Nelfinavir is the only currently licensed HIV-protease inhibitor that has an active metabolite present in potentially therapeutic concentrations. This metabolite, nelfinavir hydroxyl-t-butylamide (also known as M8 or AG1402), is the product of enzymatic conversion of nelfinavir by cytochrome P450 2C19 (CYP2C19) [1]. Previous studies have demonstrated that inherited (the homozygote’s CYP2C19*2 allele) and acquired (severe liver disease) deficiency of CYP2C19 result in diminished formation of M8 [2, 3].
However, the frequency of heterozygote mutants of CYP2C19*2 (G681A mutation) in the general caucasian population is far greater than that of homozygote mutants (15%vs. 2%) [4, 5]. If these heterozygotes differed with respect to nelfinavir pharmacokinetics, the CYP2C19*2 polymorphism would affect many more patients, and thus may have greater clinical relevance.
Data for this analysis were selected from 24 healthy subjects who participated in a study on the effect of food on the pharmacokinetics of nelfinavir. Prior to enrolment, subjects were screened for the CYP2C19 polymorphism by polymerase chain reaction. Subjects who were homozygotes for any of the variant alleles of CYP2C19 (either *2, *3, *4 or *5) were excluded from the trial. Of the 36 subjects screened, one subject was a homozygous carrier of the CYP2C19*2 mutation and, of the 24 subjects included, eight were heterozygotes. No CYP2C19*3, *4 or *5 mutants were found. The pharmacokinetics of nelfinavir and M8 were characterized by noncompartmental methods following a 1250-mg dose taken with a standardized breakfast (containing about 737 kCal, 28 g of fat). At that time of the study, subjects had been taking nelfinavir 1250 mg twice daily for at least 10 days. None of the subjects was taking concurrent medications that are potential inhibitors of CYP2C19. Nelfinavir and M8 plasma concentrations were determined by a validated high-performance liquid chromatography assay with a lower limit of quantification for both agents of 0.04 mg l−1[6]. A nelfinavir + M8 concentration at 12 h after dosing (C12 h) that was below 1.0 mg l−1 was defined as subtherapeutic based on previous studies in HIV-infected patients [7, 8].
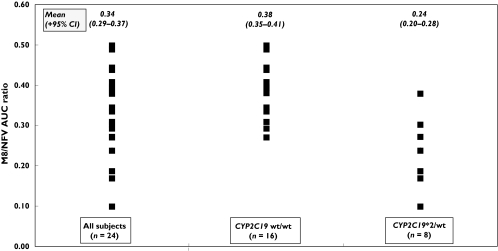
Overall, the median ratio of the AUC of M8 and nelfinavir was 0.34 with an interquartile range of 0.27–0.39. Minimum and maximum values were 0.10 and 0.50, respectively. These figures are consistent with previous reports in HIV-infected patients. No association was observed between the M8/nelfinavir AUC ratio and either gender, age (median age 40 years; range 22–63 years) or smoking habits (six smokers; maximum of 10 cigarettes per day) (t-test: all P-values were > 0.51). In contrast, there was a significant difference in the M8/nelfinavir AUC ratios between CYP2C19 wt/wt subjects and CYP2C19*2/wt subjects {mean difference [95% confidence interval (CI)] 0.14 (0.07, 0.21); P < 0.001} (Figure 1). However, the oral clearance of nelfinavir was not significantly different between the two genotypes [mean difference (95% CI) 9.1 l h−1 (−5.5, 23.8); P = 0.21], nor was there a significant difference in the nelfinavir + M8 C12 h between groups [mean difference (95% CI) 0.06 mg l−1 (−0.68, 0.80); P = 0.96]. There was a trend towards fewer subtherapeutic C12 h concentrations of nelfinavir + M8 (i.e. < 1.0 mg l−1) in CYP2C19*2/wt genotypes than in wild-type subjects (12.5%vs. 37.5%; χ2 test: P = 0.20).
Figure 1.
Effect of CYP2C19 mutations on the M8/nelfinavir AUC ratio
This is the first published report studying the effect of the CYP2C19*2/wt genotype on the pharmacokinetics of nelfinavir and M8. Preliminary data from an ACTG (AIDS Clinical Trials Group) study indicate the same nonsignificant trend of higher exposure to nelfinavir in heterozygotes of CYP2C19*2[9].
On the assumption that both nelfinavir and M8 are equally active, and differences in their molecular weight are negligible, the sum of the C12 h concentrations of these agents can be compared with the target threshold of 1.0 mg l−1 observed in previous studies [7, 8]. Subjects who are heterozygote for the CYP2C19*2 allele would be expected to produce less of the active metabolite, but the total sum of nelfinavir + M8 concentrations should be the same. This appears not to be the case, as can be observed from the nonsignificant trend in this study and in the ACTG data [9]. The nonsignificance of the threefold difference in frequency of subtherapeutic nelfinavir + M8 C12 h concentrations between the two subgroups may indicate that a larger study is needed to clarify these observations.
The total sum of nelfinavir + M8 C2 h plasma concentrations after intake of nelfinavir 750 mg tid has been shown to be significantly higher when CYP2C19 activities was impaired (3.9 vs. 2.8 mg l−1 for CYP2C19*2/*2 and CYP2C19 wt/wt genotypes, respectively, and 3.9 vs. 2.9 mg l−1 when patients were and were not taking inhibitors of CYP2C19[2]). Further clinical, pharmaco-epidemiological and pharmacogenetic studies are needed to determine whether these differences in the conversion rates of nelfinavir to M8 result in clinically significant improvements in the antiviral efficacy of nelfinavir-based treatment regimens.
Acknowledgments
This study was funded by an unrestricted grant from Roche Pharmaceuticals
References
- 1.Hirani VN, Raucy JL, Lasker JM. Conversion of the HIV protease inhibitor nelfinavir to a bioactive metabolite by human liver CYP2C19. Drug Metab Dispos. 2004;32:1462–7. doi: 10.1124/dmd.104.001743. [DOI] [PubMed] [Google Scholar]
- 2.Lillibridge JH, Lee CA, Pithavala YK, Daniels RG, Samuel TM, Wu EY, Zhang KE, Mazabel EL, Zhang M, Kerr BM. The Role of Polymorphic CYP 2C19 in the Metabolism of Nelfinavir Mesylate. p. abstract 3035. 12th AAPS Conference 1998.
- 3.Khaliq Y, Gallicano K, Seguin I, Fyke K, Carignan G, Bulman D, Badley A, Cameron DW. Single and multiple dose pharmacokinetics of nelfinavir and CYP2C19 activity in human immunodeficiency virus-infected patients with chronic liver disease. Br J Clin Pharmacol. 2000;50:108–15. doi: 10.1046/j.1365-2125.2000.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20:153–67. doi: 10.2133/dmpk.20.153. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa S, Soyama A, Saeki M, Fukushima-Uesaka H, Itoda M, Koyano S, Sai K, Ohno Y, Saito Y, Sawada J. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab Pharmacokinet. 2004;19:83–95. doi: 10.2133/dmpk.19.83. [DOI] [PubMed] [Google Scholar]
- 6.Droste JAH, Verweij-van Wissen CPWGM, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8 and nevirapine in human plasma by reversed phase high performance liquid chromatography. Ther Drug Monit. 2003;25:393–9. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Burger DM, Hugen PW, Aarnoutse RE, Hoetelmans RM, Jambroes M, Nieuwkerk PT, Schreij G, Schneider MM, Van Der Ende ME, Lange JM. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther Drug Monit. 2003;25:73–80. doi: 10.1097/00007691-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrin I, Breilh D, Montestruc F, Caumont A, Garrigue I, Morlat P, Le Camus C, Saux MC, Fleury HJ, Pellegrin JL. Virologic response to nelfinavir-based regimens: pharmacokinetics and drug resistance mutations (VIRAPHAR study) AIDS. 2002;16:1331–40. doi: 10.1097/00002030-200207050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Haas DW, Smeaton L, Shafer R, Robbins G, Morse G, Labbe L, Wilkinson G, Clifford D, Dube M, D’Aquila R, DeGruttola D, Pollard R, George A, Donahue J, Kim R. NWCS213, an Analysis of ACTG38412th Conference on Retroviruses and Opportunistic Infections. MA USA: Boston; Pharmacogenetics of Long-Term Response to Efavirenz- and Nelfinavir-Containing Regimens; pp. 22–25. February 2005 Abstract 81. [Google Scholar]