19], where slight differences observed in the pharmacokinetics of tipifarnib between healthy subjects and cancer patients, together with an effect of tipifarnib formulation on the absorption profile, were also reported.
In summary, a population pharmacokinetic approach has been used to integrate tipifarnib pharmacokinetic data gathered during the clinical development and to characterize the pharmacokinetics of the drug. Individualized dosing with tipifarnib based on body weight or total bilirubin concentration in adult cancer patients is not warranted, as the dose is not expected to have a clinically relevant influence on intersubject variability in exposure to tipifarnib.
The authors would like to thank the hundreds of patients, investigators and their medical, nursing and laboratory staff who participated in the clinical studies included in the present study. In particular, we recognize the following lead principal investigators for each of the studies from which data were derived for the current study: KH Cowan and J Zujewski, Medicine Branch, National Cancer Institute, NIH, Bethesda, MD; J Schellens, The Netherlands Cancer Institute, Antoni Van Leeuwenhoek Hospital, Amsterdam, The Netherlands; SG Eckhardt, A Patnaik, E Rowinsky, Cancer Therapy and Research Center, San Antonio, TX; L Weiner, Fox Chase Cancer Center, Department of Medical Oncology, Philadelphia, PA; F Vanhoutte, Department of Clinical Pharmacology, Janssen Research Foundation, Beerse, Belgium; CJA Punt, University Hospital St. Radboud, Nijmegen, The Netherlands; A Wadham, Pharma BioResearch, Zuidlaren, The Netherlands; M Piccart-Gebhardt, Department of Chemotherapy, Institute Bordet, Brussels, Belgium; S Johnston, Department of Medicine, Royal Marsden Hospital, London, Great Britain; GR Hudes and R Greenberg, Fox Chase Cancer Center, Philadelphia, PA; B Johnson, Dana Farber Cancer Institute; Boston, MA; R DeVore, The Vanderbilt Clinic, Nashvile TN; D Cunningham, Royal Marsden Hospital, London, Great Britain; E Van Cutsem, UZ Gasthuisburg, Leuven, Belgium; E Small, UCCF Mount Zion Cancer Center, San Francisco, CA; J Lancet, University of Rochester Medical Center, Rochester, NY, USA; JL Harousseau, Service Hématologie Clinique, CHU, Hôtel Dieu, Nantes, France.
We also thank Tom Verhaeghe for the bioanalytical determination of plasma concentrations of tipifarnib, Koen Jolling for NONMEM®database preparation, and Alain Thibault, Andrew Chow, and Paul Soons for reviewing the manuscript.
References
- 1.Caponigro F, Casale M, Bryce J. Farnesyl transferase inhibitors in clinical development. Expert Opin Invest Drugs. 2003;12:943–54. doi: 10.1517/13543784.12.6.943. [DOI] [PubMed] [Google Scholar]
- 2.End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, Venet M, Sanz G, Poignet H, Skrzat S, Devine A, Wouters W, Bowden C. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–7. [PubMed] [Google Scholar]
- 3.Zujewski J, Horak ID, Bol CJ, Woestenborghs R, Bowden C, End DW, Piotrovsky VK, Chiao J, Belly RT, Todd A, Kopp WC, Kohler DR, Chow C, Noone M, Hakim FT, Larkin G, Gress RE, Nussenblatt RB, Kremer AB, Cowan KH. Phase I and pharmacokinetic study of farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol. 2000;18:927–941. doi: 10.1200/JCO.2000.18.4.927. [DOI] [PubMed] [Google Scholar]
- 4.Crul M, de Klerk GJ, Swart M, van’t Veer LJ, de Jong D, Boerrigter L, Palmer PA, Bol CJ, Tan H, de Gast GC, Beijnen JH, Schellens JH. Phase I clinical and pharmacologic study of chronic oral administration of the farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol. 2002;20:2726–2735. doi: 10.1200/JCO.2002.09.116. [DOI] [PubMed] [Google Scholar]
- 5.Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K, Horak I, Tidwell ML, Liesveld J, Kottke TJ, Ange D, Buddharaju L, Gojo I, Highsmith WE, Belly RT, Hohl RJ, Rybak ME, Thibault A, Rosenblatt J. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase I clinical-laboratory correlative trial. Blood. 2001;97:3361–9. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 6.Punt CJA, van Maanen L, Bol CJJG, Seifert WF, Wagenet DJTh. Phase I and pharmacokinetic study of the orally administered farnesyl transferase inhibitor R115777 in patients with advanced solid tumors. Anti-Cancer Drugs. 2001;12:193–7. doi: 10.1097/00001813-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Crul M, de Klerk GJ, Swart M, Weiner L, Palmer PA, Bol CJ, Beijnen JH, Schellens JH. Evaluation of the bioequivalence of tablets and capsules containing the novel anticancer agent R115777 (Zarnestra) in patients with advanced solid tumors. Eur J Drug Metab Pharmacokinet. 2002;27:61–5. doi: 10.1007/BF03190407. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik A, Eckhardt SG, Izbicka E, Tolcher AA, Hammond LA, Takimoto CH, Schwartz G, McCreery H, Goetz A, Mori M, Terada K, Gentner L, Rybak ME, Richards H, Zhang S, Rowinsky EK. A phase I, pharmacokinetic, and biological study of the farnesyltransferase inhibitor tipifarnib in combination with gemcitabine in patients with advanced malignancies. Clin Cancer Res. 2003;9:4761–71. [PubMed] [Google Scholar]
- 9.Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, Salter J, Michiels B, Perez-Ruixo JJ, Palmer P, Howes A. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol. 2003;21:2492–9. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Heymach JV, Johnson DH, Khuri FR, Safran H, Schlabach LL, Yunus F, DeVore RF 3rd, De Porre PM, Richards HM, Jia X, Zhang S, Johnson BE. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann Oncol. 2004;15:1187–93. doi: 10.1093/annonc/mdh315. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg JE, von der Maase H, Seigne JD, Mardiak J, Vaughn DJ, Moore M, Sahasrabudhe D, Palmer PA, Perez-Ruixo JJ, Small EJ. A phase II trial of R115777, an oral farnesyl transferase inhibitor, in patients with advanced urothelial tract transitional cell carcinoma. Cancer. 2005;103:2035–41. doi: 10.1002/cncr.21023. [DOI] [PubMed] [Google Scholar]
- 12.Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, Kourteva G, Iveson T, Andre T, Dostalova J, Illes A, Belly R, Perez-Ruixo JJ, Park YC, Palmer PA. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22:3950–7. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–8. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 14.Boeckman A, Sheiner L, Beal S. San Francisco: University of California at San Francisco; 1992–99. NONMEM Users Guides. [Google Scholar]
- 15.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–50. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 16.Wählby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS Pharmsci. 2002;4:E27. doi: 10.1208/ps040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frame B, Koup J, Miller R, Lalonde R. Population pharmacokinetics of clinafloxacin in healthy volunteers and patients with infections. Experience with heterogenous pharmacokinetic data. Clin Pharmacokinet. 2001;40:307–15. doi: 10.2165/00003088-200140040-00006. [DOI] [PubMed] [Google Scholar]
- 18.Israili ZH, Dayton PG. Human alpha-1–glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Ruixo JJ, Piotrovsky V, Cowan KH, Weiner L, Punt CJA, Piccart M. Population pharmacokinetics of Zarnestra® using data from phase I trials. IX meeting of Population Approach Group in Europe. 2002. ( http://userpage.fu-berlin.de/~page/) (Abstract 21)