Abstract
Aim
To develop an appropriate dosing strategy for continuous intravenous infusions (CII) of enoxaparin by minimizing the percentage of steady-state anti-Xa concentration (Css) outside the therapeutic range of 0.5–1.2 IU ml−1.
Methods
A nonlinear mixed effects model was developed with NONMEM® for 48 adult patients who received CII of enoxaparin with infusion durations that ranged from 8 to 894 h at rates between 100 and 1600 IU h−1. Three hundred and sixty-three anti-Xa concentration measurements were available from patients who received CII. These were combined with 309 anti-Xa concentrations from 35 patients who received subcutaneous enoxaparin. The effects of age, body size, height, sex, creatinine clearance (CrCL) and patient location [intensive care unit (ICU) or general medical unit] on pharmacokinetic (PK) parameters were evaluated. Monte Carlo simulations were used to (i) evaluate covariate effects on Css and (ii) compare the impact of different infusion rates on predicted Css. The best dose was selected based on the highest probability that the Css achieved would lie within the therapeutic range.
Results
A two-compartment linear model with additive and proportional residual error for general medical unit patients and only a proportional error for patients in ICU provided the best description of the data. Both CrCL and weight were found to affect significantly clearance and volume of distribution of the central compartment, respectively. Simulations suggested that the best doses for patients in the ICU setting were 50 IU kg−1 per 12 h (4.2 IU kg−1 h−1) if CrCL <30 ml min−1; 60 IU kg−1 per 12 h (5.0 IU kg−1 h−1) if CrCL was 30–50 ml min−1; and 70 IU kg−1 per 12 h (5.8 IU kg−1 h−1) if CrCL >50 ml min−1. The best doses for patients in the general medical unit were 60 IU kg−1 per 12 h (5.0 IU kg−1 h−1) if CrCL <30 ml min−1; 70 IU kg−1 per 12 h (5.8 IU kg−1 h−1) if CrCL was 30–50 ml min−1; and 100 IU kg−1 per 12 h (8.3 IU kg−1 h−1) if CrCL >50 ml min−1. These best doses were selected based on providing the lowest equal probability of either being above or below the therapeutic range and the highest probability that the Css achieved would lie within the therapeutic range.
Conclusions
The dose of enoxaparin should be individualized to the patients’ renal function and weight. There is some evidence to support slightly lower doses of CII enoxaparin in patients in the ICU setting.
Keywords: creatinine clearance, enoxaparin, NONMEM, pharmacokinetics
Introduction
Venous thromboembolism is a common cause of morbidity and mortality. Low-molecular-weight heparins (LMWHs) are as effective and safe as unfractionated heparin (UFH) for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) [1–4]. LMWHs are also superior to and as safe as UFH for acute coronary syndromes [5–7]. When compared with UFH, LMWHs have superior bioavailability [8], a more predictable anticoagulation response and a lower incidence of heparin-induced thrombocytopenia and osteoporosis with long-term treatment [9].
Enoxaparin is one of the most widely used LMWHs in Europe and the USA [10, 11], with anti-Xa activity widely used as a marker of enoxaparin concentration [12–14]. It is eliminated predominantly by the kidney [15]. Studies suggest that renal dysfunction leads to increased anti-Xa concentrations [12, 16, 17], which in turn is associated with bleeding complications. Therefore, dosage adjustment based on renal function is suggested to decrease the risk of adverse bleeding events [18–20].
Compared with general medical unit patients, critically ill patients have more medical complications due to premorbid and surgical conditions, invasive treatments and prolonged immobility [21]. Cook et al. [22] found that intensive care unit (ICU) patients with multiple predisposing factors have a high risk of venous thromboembolism and PE, which may result in a higher risk of mortality. Moreover, a range of organ dysfunction in ICU patients may result in more variable exposure to drugs and thus response [23]. Investigators at the University of Buffalo [24] have observed substantial variability in anti-Xa concentrations measured in multiple trauma critically ill patients. Unreliable and extensive variable anti-Xa concentrations were found in these trauma critically ill patients when the standard recommended dose and route of administration [subcutaneous (s.c.)] of enoxaparin for the prevention of venous thromboembolism was applied. This has led the group to examine alternative means of administration (intravenous infusion) to attempt to reduce variability in the observed anti-Xa concentrations after enoxaparin administration in trauma critically ill populations. Under the circumstance where patients were reported to have substantial variability [24] (e.g. ICU patients) in the observed anti-Xa concentrations with s.c. enoxaparin, intravenous infusion/continuous intravenous infusion (CII) could be utilized as a possible approach to reducing the variability. Therefore, it is desirable to attempt to understand these factors and attempt to control exposure to drug more closely.
The modelling and simulation work presented here represents a pilot examination of enoxaparin administered via CII and provides a first look at the nature of the interindividual variability (including covariate examination) for this method of administration.
Dosing strategy and extensive population pharmacokinetic analysis for patients receiving enoxaparin by CII has not been reported in the literature. The purpose of this study was to describe the pharmacokinetics (PK) for CII enoxaparin by developing a population PK model. This model was then used to guide a dosing strategy for CII enoxaparin.
Subjects and methods
Subjects
Anti-Xa concentrations were available from two studies. Patient characteristics for the two studies are shown in Table 1. The first study was conducted at the Cleveland Clinic Foundation [25]. In the CII study, patients who received enoxaparin from January 1997 to December 1998 were identified and a retrospective chart review was completed subsequent to institutional review board approval. The study provided 48 patients (23 male) with 363 anti-Xa concentrations with an average (mean ± SD) age and weight of 60.3 ± 17.7 years, 73.9 ± 14.6 kg, respectively. Patients were located in both the general medical unit (n = 29) and ICU (n = 19) and initially received enoxaparin 100 IU kg−1 per 12 h (8.3 IU kg−1 h−1) by CII. Routine monitoring of anti-Xa concentration was determined by chromogenic assay of LMWHs [26].
Table 1.
Patient characteristics for the two studies
| Demographics | S.c. General medical unit | CII General medical unit | ICU | Combined |
|---|---|---|---|---|
| Sample size | 35 | 29 | 19 | 83 |
| Age (years) | 75.1 | 60.9 | 59.3 | 66.6 |
| (44–86) | (16–90) | (23–77) | (16–90) | |
| Weight (kg) | 67.7 | 74.1 | 73.3 | 71.0 |
| (32–95) | (46.5–108) | (46.5–97.5) | (32–108) | |
| Height (cm) | 164.0 | 168.7 | 166.2 | 166.0 |
| (147–184) | (152–182) | (151–177) | (147–184) | |
| Gender (male/female) | 17/18 | 16/13 | 7/12 | 40/43 |
| CrCL (ml min−1) | 39.2 | 63.5* | 26.8 | 45.0 |
| (14.9–95.7) | (31.1–128.3) | (7.6–49.6) | (7.6–128.3) |
S.c., Subcutaneous; CII, continuous intravenous infusion; CrCL, creatinine clearance.
Twenty-seven patients in the CII study did not have a serum creatinine (SCr) concentration measurement.
The second study, reported by Green et al., provided detailed subject information for the s.c. use of enoxaparin [20]. The study included 35 patients with 309 anti-Xa concentrations. The patients’ age, weight and creatinine clearance (CrCL) were (mean ± SD): 75.1 ± 10.5 years, 67.7 ± 15.5 kg, 39.2 ± 21.6 ml min−1, respectively.
The Brater equation [27] was used to calculate the CrCL for individuals with unstable serum creatinine (SCr) in the CII study when two SCr concentrations measured over 12 h apart were different by more than 0.2 mg dl−1. CrCL for individuals with stable SCr concentrations was calculated using the Cockcroft and Gault (CG) equation in the CII and s.c. study, using ideal body weight (IBW) as a body size descriptor [28].
Population PK analysis
The population PK analysis for the combined dataset was performed by using NONMEM® (version V; GloboMax, Hanover, MD, USA) [29] with the subroutine ADVAN4, TRANS4. The first order conditional estimation with interaction (FOCEI) method was used to estimate parameters.
The likelihood ratio test was used to discriminate between alternative models. An objective function decrease of 3.84 units was considered significant (χ2P < 0.05, d.f. = 1). The covariates age, height, sex, CrCL and body size [total body weight (weight), body surface area (BSA), body mass index (BMI), IBW, lean body weight (LBW), adjusted body weight (ABW) and percent ideal body weight (%IBW) [13, 30] were introduced into each parameter one by one. The continuous covariate weight on clearance (CL) was incorporated into the model in several ways. These are shown below:
TVCL is the typical value for the population and ηi is the random effect representing the difference of the ith patient from the population mean. The random effects of between-subject variability were assumed to be log-normally distributed, with a mean of zero and standard deviation of ω. Weight is the total body weight in kg and Medweight is the median total body weight. Weight and other body size descriptors were included in the analysis to help examine whether the departure from the normal body size affected disposition.
CrCL (creatinine clearance in l h−1) was included in CL as below:
The nonrenal component of clearance (θ1) was evaluated in this model as a fixed parameter (0.229) reported by Green et al.[20] as well as being directly estimated by NONMEM. If CrCL was missing, then TVCL =θmissing was used. A sensitivity analysis was used to evaluate the impact on the other parameter estimates if θ1 was fixed. The reported parameter estimates for θNR (nonrenal clearance component) and θCrCL (renal component clearance) were 0.229 and 0.681, respectively, in the literature [20]. To assess how the previously published parameters (see above) would impact on the analysis, θNR was fixed to the published value of 0.229. The fixed value for θNR was then changed in 10% increments over a range of ±50% to assess whether or not this affected the other parameter estimates.
Residual variability was modelled using additive, proportional and combined error structures.
Graphical assessment of Bayesian individual parameter estimates vs. covariates was performed to help identify possible covariate relationships. Covariates were retained in the model if inclusion in the model decreased the objective function value (OFV) by 3.84 (χ2P < 0.05, d.f. = 1). The model improvement was assessed by the OFV values and parameter estimates. In addition, the significance of the covariates was assessed using a randomization test with Wings for NONMEM [31, 32]. This approach provided a calibration for the changes in OFV vs. P-value for determination of statistical significance. In addition, graphics of goodness of fit were utilized to assess model robustness [33].
Simulation of steady-state anti-Xa concentration
Two types of simulation were performed; the first was a deterministic simulation which assessed the impact of covariate effects on predicted Css. Anti-Xa concentrations were simulated using mean model parameters obtained from the final covariate model with random effects fixed to zero. This was done to evaluate more clearly the covariate effect on Css. The calculation of Css is shown below:
| (1) |
The second simulation set used a Monte Carlo approach [34–36] to identify an appropriate dose for CII enoxaparin. The final covariate model was used as the input–output model to predict concentrations. The final model and parameter estimates obtained from the final model were used for the Monte Carlo simulations. The distribution of PK parameters was set to a log-normal distribution. Simulations were conducted to compare the percentage of the predicted Css values that were outside of the therapeutic range for the general medical unit and ICU patients receiving enoxaparin at infusion rates of 8.3, 5.8, 5.0 and 4.2 IU kg−1 h−1. The lowest infusion rate (4.2 IU kg−1 h−1) was selected based on the best dose suggested by Green et al. [20] for renal dysfunction patients receiving s.c. enoxaparin. The highest infusion rate (8.3 IU kg−1 h−1) is the current dosing strategy of enoxaparin administrated by s.c. administration. A unique covariate distribution model was developed for general medical unit and ICU patients. The model constituted a joint distribution of weight and CrCL based on the ICU and the general medical unit patients in CII study. The correlation of weight and CrCL in the covariate distribution model was 0.33 for general medical unit patients and 0.30 for ICU patients in the CII study [25]. One thousand general medical unit patients and 1000 ICU patients were simulated from the joint distribution model. Two hundred simulations of 2000 patients were performed for each infusion rate using NONMEM®. For twice-daily s.c. administration, the therapeutic range of anti-Xa is 0.5–1.2 IU ml−1[26, 37, 38, 39, 40, 41]. This therapeutic range was applied as the target range for dose selection in simulation study for CII. The percentage of predicted Css which was >1.2 IU ml−1 or which was <0.5 IU ml−1 was calculated for each simulation using code written by the researchers in True-BASIC® (developed in 1965 by J. Kemeny & T. E. Kurtz). The mean, 5th and 95th percentiles [90% predicted interval (PI)] were calculated from 200 simulations for the percent of predicted Css falling out of therapeutic range at each infusion rate. The patients were classified into three categories (CrCL <30 ml min−1; CrCL 30–50 ml min−1; CrCL >50 ml min−1) prior to the simulation study, which was based on the severity of kidney impairment. These probabilities were then calculated for patients with varying degrees of renal function (CrCL <30 ml min−1; CrCL 30–50 ml min−1; CrCL >50 ml min−1) and the percentile of the mean, 5th and 95th (90% PI) is represented graphically. The best dosing regimens were selected based on the highest probability that the achieved concentrations would fall within the desired therapeutic range.
Results
Patient characteristics
Eight patients in the CII study had unstable SCr; three of them were general medical unit patients and five were ICU patients. The CrCL for 27 patients in the CII study was unavailable. The duration of infusion for the 48 patients ranged from 8 to 894 h (138 ± 158 h) and infusion rates ranged from 100 to 1600 IU h−1 (500 ± 210 IU h−1).
Population PK modelling
A two-compartment linear model with exponential interindividual variability on CL and volume of distribution of central compartment (V2) adequately described the data. The basic PK parameters of CL, V2 and volume of distribution of peripheral compartment (V3), absolute bioavailability (F1) and absorption rate constant Ka (for the s.c. study) are shown in Table 2. The residual error model accounted for differences in the residual error variance between the general medical unit and ICU patients. The residual error model was a combined additive and proportional model for general medical unit patients and proportional only for ICU patients. Allowing the residual error variance to partition based on location of the patient improved the OFV by 62.6 units (P< 0.005).
Table 2.
Pharmacokinetic parameter estimates for the two-compartment model
| Parameters | Base model | SE% | Parameters | Final model | SE% |
|---|---|---|---|---|---|
| CL (l h−1) | 0.693 | 9.5 | CLmissing (l h−1) | 0.972 | 10.5 |
| θNR | N/A | N/A | θNR (l h−1) | 0.229 | N/A |
| θCrCL | N/A | N/A | θCrCL (1/4.8 CrCL) | 0.744 | 18.7 |
| V2 (l) | 7.07 | 22.5 | V2 (l per 70 kg weight) | 6.78 | 19.2 |
| V3 (l) | 5.99 | 25.4 | V3 (l) | 6.19 | 24.9 |
| Q (l h−1) | 0.494 | 27.9 | Q (lh−1) | 0.429 | 24.7 |
| Ka (h−1) | 0.428 | 29.0 | Ka (h−1) | 0.476 | 27.3 |
| F1 | 1 | 1.1 | F1 | 0.94 | 9.7 |
| ωcl% | 65.5 | 44.1 | ωcl% | 40.7 | 23.8 |
| ωv2% | 61.9 | 31.3 | ωv2% | 29.4 | 82.2 |
| σ1% | 22.6 | 28.4 | σ1% | 12.1 | 100 |
| σ2 (IU l−1) | 75.4 | 36.6 | σ2 (IU l−1) | 132 | 44.7 |
| σ3% | 43.1 | 26.0 | σ3% | 44.0 | 26.3 |
CL, Clearance; CrCL, creatinine clearance; IU, international units; SE, standard error; weight, total body weight; V2, volume of distribution of central compartment; V3, volume of distribution of peripheral compartment; ω, coefficient of variation of interindividual variability; σ1, proportional coefficient of variation of residual error for general medical unit patients; σ2, additive coefficient of variation of residual error for general medical unit patients; σ3, proportional coefficient of variation of residual error for ICU patients; N/A, not available; θNR, 0.229 (fixed); unit of weight = kg, unit of CrCL = l h−1; F1, absolute bioavailability.
The best residual error was described by the equations
where IPREDij represents the jth predicted concentration for the ith individual, Yij is the observed anti-Xa concentration, and ɛ are the independent and identically distributed normal distribution random effects with normal distribution with a mean zero and SD σ. ɛ1 and ɛ3 are the proportional component and ɛ2 is the additive component.
Visual inspection of individual empirical Bayes estimates of clearance showed a systematic change with CrCL. Thus CrCL was chosen for inclusion in the model, as below:
The θNR and θCrCL are nonrenal and renal clearance components, respectively [20]. The reported parameter estimates for θNR and θCrCL were 0.229 and 0.681, respectively, in the literature [20]. From the sensitivity analysis, the CV% of all other parameter estimates, including mean parameter estimates (CV% 0.4–2.5%), interindividual [CV% 3.4% (ωcl); 3.0% (ωv2)] and intraindividual variability [CV% 0.1% (σ1); 0.2% (σ2); 1.2% (σ3)], was <10% as a result of changing the value of θNR with one exception. θCrCL, which is correlated with the θNR value, had a larger change in value (CV% 19%) than all the other parameters in the analysis. However, the CV% of total CL estimates was <10%, which may explain the compensatory change of θCrCL with θNR value. Therefore, fixing θNR to 0.229 did not affect the estimation of other parameters (mean parameter estimates, inter- and intraindividual variability), based on the sensitivity analysis. We left this value fixed at 0.229 as it was estimated under a much more robust experimental design and thus more likely to be an accurate reflection of nonrenal clearance [20].
CrCL was the most significant covariate on CL (ΔOFV = −10.1; P < 0.005). Weight was the most significant covariate on V 2 (ΔOFV = −11.8; P < 0.005). After incorporating the effect of CrCL on CL, weight was the most significant covariate on V 2 (ΔOFV =−21.56; P < 0.005). The final model included CrCL on CL and weight on V 2. The critical values of the ΔOFV, according to the randomization test, to accept CrCL and weight were 2.6 and 2.3, respectively. The final model for CL and V2 was therefore:
where θ denotes the fixed effects, η denotes random effects with log normal distribution with zero mean and SD ω, 0.229 (l h−1) is the fixed value for nonrenal clearance component, 80 ml min−1 (4.8 l h−1) is considered as the cut-off value for normal renal clearance [42, 43].
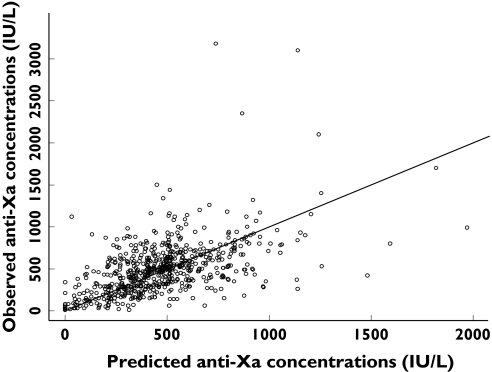
The final PK parameter estimates are shown in Table 2. Observed vs. population predicted anti-Xa concentrations are shown in Figure 1. ICU patients had an approximately twofold higher proportional residual variability than those general medical unit patients. Interindividual variability of CL and V2 decreased by 38% and 53%, respectively, in the covariate model compared with the base model.
Figure 1.
Observed vs. population predicted anti-Xa concentrations for the two-compartment model with CrCL and weight covariates in the model. Individual data points are shown as dots and the unity as a solid line
Upon inspection, ICU patients had a lower CL (0.79 ± 0.40 l h−1) than general medical unit patients (0.99 ± 0.39 l h−1) receiving CII enoxaparin. This is consistent with our previous results [25, 44]. The individual dosage adjustment was calculated using individual estimates from NONMEM®. To achieve a target of 0.5 IU ml−1 anti-Xa concentration, the infusion rates for typical ICU and general medical unit patients with weight of 70 kg were 5.6 ± 2.7 IU kg−1 h−1 and 7.0 ± 2.7 IU kg−1 h−1, respectively.
Simulation of steady-state anti-Xa concentrations
Assessing significant covariates that affect anti-Xa concentrations
Since weight and CrCL were significant covariates for PK parameters, simulations were applied to evaluate their impact on target anti-Xa concentration at steady state with weights varying from 30 to 120 kg and CrCL varying from 10 to 120 ml min−1. Steady-state anti-Xa concentrations were simulated using a two-compartment model with parameters fixed to the final parameters under the covariate model and all random effects defined to zero.
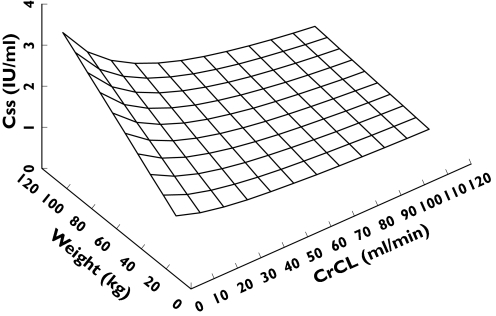
The anti-Xa concentration at steady state was calculated using Equation 1. The effect of weight and CrCL on Css when administering enoxaparin at a rate of 100 IU kg−1 per 12 h by CII is shown in Figure 2. Clearance increased from 0.6 to 0.9 l h−1 when CrCL increased from 30 to 80 ml min−1. As CrCL decreased and weight increased, predicted Css increased. This was particularly pronounced when CrCL was <30 ml min−1.
Figure 2.
Three-dimensional surface showing the relationship between CrCL, weight and predicted Css. The surface shows how the Css changes with both weight and CrCL simultaneously
Comparing the percent of predicted Css outside of therapeutic range at infusion rates of 8.3, 5.8, 5.0 and 4.2 IU kg−1 h−1
CrCL was simulated using the covariate distribution model. The distribution of the covariates in patients with simulated values was comparable to that of general medical unit and ICU patients in the CII study. The final PK model with covariates was used as the input–output model. The percent for a predicted Css >1.2 IU ml−1 or <0.5 IU ml−1 was calculated for each simulation when general medical unit and ICU patients received infusions at rates of 8.3, 5.8, 5.0 and 4.2 IU kg−1 h−1, respectively.
The percentage of predicted Css outside of the therapeutic range (mean, 5th and 95th percentiles) at each infusion rate for general medical unit and ICU patients is shown in Table 3. The percentage of predicted Css outside of the therapeutic range at each infusion rate for these subjects with different renal function is shown in Table 4. For both general medical unit and ICU patients, when the infusion rate decreased, the percentages of the predicted Css that were >1.2 IU ml−1 decreased and the percentages of the predicted Css that were <0.5 IU ml−1 increased (Figures 3a, b and 4a, b). General medical unit patients achieved the lowest total percentage (with an equal probability of being either above or below the therapeutic range) of the predicted Css falling outside of therapeutic range at an infusion rate of 8.3 IU kg−1 h−1, while ICU patients achieved the lowest total percent at 4.2 IU kg−1 h−1.
Table 3.
Percent of predicted anti-Xa Css >1.2 IU ml−1 or percent of predicted anti-Xa Css <0.5 IU ml−1 when general medical unit and ICU patients receive enoxaparin at different infusion rates of 8.3, 5.8, 5.0 and 4.2 IU kg−1 h−1
| General medical unit patients | ICU patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent of Css < 0.5 IU ml−1 | Percent of Css >1.2 IU ml−1 | Percent of Css < 0.5 IU ml−1 | Percent of Css >1.2 IU ml−1 | |||||
| Infusion rate (IU kg−1 h−1) | Mean | 90% PI | Mean | 90% PI | Mean | 90% PI | Mean | 90% PI |
| 8.3 | 18.1 | 16.7–20.7 | 33.9 | 31.3–36.1 | 5.8 | 4.5–6.8 | 61.1 | 59.4–63.9 |
| 5.8 | 35.8 | 33.8–38.2 | 17.0 | 15.0–19.0 | 13.8 | 12.5–15.1 | 41.4 | 40.4–43.5 |
| 5.0 | 44.9 | 42.5–47.1 | 11.9 | 10.3–13.5 | 20.5 | 19.2–22.2 | 31.0 | 30.3–33.3 |
| 4.2 | 55.2 | 52.5–57.5 | 7.50 | 6.40–8.70 | 28.3 | 26.5–29.8 | 22.7 | 21.2–24.4 |
ICU, Intensive care unit; Css, steady-state anti-Xa concentration; PI, predicted interval.
Table 4.
Percent of predicted anti-Xa Css >1.2 IU ml−1 or percent of predicted anti-Xa Css <0.5 IU ml−1 when general medical unit and ICU patients receive enoxaparin at different infusion rates of 8.3, 5.8, 5.0 and 4.2 IU kg−1 h−1 for subjects in each renal function group
| General medical unit patients | ICU patients | ||||
|---|---|---|---|---|---|
| Infusion rate (IU kg−1 h−1) | CrCL (ml min−1) | Mean % of Css < 0.5 IU ml−1 | Mean % of Css > 1.2 IU ml−1 | Mean % of Css < 0.5 IU ml−1 | Mean % of Css > 1.2 IU ml−1 |
| 8.3 | < 30 | 6.74 | 54.4 | 4.1 | 65.8 |
| 30–50 | 11.9 | 42.7 | 7.4 | 55.5 | |
| > 50 | 22.4 | 28 | 13.3 | 44 | |
| 5.8 | < 30 | 16.6 | 32.3 | 10.97 | 46.1 |
| 30–50 | 28.2 | 21.6 | 16.8 | 36.2 | |
| > 50 | 43.8 | 11.6 | 25.8 | 22.9 | |
| 5.0 | < 30 | 23.7 | 24.3 | 16.8 | 35.1 |
| 30–50 | 36.8 | 15.3 | 24.2 | 26.4 | |
| > 50 | 53.3 | 7.59 | 36.8 | 14.5 | |
| 4.2 | < 30 | 32.8 | 16.3 | 24.1 | 26.4 |
| 30–50 | 47.4 | 9.93 | 32.4 | 18.4 | |
| > 50 | 63.8 | 4.47 | 48.0 | 9.13 | |
ICU, Intensive care unit; Css, steady-state anti-Xa concentration.
Figure 3.
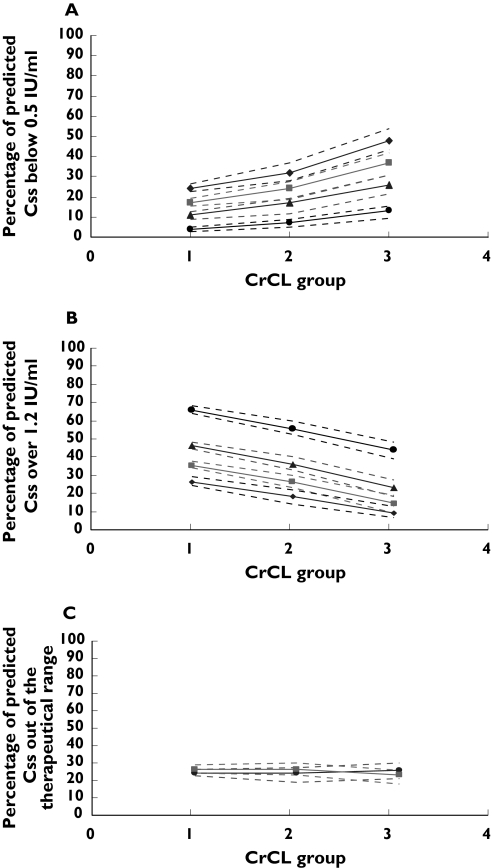
The percentage of predicted Css falling out of therapeutic range at different infusion rates (8.3, 5.8, 5.0, 4.2 IU kg−1 h−1) for intensive care unit patients with different renal function (1, CrCL <30 ml min−1; 2, CrCL 30–50 ml min−1; 3, CrCL >50 ml min−1). Dashed lines represent the 5th and 95th percentiles (90% PI). (a) Percentage of predicted Css which is <0.5 IU ml−1. ♦, 4.2 IU/kg/h; ▪, 5.0 IU/kg/h; ▪, 5.8 IU/kg/h; •, 8.3 IU/kh/h. (b) Percentage of predicted Css which is >1.2 IU ml−1. ♦, 4.2 IU kg−1 h−1; ▪, 5.0 IU kg−1 h−1; ▴, 5.8 IU kg−1 h−1; •, 8.3 IU kg−1 h−1. (c) Percentage of predicted Css falling out of therapeutic range (0.5–1.2 IU ml−1) when patients with CrCL <30 ml min−1 received enoxaparin at 4.2 IU kg−1 h−1 infusion rate, with CrCL between 30 and 50 ml min−1 received enoxaparin at 5.0 IU kg−1 h−1 infusion rate and with CrCL >50 ml min−1 received enoxaparin at 5.8 IU kg−1 h−1 infusion rate. ▪, >1.2 IU ml−1; •, <0.5 IU ml−1
Figure 4.
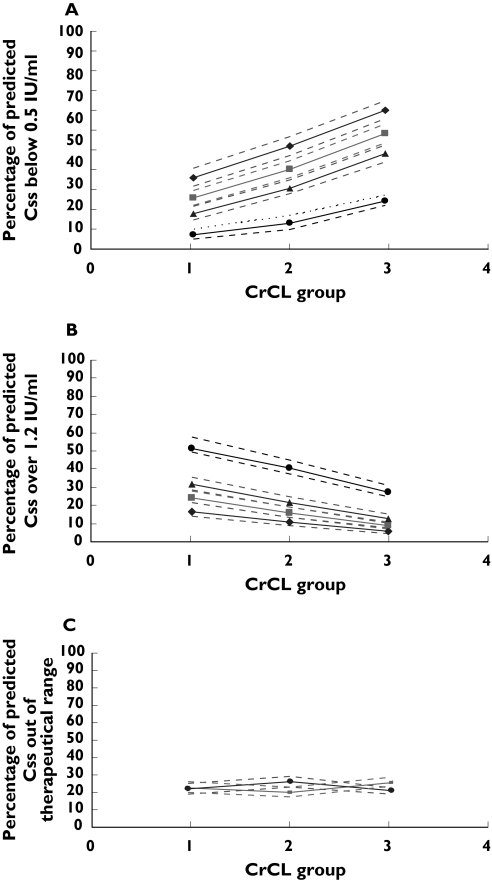
The percentage of predicted Css falling out of therapeutic range at different infusion rates (8.3, 5.8, 5.0, 4.20 IU kg−1 h−1) for general medical unit patients with different renal function (1, CrCL <30 ml min−1; 2, CrCL 30–50 ml min−1; 3, CrCL >50 ml min−1). Dashed lines represent the 5th and 95th percentiles (90% PI). (a) Percentage of predicted Css which is <0.5 IU ml−1. ♦, 4.2 IU/kg/h; ▪, 5.0 IU/kg/h; ▴, 5.8 IU/kg/h; •, 8.3 IU/kh/h. (b) Percentage of predicted Css which is >1.2 IU ml−1. ♦, 4.2 IU kg−1 h−1; ▪, 5.0 IU kg−1 h−1; m, 5.8 IU kg−1 h−1; •, 8.3 IU kg−1 h−1. (c) Percentage of predicted Css falling out of therapeutic range (0.5–1.2 IU ml−1) when patients with CrCL <30 ml min−1 received enoxaparin at 5.0 IU kg−1 h−1 infusion rate, with CrCL between 30 and 50 ml min−1 received enoxaparin at 5.8 IU kg−1 h−1 infusion rate and with CrCL >50 ml min−1 received enoxaparin at 8.3 IU kg−1 h−1 infusion rate. ▪, >1.2 IU ml−1; •, <0.5 IU ml−1
Figures 3 and 4 and Table 4 illustrate the percentage of patients’ predicted Css falling out of therapeutic range for ICU and general medical unit patients. These figures reflect that, given optimization of dosage to give an equal probability of being above or below the therapeutic range, general ward unit subjects achieved the lowest total percentage of Css falling outside of therapeutic range at infusion rates of 5.0 IU kg−1 h−1 if CrCL was <30 ml min−1, 5.8 IU kg−1 h−1 if CrCL was 30–50 ml min−1 and 8.3 IU kg−1 h−1 if CrCL was >80 ml min−1, while ICU subjects achieved the lowest total percentage of Css falling outside of therapeutic range at infusion rates of 4.2 IU kg−1 h−1 if CrCL was <30 ml min−1, 5.0 IU kg−1 h−1 if CrCL was 30–50 ml min−1 and 5.8 IU kg−1 h−1 if CrCL was >80 ml min−1. The difference between different dosing strategies is shown graphically in Figures 3a,b and 4a,b. If the current dosing guideline (100 IU kg−1 twice a day) of enoxaparin administrated subcutaneously was used for patients with CrCL <30 ml min−1 receiving CII, 64.6–68.1% of ICU patients and 52.1–60.9% of general medical unit patients would have an anti-Xa concentration of >1.2 IU ml−1 (Figures 3b and 4b). This can be reduced to 24.1–29.2% for ICU patients when dosing is decreased to 4.2 IU kg−1 h−1 and to 21.4–28.3% for general medical unit patients when the dosing is decreased to 5.0 IU kg−1 h−1. When using the revised dosing strategy, simulated ICU and general medical unit patients with a CrCL <30 ml min−1 experienced a 28% and 22% (Table 4) decrease in the percentage of the total predicted Css falling out of therapeutic range, respectively, when compared with the patients receiving 8.3 IU kg−1 h−1 of enoxaparin.
In some situations, the best dose selected based on the total percentage of Css outside of the therapeutic range was found to be indistinguishable from other doses (change of total percentage Css outside of the therapeutic range <10%). For example, if ICU patients with CrCL <30 ml min−1 received enoxaparin at an infusion rate of 5.0 IU kg−1 h−1, the total percent Css outside of therapeutic range was reduced by 3% compared with the situation when the best dose of 4.2 IU kg−1 h−1 was applied. This is also true for general medical unit patients with CrCL <30 ml min−1; the total percentage of Css falling outside of therapeutic range at an infusion rate of 5.0 IU kg−1 h−1 was 48% and became 49% at the rate of 5.8 IU kg−1 h−1. However, in the ‘best dose’ situations, patients have a similar probability of being either above or below the therapeutic range (Figures 3c and 4c). If the change of the total percentage Css outside of the therapeutic range was <10% when a dose other than best dose was applied, the dose was considered to be indistinguishable from the best doses suggested above. Thus, the range of dosages at each of the patient types were indicated, where the total probability of being outside the therapeutic range was indistinguishable: for general medical patients 4.2–5.8 IU kg−1 h−1 if CrCL was <30 ml min−1, 5.0–8.33 IU kg−1 h−1 if CrCL was 30–50 ml min−1 and 5.8–8.33 IU kg−1 h−1 if CrCL was >50 ml min−1; for ICU patients 4.2–5.0 IU kg−1 h−1 if CrCL was <30 ml min−1, 4.2–5.8 IU kg−1 h−1 if CrCL was 30–50 ml min−1 and 5.0–5.8 IU kg−1 h−1 if CrCL was >50 ml min−1. However, the clinician will have to consider the relative probability of above or below the range when tailoring the actual dose administered to the patient.
Discussion
Dosing strategies developed by many s.c. enoxaparin studies have been based on weight and renal function, which may help to reduce bleeding complications [14, 16, 17, 37] and these changes are amplified in complicated patient populations that are present in critically ill multiple trauma patients [24]. Highly variable and unreliable anti-Xa concentrations were observed when the standard dose of enoxaparin for prevention of venous thromboembolism was applied. In this study, the bioavailability estimation for general medical unit patients in the s.c. study was 0.94. Whether the extensive variability of anti-Xa concentrations in critically ill patients from the Haas et al. [24] study was due to the variable bioavailability for s.c. enoxaparin is unknown. Applying CII enoxaparin is one approach to evaluate this issue and may reduce the variability observed after s.c. administration in critically ill patients. This has led some investigators to begin examining the i.v. administration as continuous infusion of enoxaparin. Despite this, no extensive population pharmacokinetic analysis or dosing adjustment suggestions have been reported for enoxaparin given by CII. This is the first study to evaluate factors affecting anti-Xa concentrations following CII administration of enoxaparin. This information is used to develop a dosing guideline based on the percentage of the predicted steady-state anti-Xa concentrations falling out of the therapeutic range with CII using Monte Carlo simulations.
In previous population data analyses, combined datasets were used to help stabilize estimations [45]. In our study, combining additional data from the s.c. study with the CII data allowed us to better describe and characterize the PK parameters for CII. Compared with the CII data analysis alone, there was a 50% decrease of standard error of estimation for CL and V2 in the combined data analysis. Moreover, the interindividual variability of CL and V2 decreased 37% and 47%, respectively, compared with the CII data analysis alone [25].
Approximately half of the subjects in the CII study were from the ICU. This may contribute to additive PK complexity, as those patients were prone to have fluid shifts, organ dysfunction and drug binding alteration [21, 46]. Different PK parameters (CL) were found in ICU and general medical unit patients in this study and our previous report [25]. The different clearance between ICU and general medical unit patients has also been found by Priglinger et al.[23], where they demonstrated that s.c. administration of LMWH may not work well in critically ill patients due to different PK behaviour compared with general medical unit patients. Simulations suggest that infusion rates of 5.6 ± 2.7 IU kg−1 h−1 for ICU patients and of 7.0 ± 2.7 IU kg−1 h−1 for general medical unit patients were needed to achieve lower limit of therapeutic range of 0.5 IU ml−1 anti-Xa concentration. The model for ICU patients showed a higher proportional residual error than that from general medical unit patients. This may be a function of model misspecification in the highly dynamic ICU population compared with the more stable general medical unit patients.
Similar to previous reports of s.c. administration of enoxaparin [17, 20], this study has shown that enoxaparin CL increased with increasing CrCL. One study in 96 obese patients reported by Green et al. [13] demonstrated that LBW is a significant covariate on CL and weight on V2. After including CrCL on CL and weight on V2, no body size descriptor other than weight was found as a significant covariate on PK parameters. Green et al. [20] reported a series of recommended dosing regimens based on the glomerular filtration rate (GFR) estimated using CG equation, where a dose of 0.4 mg kg−1 per 12 h was suggested to subjects with GFR <30 ml min−1. A simulation study for CII administration found that CrCL had a higher impact on Css in patients with renal dysfunction (CrCL <30 ml min−1) than in patients with moderate renal impairment and normal renal function patients. Results from 200 simulations at each infusion rate (8.3, 5.8, 5.0, 4.2 IU kg−1 h−1) demonstrated that general medical unit patients achieved the lowest total percent of predicted Css outside of the therapeutic range at 8.3 IU kg−1 h−1 (90% PI 48.0–56.8%), while ICU patients achieved the lowest total percent at 4.2 IU kg−1 h−1 (90% PI 47.7–54.2%) (Table 3). Furthermore, if CrCL was <30 ml min−1 (renal dysfunction), the best doses for patients in the ICU and general medical unit were 4.2 IU kg−1 h−1 and 5.0 IU kg−1 h−1, respectively; 5.0 IU kg−1 h−1 and 5.8 IU kg−1 h−1, respectively, if CrCL was between 30 and 50 ml min−1 (moderate renal impairment). For ICU and general medical unit patients with CrCL >50 ml min−1, the best dose was 5.8 IU kg−1 h−1 and 8.3 IU kg−1 h−1, respectively (Table 4). Based on these results, most patients will achieve expected steady-state anti-Xa concentrations of between 0.5 IU ml−1 and 1.2 IU ml−1, if (i) ICU patients with CrCL >50 ml min−1 receive enoxaparin at 5.8 IU kg−1 h−1 and general medical unit patients with CrCL >50 ml min−1 receive enoxaparin at 8.3 IU kg−1 h−1 infusion rate; (ii) ICU patients with CrCL between 30 and 50 ml min−1 receive enoxaparin at 5.0 IU kg−1 h−1 and general medical unit patients with CrCL between 30 and 50 ml min−1 receive enoxaparin at 5.8 IU kg−1 h−1; and (iii) ICU patients with CrCL <30 ml min−1 receive enoxaparin at 4.2 IU kg−1 h−1 and medical unit patients with CrCL <30 ml min−1 receive enoxaparin at 5.0 IU kg−1 h−1. These best doses also represented the optimal solution where the probability of being above the therapeutic range is not different from that of being below the range (Figures 3c and 4c). Given different therapeutic risks in the clinic, it was felt that this would provide a starting point. The additional information on the total risk of being outside the therapeutic range can then be considered in concert with this information, tailoring to the patient with respect to whether or not it is worse for that patient to be above or below the range. Given the equal total probabilities of being outside the range for multiple dosage levels, we have provided a range of dosages where that total probability is indistinguishable across groups. This can be read from Table 4. However, the clinician will have to consider the relative probability of above or below the range when tailoring the actual dose administered to the patient.
CII administration of enoxaparin had been used in the treatment of acute PE [47, 48]. Patients with acute PE received an i.v. bolus of 0.5 mg kg−1 enoxaparin followed by an initial dosage of 2–3 mg kg−1 day−1 CII enoxaparin. Anti-Xa concentrations were measured daily. The dosage was adjusted to maintain the anti-Xa concentration between 0.2 and 0.6 IU ml−1[47, 48]. No deleterious haemorrhagic side-effects were found during the treatment of acute PE [48]. This might be due to the dosage adjustment by daily measurements of anti-Xa and anti-IIa concentrations, which lead to more constant levels of anticoagulation. The dosing adjustment recommended in this study can be applied when CII is used in clinical practice to patients with varying renal function, which is not yet available in the literature.
Unfortunately, the limitations of a retrospective study are the availability of documented data in a medical unit record review. Even with the electronic laboratory information, SCr concentrations were unavailable in 27 patients in the CII study. The need to evaluate SCr was at the discretion of the physician since this was an observational evaluation. We acknowledge the small sample size of patients with available SCr in the CII study and accounted for this by combining data in the PK analysis with additional patients from a second study. This approach has been discussed previously [45]. Combining datasets assisted in identifying CrCL as a significant covariate of CL [25].
Conclusion
This study has evaluated the pharmacokinetic profile and defined a dosage strategy for administering enoxaparin by continuous i.v. infusion in patients with varying renal function. CrCL was identified as a significant covariate on CL and total body weight on V2.
Acknowledgments
This work was financially supported by National Institute for Biomedical Imaging and Bioengineering Grant no. P41 EB001975-06 (R.R.B.); and supported in part by a grant from Aventis Pharma Australia Pty Ltd. Part of the work has been presented at the 2005 ASCPT meeting. The authors would like to acknowledge Dr Curtis E. Haas for sharing his paper and Dr Alan Forrest for helpful advice on the manuscript.
References
- 1.Hull RD, Raskob GE, Brant RF, Pineo GF, Elliott G, Stein PD, Gottschalk A, Valentine KA, Mah AF. Low-molecular-weight heparin vs. heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med. 2000;160:229–36. doi: 10.1001/archinte.160.2.229. [DOI] [PubMed] [Google Scholar]
- 2.Mismetti P, Laporte-Simitsidis S, Tardy B, Cucherat M, Buchmuller A, Juillard-Delsart D, Decousus H. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost. 2000;83:14–9. [PubMed] [Google Scholar]
- 3.Hull RD, Raskob GE, Pineo GF, Green D, Trowbridge AA, Elliott CG, Lerner RG, Hall J, Sparling T, Brettell HR, Norton J, Carter CJ, George R, Merli G, Ward J, Mayo W, Rosenbloom D, Brant R. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med. 1992;326:975–82. doi: 10.1056/NEJM199204093261502. [DOI] [PubMed] [Google Scholar]
- 4.Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysis. Ann Intern Med. 1999;130:789–99. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, Bayes De Luna A, Fox K, Lablanche JM, Radley D, Premmereur J, Braunwald E. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation. 1999;100:1593–601. doi: 10.1161/01.cir.100.15.1593. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, Langer A, Califf RM, Fox KA, Premmereur J, Bigonzi F. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med. 1997;337:447–52. doi: 10.1056/NEJM199708143370702. [DOI] [PubMed] [Google Scholar]
- 7.Kaul S, Shah PK. Low molecular weight heparin in acute coronary syndrome: evidence for superior or equivalent efficacy compared with unfractionated heparin? J Am Coll Cardiol. 2000;35:1699–712. doi: 10.1016/s0735-1097(00)00648-3. [DOI] [PubMed] [Google Scholar]
- 8.Bendetowicz AV, Beguin S, Caplain H, Hemker HC. Pharmacokinetics and pharmacodynamics of a low molecular weight heparin (enoxaparin) after subcutaneous injection, comparison with unfractionated heparin—a three way cross over study in human volunteers. Thromb Haemost. 1994;71:305–13. [PubMed] [Google Scholar]
- 9.Greer IA. Prevention of venous thromboembolism in pregnancy. Best Pract Res Clin Haematol. 2003;16:261–78. doi: 10.1016/s1521-6926(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 10.Wong G, Giugliano R, Antman E. Use of low-molecular-weight heparins in the management of acute coronary artery syndromes and percutaneous coronary intervention. JAMA. 2003;289:331–42. doi: 10.1001/jama.289.3.331. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann JF, Mouly S. Thromboprophylaxis in medical patients: focus on France. Semin Thromb Hemost. 2002;28(Suppl. 3):51–5. doi: 10.1055/s-2002-34077. [DOI] [PubMed] [Google Scholar]
- 12.Bruno R, Baille P, Retout S, Vivier N, Veyrat-Follet C, Sanderink GJ, Becker R, Antman EM. Population pharmacokinetics and pharmacodynamics of enoxaparin in unstable angina and non-ST-segment elevation myocardial infarction. Br J Clin Pharmacol. 2003;56:407–14. doi: 10.1046/j.1365-2125.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;56:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderink GJ, Le Liboux A, Jariwala N, Harding N, Ozoux ML, Shukla U, Montay G, Boutouyrie B, Miro A. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72:308–18. doi: 10.1067/mcp.2002.127114. [DOI] [PubMed] [Google Scholar]
- 15.Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl. 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20:771–5. doi: 10.1592/phco.20.9.771.35210. [DOI] [PubMed] [Google Scholar]
- 17.Hulot JS, Vantelon C, Urien S, Bouzamondo A, Mahe II, Ankri A, Montalescot G, Lechat P. Effect of renal function on the pharmacokinetics of enoxaparin and consequences on dose adjustment. Ther Drug Monit. 2004;26:305–10. doi: 10.1097/00007691-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Ma JM, Jackevicius CA, Yeo E. Anti-Xa monitoring of enoxaparin for acute coronary syndromes in patients with renal disease. Ann Pharmacother. 2004;38:1576–81. doi: 10.1345/aph.1E096. [DOI] [PubMed] [Google Scholar]
- 19.Brophy D, Wazny L, Gehr T, Cornstock T, Venitz J. The pharmacokinetics of subcutaneous enoxaparin in end-stage renal disease. Pharmacotherapy. 2001;21:169–74. doi: 10.1592/phco.21.2.169.34113. [DOI] [PubMed] [Google Scholar]
- 20.Green B, Greenwood M, Saltissi D, Westhuyzen J, Kluver L, Rowell J, Atherton J. Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol. 2005;59:281–90. doi: 10.1111/j.1365-2125.2004.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power B, Forbes A, Heerden P, Ilett K. Pharmacokinetics of drugs used in critically III adults. Clin Pharmacokinet. 1998;34:25–56. doi: 10.2165/00003088-199834010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care. 2000;15:127–32. doi: 10.1053/jcrc.2000.19224. [DOI] [PubMed] [Google Scholar]
- 23.Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R, Hulsmann M, Spitzauer S, Pabinger I, Heinz G. Prophylactic anticoagulation with enoxaparin: is the subcutaneous route appropriate in the critically ill? Crit Care Med. 2003;31:1405–9. doi: 10.1097/01.CCM.0000059725.60509.A0. [DOI] [PubMed] [Google Scholar]
- 24.Haas CE, Nelsen JL, Raghavendran K, Mihalko W, Beres J, Ma Q, Forrest A. Pharmacokinetics and pharmacodynamics of enoxaparin in multiple trauma patients. J Trauma. 2005;59:1336–43. doi: 10.1097/01.ta.0000197354.69796.bd. discussion 1343 34. [DOI] [PubMed] [Google Scholar]
- 25.Kane-Gill SL, Feng Y, Bobek MB, Bies RR, Pruchnicki MC, Dasta JF. Administration of enoxaparin by continuous infusion in a naturalistic setting: analysis of renal function and safety. J Clin Pharm Ther. 2005;30:207–13. doi: 10.1111/j.1365-2710.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 26.Laposata M, Green D, Van Cott EM, Barrowcliffe TW, Goodnight SH, Sosolik RC. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: the clinical use and laboratory monitoring of low-molecular-weight heparin, danaparoid, hirudin and related compounds, and argatroban. Arch Pathol Lab Med. 1998;122:799–807. [PubMed] [Google Scholar]
- 27.Brater DC, Chennavasin P. Dosing regimens: determination of creatinine clearance as an index of renal function. In: Brater DC, editor. Drug Use in Renal Disease, Boston: Adis Health Science Press; 1983. [Google Scholar]
- 28.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 29.Beal B, Sheiner L. NONMEM User’s Guide. San Francisco: University of California at San Francisco; 1992. Part I. [Google Scholar]
- 30.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–33. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gobburu JV, Lawrence J. Application of resampling techniques to estimate exact significance levels for covariate selection during nonlinear mixed effects model building: some inferences. Pharm Res. 2002;19:92–8. doi: 10.1023/a:1013615701857. [DOI] [PubMed] [Google Scholar]
- 32.WFN Randomization Test. [September 2005]; http://wfnsourceforgenet/wfnrthtm2003.
- 33.Ette EI, Ludden TM. Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res. 1995;12:1845–55. doi: 10.1023/a:1016215116835. [DOI] [PubMed] [Google Scholar]
- 34.Kimko HC, Reele SS, Holford NH, Peck CC. Prediction of the outcome of a phase 3 clinical trial of an antischizophrenic agent (quetiapine fumarate) by simulation with a population pharmacokinetic and pharmacodynamic model. Clin Pharmacol Ther. 2000;68:568–77. doi: 10.1067/mcp.2000.110975. [DOI] [PubMed] [Google Scholar]
- 35.Kimko HC, Duffull SB. Simulation for Designing Clinical Trials: A Pharmacokinetic–pharmacodynamic Modeling Perspective. New York: Marcel Dekker; 2002. Drugs & the Pharmaceutical Sciences Series. [Google Scholar]
- 36.Holford NH, Kimko HC, Monteleone JP, Peck CC. Simulation of clinical trials. Annu Rev Pharmacol Toxicol. 2000;40:209–34. doi: 10.1146/annurev.pharmtox.40.1.209. [DOI] [PubMed] [Google Scholar]
- 37.Collet JP, Montalescot G, Choussat R, Lison L, Ankri A. Enoxaparin in unstable angina patients with renal failure. Int J Cardiol. 2001;80:81–2. doi: 10.1016/s0167-5273(01)00455-7. [DOI] [PubMed] [Google Scholar]
- 38.Montalescot G, Polle V, Collet JP, Leprince P, Bellanger A, Gandjbakhch I, Thomas D. Low molecular weight heparin after mechanical heart valve replacement. Circulation. 2000;101:1083–6. doi: 10.1161/01.cir.101.10.1083. [DOI] [PubMed] [Google Scholar]
- 39.Collet JP, Montalescot G, Lison L, Choussat R, Ankri A, Drobinski G, Sotirov I, Thomas D. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation. 2001;103:658–63. doi: 10.1161/01.cir.103.5.658. [DOI] [PubMed] [Google Scholar]
- 40.Boneu B. Low molecular weight heparin therapy: is monitoring needed? Thromb Haemost. 1994;72:330–4. [PubMed] [Google Scholar]
- 41.Abbate R, Gori AM, Farsi A, Attanasio M, Pepe G. Monitoring of low-molecular-weight heparins in cardiovascular disease. Am J Cardiol. 1998;82:33L–36L. doi: 10.1016/s0002-9149(98)00111-8. [DOI] [PubMed] [Google Scholar]
- 42.FDA, Center for Drug Evaluation and Research. Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling. Rockville, MD: Food and Drug Administration; 1998. [Google Scholar]
- 43.K/DOQI. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kindney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–246. [PubMed] [Google Scholar]
- 44.Feng Y, Bies R, Bobek M, Kane S. Population pharmacokinetics analysis of patients receiving enoxaparin by continuous intravenous infusion. Clin Pharmacol Therapeutics. 2004;75:30. [Google Scholar]
- 45.Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29:473–505. doi: 10.1023/a:1022972420004. [DOI] [PubMed] [Google Scholar]
- 46.Bodenham A, Shelly MP, Park GR. The altered pharmacokinetics and pharmacodynamics of drugs commonly used in critically ill patients. Clin Pharmacokinet. 1988;14:347–73. doi: 10.2165/00003088-198814060-00003. [DOI] [PubMed] [Google Scholar]
- 47.Huet Y, Gouault-Heilmann M. Low molecular weight heparin fraction PK 10169: a new therapeutic means for anticoagulant therapy? Haemostasis. 1986;16:165–72. doi: 10.1159/000215287. [DOI] [PubMed] [Google Scholar]
- 48.Huet Y, Gouault-Heilmann M, Contant G, Brun-Buisson C. Treatment of acute pulmonary embolism by a low molecular weight heparin fraction. A preliminary study. Intens Care Med. 1987;13:126–30. doi: 10.1007/BF00254798. [DOI] [PubMed] [Google Scholar]