Abstract
Aims
To assess the antihistaminic activity of levocetirizine and fexofenadine 2 h and 24 h after drug administration using facial thermography and to compare the results with those using well-established parameters of antihistaminic activity in the nose and skin.
Methods
This was a randomized, double-blind, three-treatment, three-period, single-dose, cross-over study in healthy males taking levocetirizine 5 mg, fexofenadine 120 mg or placebo. The primary endpoint was nasal skin temperature after nasal histamine challenge recorded for 20 min at 2 and 24 h after drug intake. The secondary endpoints were nasal symptoms and a histamine skin prick test.
Results
Thirty subjects were randomized. At 2 h after drug intake the inhibition of the nasal temperature increase from baseline was not significantly different between levocetirizine and fexofenadine. At 24 h it was significantly more pronounced after levocetirizine than fexofenadine (difference: least-squares mean: −0.13 °C; P ≤ 0.024, 95% CI −0.24, −0.02). Both drugs significantly reduced (P≤ 0.001) the mean temperature increase from baseline compared with placebo at 2 and 24 h (least-squares mean increase and (95% CI): levocetirizine, −0.28 °C (−0.42, −0.14) and −0.32 °C (−0.43, −0.21); fexofenadine −0.35 °C (−0.49, −0.21) and −0.19 °C (−0.30, −0.08), respectively). Results of nasal symptom score and wheal and flare were consistent with the thermography results.
Conclusions
Facial thermography is an objective, non-invasive and sensitive method to study antihistaminic activity at the nose level. Levocetirizine and fexofenadine demonstrate the same activity at 2 h after drug intake, but levocetirizine has a more sustained activity at 24 h.
Keywords: facial thermography, fexofenadine, healthy subjects, levocetirizine, nasal histamine provocation, nasal skin temperature, wheal and flare
Introduction
Histamine is one of the most important mediators in allergy, and antihistamines are the mainstay of anti-allergic treatment [1–3]. H1-antihistamines, which are historically known as H1-receptor antagonists, have recently been reclassified as inverse agonists [3]. To define and compare the activity of different antihistamines controlled challenge models are of great importance. Although these studies cannot replace field studies to prove efficacy in the general allergic population, they are very powerful in demonstrating proof of principle and evaluating specific pharmacodynamic properties of antihistamines. Wheal and flare models using allergen or histamine skin challenge have been intensively studied to demonstrate and compare antihistaminic effects at the skin level [4–7]. At the nose level antihistaminic effects have been compared using nasal provocation with histamine or allergen and by measuring nasal flow (rhinomanometry), nasal secretion and nasal symptoms [8–10].
As nasal allergen and histamine challenge is associated with temperature changes of the nose, infrared thermography has been successfully applied. Seppey et al. demonstrated a dose-dependent increase in nasal skin temperature after intranasal administration of histamine in healthy subjects and grass pollen in patients with grass pollen allergy [11]. This method has the advantage that it is objective, non-invasive and very sensitive. However, this technique has not been applied to compare antihistaminic activities of different antihistamines at the nose level.
The aim of this study was to use facial thermography to compare the antihistaminic activity of the two antihistamines levocetirizine and fexofenadine. Both substances are second-generation antihistamines that bind with a high affinity and selectivity to H1-receptors [3, 12, 13]. Both drugs show few side-effects and have similar pharmacodynamic properties. However, there is limited information about their onset of action and duration of action [3]. Therefore, nasal skin temperature was recorded in healthy subjects after nasal histamine challenge at an early (2 h) and late time point (24 h) after drug intake. To relate these results to established antihistaminic parameters at the nose and skin level, nasal symptoms and wheal and flare reactions after a histamine skin prick test were recorded at the same time points.
Methods
Subjects
Thirty healthy adult male subjects (aged 18–55 years) were enrolled in the study. Included were persons with a positive skin prick test to histamine (wheal diameter ≥ 6 mm with histamine dihydrochloride 100 mg ml−1 in NaCl 0.9% and ≤ 3 mm with saline control), without documented or suspected history of allergic disease or asthma, nasal structural abnormalities, or current acute rhinitis. The use of intranasal or systemic decongestants and antihistamines within 3 days before study initiation, desloratadine or loratadine (within 10 days), oral corticosteroids (within 1 month), astemizole (within 6 weeks), or drugs potentially affecting vasomotor functions, was not allowed. The study was approved by the ethics committee of the Hanover Medical School. It was conducted in accordance with Good Clinical Practice and the relevant national laws. Each subject gave written informed consent before participation in the study.
Study design
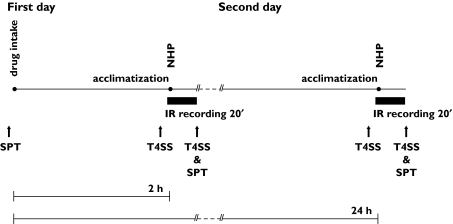
This was a randomized, double-blind, placebo-controlled, three-treatment, three-period, cross-over study. The drugs were blinded using over-encapsulation. On the first day subjects received a light breakfast and 1 h later either a single oral dose of levocetirizine (LCTZ) 5 mg, fexofenadine (FEX) 120 mg or placebo (PLC). These are the doses for the treatment of allergic rhinitis recommended by the FDA. The subjects were allowed to acclimatize for 30 min in the infrared recording room before a nasal histamine provocation (NHP) was performed at 2 h after the drug intake. Facial thermography recording was started 2 min before provocation and continued until 20 min after the provocation. On the second day no drug was administered but the rest of the procedure was identical to the first day. Histamine provocation was performed 24 h after drug intake. Skin prick tests were performed before drug intake on the first day and at the end of each infrared (IR) recording. Nasal symptoms were scored at drug intake, before each provocation and at the end of each recording. The same procedure was repeated for each treatment period. The workflow of the study procedures is summarized in Figure 1.
Figure 1.
Study procedure of each treatment period. IR, infrared; NHP, nasal histamine provocation; T4SS, nasal symptom score; SPT, skin prick test
Nasal provocation and facial thermography
Nasal provocations were performed according to standard procedures [14]. The histamine solution was preheated to 30 °C before use. Histamine 70 µg was sprayed into each nostril using a hand-held metered dose pump spray that delivered 140 ± 10 µl. One actuation (histamine dihydrochloride 0.5 mg ml−1 in NaCl 0.9%) was administered into each nostril.
Temperature and relative humidity were continuously monitored and maintained constant at 22 ± 0.4 °C and 50 ± 6%, respectively, in the room used for IR recordings. External IR radiation was averted by using adequate curtains and shutters. Only cold light sources (fluorescent tubes) were used. During IR recordings, both the subject and the camera were kept away from draft and external IR sources.
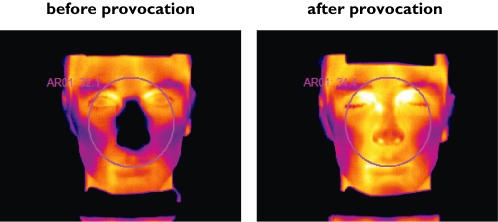
Facial thermography was carried out with a calibrated IR camera (ThermaCAM® SC500 from FLIR Systems™) equipped with 12° close-up optics. Resolution, sensitivity and accuracy of the camera were 320 × 240 pixels, 0.07 °C and ±2 °C, respectively. During IR recordings, subjects sat with their head positioned on an ophthalmic pedestal. The distance between the subject’s face and the camera was 1.20 m. Infrared images of the subject’s face were taken at a sampling rate of 0.5 Hz. They were directly recorded onto CD-R media. Average temperature of the nasal region (circle centred on subject’s nose, tangent to the upper border of the inferior lip and the lateral canthus of both eyes) was subsequently computed for every IR image using ThermaCAMTM Researcher 2001 software from FLIR Systems™. Figure 2 shows photographs of the nasal region before and after nasal histamine provocation.
Figure 2.
Nasal region before and after nasal histamine provocation. Differences in nasal skin temperature were measured with infrared thermography. The temperature is colour coded. A change from black to yellow indicates an increase in temperature
Baseline was defined as the mean temperature recorded during the 2 min preceding NHP. Histamine provocation and removal and repositioning of the head on the ophthalmic pedestal lasted no more than 1 min. The primary efficacy variable of this study was the mean change from baseline of the ‘average temperature of the nasal region’ (referred to in this publication only as the ‘temperature’) recorded in the 20-min interval following NHP. The secondary variables were nasal symptom score and histamine skin prick test as described below.
Nasal symptom score
The subjects rated the severity of sneezing, rhinorrhea, nasal pruritus, and nasal congestion on a 4-point scale ranging from 0 (absence of symptoms) to 3 (severe symptoms), at drug intake, before provocation and at the end of each recording (Figure 1). Total nasal symptom score (T4SS) was calculated by adding these four symptom scores.
Histamine skin prick test
Prick tests were performed on the inner forearm. A drop of histamine solution (histamine 100 mg ml−1) was placed on the skin. A sterile lancet was used to vertically prick the skin through the drop. After 2 s, the lancet was removed. The drop was dried using swab gauze 1 min later. After 10 min, the contours of the wheal and flare area were outlined on the skin using a fine marker pen and traced onto transparent acetate sheets. The area (mm2) was computed using calibrated templates. The prick test was performed before drug intake and at the end of each thermography recording (Figure 1).
Statistics
Statistical analyses were carried out using an analysis of variance model for cross-over design [15, 16]. Period, sequence and study medication were considered as fixed effects, and subject nested within sequence was considered as a random effect in this model. The differences in the least-squares (LS) mean of the thermography parameters and 95% confidence intervals (95% CI) were calculated for all pairwise comparisons (levocetirizine–fexofenadine, levocetirizine–placebo and fexofenadine–placebo). The primary comparison was between levocetirizine and fexofenadine. The comparison with placebo was used as a control.
When the cross-over design of the study was taken into account, a sample size of 27 subjects was sufficient to detect a change in mean nasal skin temperature of 0.3 °C with a power of 90% and a type I error of 0.05. We assumed an intrasubject standard deviation (SD) of 0.32 °C (SD determined in a previous clinical trial under similar conditions).
Results
Demographics
Thirty healthy male subjects aged 29.8 ± 6.8 years (mean ± SD) were randomized. The mean weight, height and body mass index were 78.1 ± 9.5 kg, 179.8 ± 7.3 cm and 24.1 ± 1.9 kg m−2, respectively. One subject discontinued the study because of acute rhinitis.
Reduction of nasal skin temperature after drug intake
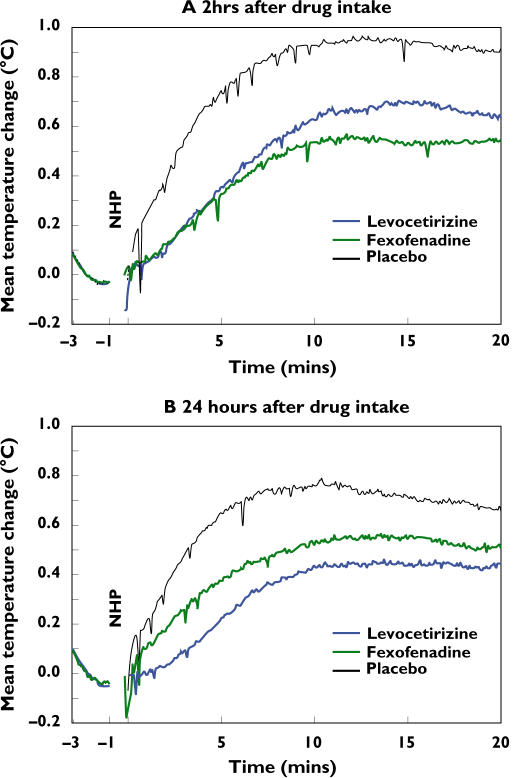
Figure 3 shows the change in nasal temperature from baseline over a 20-min period after nasal histamine provocation at 2 h (A) and 24 h (B) after levocetirizine, fexofenadine or placebo treatment. The mean temperature increase was reduced at 2 h and 24 h after drug treatment compared with treatment with placebo.
Figure 3.
Mean temperature change (°C) after nasal histamine provocation at 2 h (A) and 24 h (B) after drug intake (n = 30)
Least-squares mean temperature changes from baseline, which were recorded over 20 min after provocation, and differences between the drugs are shown in Table 1. Two hours after levocetirizine or fexofenadine intake, the mean temperature increase from baseline was significantly reduced compared with placebo (Table 1, −0.28 °C and −0.35 °C, respectively, P < 0.001). There was no significant difference between the two drugs at this time point. Twenty-four hours after drug treatment the mean temperature increase was also significantly reduced compared with placebo (levocetirizine −0.32 °C and fexofenadine −0.19 °C, P ≤ 0.001); at this time point, inhibition of the nasal temperature increase after levocetirizine was significantly more pronounced than after fexofenadine (difference −0.13 °C; P = 0.024).
Table 1.
Mean temperature increase (°C) from baseline after nasal histamine provocation
| Time point after dose | LCTZa | FEXa | PLCa | LCTZ-FEXb | LCTZ-PLCb | FEX-PLCb |
|---|---|---|---|---|---|---|
| 2 h | 0.50(0.38, 0.61) | 0.43(0.32, 0.55) | 0.78(0.66, 0.90) | 0.07(−0.07, 0.21)(P= 0.327) | −0.28(−0.42, −0.14)(P< 0.001) | −0.35(−0.49, −0.21)(P< 0.001) |
| 24 h | 0.32(0.22, 0.41) | 0.44(0.35, 0.54) | 0.63(0.54, 0.73) | −0.13(−0.24, −0.02)(P= 0.024) | −0.32(−0.43, −0.21)(P< 0.001) | −0.19(−0.30, −0.08)(P= 0.001) |
Values given are LS mean (95% CI).
Values given are change in LS mean (95% CI). CI, confidence interval; LCTZ, levocetirizine; FEX, fexofenadine; PLC, placebo.
Reduced nasal symptom score after drug intake
Mean T4SS showed no relevant differences before drug intake or before nasal histamine provocation between the various treatment periods. Two and 24 h after drug intake, following histamine challenge T4SS was significantly lower after levocetirizine and fexofenadine than after placebo (LS means (95% CI) (P value vs. placebo): LCTZ 2 h, 0.67 (0.20, 1.13) (P< 0.001); LCTZ 24 h, 0.72 (0.25, 1.18) (P< 0.001); FEX 2 h, 0.73 (0.27, 1.20) (P < 0.001); FEX 24 h, 1.27 (0.81, 1.73) (P = 0.024); PLC 2 h, 2.11 (1.64, 2.58); PLC 24 h, 1.88 (1.42, 2.35)). There was no significant difference between the two drugs at 2 h after dose. However, at 24 h after levocetirizine, subjects had significantly reduced T4SS compared with subjects who received fexofenadine (P= 0.043).
Inhibition of wheal and flare after drug intake
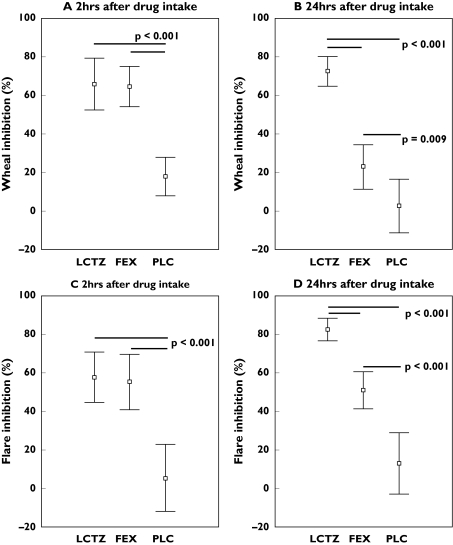
Wheal and flare were significantly inhibited after histamine provocation at 2 h and 24 h after drug treatment compared with placebo (Figure 4, P < 0.01). There was no significant difference between the levocetirizine and fexofenadine at 2 h after drug intake (Tables 2 and 3). However, at 24 h after dose, inhibition of wheal and flare was significantly higher after levocetirizine than after fexofenadine (LS means wheal 49.8% and flare 32.9%, P < 0.001).
Figure 4.
Inhibition in wheal (A + B) and flare (C + D) after nasal histamine provocation at 2 h and 24 h after drug intake (mean ± 95% CI)
Table 2.
Wheal inhibition (%) after drug intakea
| Time point after dose | LCTZb | FEXb | PLCb | LCTZ-FEXc | LCTZ-PLCc | FEX-PLCc |
|---|---|---|---|---|---|---|
| 2 h | 65.8(54.6, 77.1) | 64.7(53.5, 75.9) | 17.9(6.5, 29.3) | 1.14(−14.2, 16.5)(P= 0.882) | 48.0(32.5, 63.4)(P< 0.001) | 46.8(31.3, 62.3)(P< 0.001) |
| 24 h | 72.8(62.0, 83.6) | 23.0(12.2, 33.8) | 2.4(−8.6, 13.4) | 49.8(34.8, 64.9)(P< 0.001) | 70.4(55.3, 85.6)(P< 0.001) | 20.6(5.4, 35.8)(P= 0.009) |
Wheal inhibition is defined as the reduction of wheal area measured before and after drug intake expressed in (%).
Values given are LS mean (95% CI).
Values given are change in LS mean (95% CI). CI, confidence interval; LCTZ, levocetirizine; FEX, fexofenadine; PLC, placebo.
Table 3.
Flare inhibition (%) after drug intakea
| Time-point after dose | LCTZb | FEXb | PLCb | LCTZ-FEXc | LCTZ-PLCc | FEX-PLCc |
|---|---|---|---|---|---|---|
| 2 h | 57.2(43.2, 71.3) | 54.8(40.8, 68.9) | 5.0(−9.3, 19.3) | 2.4(−15.6, 20.5)(P= 0.790) | 52.3(34.0, 70.5)(P< 0.001) | 49.9(31.6, 68.1)(P< 0.001) |
| 24 h | 83.1(72.7, 93.5) | 50.2(39.8, 60.6) | 12.5(1.85, 3.0) | 32.9(18.2, 47.6)(P< 0.001) | 70.7(55.9, 85.5)(P< 0.001) | 37.8(23.0, 52.6)(P< 0.001) |
Flare inhibition is defined as the reduction of wheal area measured before and after drug intake expressed in (%).
Values given are LS mean (95% CI).
Values given are change in LS mean (95% CI). CI, confidence interval; LCTZ, levocetirizine; FEX, fexofenadine; PLC, placebo.
Discussion
Facial thermography was used to evaluate the antihistaminic activity of levocetirizine and fexofenadine at the nasal skin level in healthy male subjects. The recommended dose for the treatment of allergic rhinitis was selected for each drug. This seemed most appropriate as the purpose of the trial was to verify the suitability of the method to investigate the effects of antihistaminic drugs. When nasal histamine provocation was performed 2 h after drug intake, both levocetirizine and fexofenadine inhibited the nasal temperature increase to the same extent. This difference compared with placebo was statistically significant. Additionally, histamine-induced nasal symptoms were significantly reduced by both drugs compared with placebo. A similar effect was seen in skin: levocetirizine and fexofenadine significantly inhibited histamine-induced wheal and flare reaction at 2 h after drug intake compared with placebo. Our results indicate that both antihistamines have a similar antihistaminic activity at the skin and nose level at an early time point after drug intake.
When nasal and skin histamine provocation was performed at 24 h after drug intake, both drugs still showed significant antihistaminic activity compared with placebo. Nasal temperature increase, nasal symptoms and wheal and flare reaction were reduced by both drugs. However, at 24 h after drug intake a significant difference between the two drugs was observed. Levocetirizine reduced the nasal temperature increase and the nasal symptoms to a greater extent than fexofenadine. This was confirmed by the skin prick test. Thus, the antihistaminic activity of levocetirizine was longer lasting than the activity of fexofenadine at the nose and the skin level.
These data have been confirmed by others using histamine and allergen provocation studies. Grant et al. observed a similar decrease in the wheal and flare surface area 2 h after a single dose of either levocetirizine or fexofenadine in healthy subjects challenged with histamine [17]. However, at 24 h they saw a more potent inhibition with levocetirizine than fexofenadine.
The time required to reach peak plasma concentration (tmax) is shorter for levocetirizine (0.8 ± 0.5 h) than for fexofenadine (1–3 h). The terminal elimination half-life (t1/2) is 7 ± 1.5 h and 14.4 h for levocetirizine and fexofenadine, respectively [18]. These parameters alone cannot explain the difference in activity of both drugs 24 h after administration. Concentrations of the drugs at the active site (histamine H1-receptor), as well as their affinity for these receptors, also have to be considered. Receptor occupancy at 4 h (24 h) is 95% (12%) and 90% (57%) for fexofenadine and levocetirizine, respectively [19]. The results found in our study are consistent with these values: a similar activity of fexofenadine and levocetirizine 2 h after intake and a higher activity of levocetirizine compared with fexofenadine at 24 h.
Facial thermography is a new non-invasive and sensitive tool. It adds information to established methodologies in challenge models, such as wheal and flare at the skin level and allergen provocation with rhinomanometry and nasal symptom score at the nose level. In contrast to these methods, thermography facilitates continuous data collection. Thus, thermography has the potential, in future studies, to detect onset of action very precisely. Prerequisites for this method are a room with stable climatic conditions, exclusion of external heat sources and immobilization of the patient’s head. Facial thermography can be performed on one patient at a time.
We conclude that facial thermography is an effective, non-invasive and sensitive method to study the activity of antihistamines at the nose level. Although both levocetirizine and fexofenadine are successful H1-receptor inverse agonists, our results indicate that at the end of the recommended dosing interval for both drugs, levocetirizine has a more sustained activity.
Acknowledgments
We would like to thank W. Koch and H. Windt (ITEM, Fraunhofer Institute) for their technical support. This study was supported by UCB Pharma, Braine L’Alleud, Belgium.
References
- 1.Akdis CA, Blaser K. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol. 2003;112:15–22. doi: 10.1067/mai.2003.1585. [DOI] [PubMed] [Google Scholar]
- 2.MacGlashan D., Jr Histamine: a mediator of inflammation. J Allergy Clin Immunol. 2003;112:S53–S59. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- 3.Estelle F, Simons R. Advances in H1-antihistamines. N Eng J Med. 2004;351:2203–17. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- 4.Denham KJ, Boutsiouki P, Clough GF, Church MK. Comparison of the effects of desloratadine and levocetirizine on histamine-induced wheal, flare and itch in human skin. Inflamm Res. 2003;52:424–7. doi: 10.1007/s00011-003-1193-5. [DOI] [PubMed] [Google Scholar]
- 5.Purohit A, Melac M, Pauli G, Frossard N. Twenty-four-hour activity and consistency of activity of levocetirizine and desloratadine in the skin. Br J Clin Pharmacol. 2003;56:388–94. doi: 10.1046/j.1365-2125.2003.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purohit A, Duvernelle C, Melac M, Pauli G, Frossard N. Twenty-four hours of activity of cetirizine and fexofenadine in the skin. Ann Allergy Asthma Immunol. 2001;86:387–92. doi: 10.1016/S1081-1206(10)62483-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaliner MA, White MV, Economides A, Crisalida T, Hale M, Liao Y, Christian CD, Georges GC, Woodworth TH, Meeves SG. Relative potency of fexofenadine HCl 180 mg, loratadine 10 mg, and placebo using a skin test model of wheal-and-flare suppression. Ann Allergy Asthma Immunol. 2003;90:629–34. doi: 10.1016/S1081-1206(10)61867-4. [DOI] [PubMed] [Google Scholar]
- 8.Horak F, Stubner P, Zieglmayer R, Karina A, De Vos C, Burtin B, Donnelly F. Controlled comparison of the efficacy and safety of cetirizine 10 mg o.d. and fexofenadine 120 mg o.d. in reducing symptoms of seasonal allergic rhinitis. Int Arch Allergy Immunol. 2001;125:73–9. doi: 10.1159/000053799. [DOI] [PubMed] [Google Scholar]
- 9.Wilson AM, Orr LC, Coutie WJ, Sims EJ, Lipworth BJ. A comparison of once daily fexofenadine versus the combination of montelukast plus loratadine on domiciliary nasal peak flow and symptoms in seasonal allergic rhinitis. Clin Exp Allergy. 2002;32:126–32. doi: 10.1046/j.0022-0477.2001.01252.x. [DOI] [PubMed] [Google Scholar]
- 10.van Steekelenburg J, Clement PA, Beel MH. Comparison of five new antihistamines (H1-receptor antagonists) in patients with allergic rhinitis using nasal provocation studies and skin tests. Allergy. 2002;57:346–50. doi: 10.1034/j.1398-9995.2002.1s3426.x. [DOI] [PubMed] [Google Scholar]
- 11.Seppey M, Hessler C, Bruchez M, Savary M, Pecoud A. Facial thermography during nasal provocation tests with histamine and allergen. Allergy. 1993;48:314–8. doi: 10.1111/j.1398-9995.1993.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 12.Day JH, Ellis AK, Rafeiro E. Levocetirizine: a new selective H1 receptor antagonist for use in allergic disorders. Drugs Today (Barc) 2004;40:415–21. doi: 10.1358/dot.2004.40.5.850489. [DOI] [PubMed] [Google Scholar]
- 13.Simpson K, Jarvis B. Fexofenadine. a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs. 2000;59:301–21. doi: 10.2165/00003495-200059020-00020. [DOI] [PubMed] [Google Scholar]
- 14.Malm L, Gerth vW, Bachert C. Guidelines for nasal provocations with aspects on nasal patency, airflow, and airflow resistance. International Committee on Objective Assessment of the Nasal Airways, International Rhinologic Society. Rhinology. 2000;38:1–6. [PubMed] [Google Scholar]
- 15.Jones B, Kenward MG. Design and Analysis of Cross-over Trials. 2. Chapman & Hall/CRC; 2003. Designs for three and more treatments; pp. 151–204. [Google Scholar]
- 16.Brown H, Prescott R. Barnett V. Applied Mixed Models in Medicine. West Sussex: John Wiley & Sons; 1999. Crossover trials; pp. 261–94. [Google Scholar]
- 17.Grant JA, Riethuisen JM, Moulaert B, De Vos C. A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann Allergy Asthma Immunol. 2002;88:190–7. doi: 10.1016/S1081-1206(10)61995-3. [DOI] [PubMed] [Google Scholar]
- 18.Simons FE, Simons KJ. Clinical pharmacology of H1-antihistamines. Clin Allergy Immunol. 2002;17:141–78. [PubMed] [Google Scholar]
- 19.Gillard M, Benedetti MS, Chatelain P, Baltes E. Histamine H1 receptor occupancy and pharmacodynamics of second generation H1-antihistamines. Inflamm Res. 2005;54:367–9. doi: 10.1007/s00011-005-1368-3. [DOI] [PubMed] [Google Scholar]