Abstract
Aims
There is evidence that different methods used to identify and quantify adverse drug reactions (ADR) in hospitals are not exhaustive (spontaneous reporting or computerized medical databases). The combination of these different sources of data could improve knowledge about ADR frequency in hospitals. The aim of this study was to estimate the incidence of serious ADRs handled in medical wards of a French university hospital using data from the Programme de Medicalization des Systemes d’Information (PMSI) and spontaneous reports recorded in the French Pharmacovigilance Database.
Methods
The study period was the first semester of 2001. From PMSI, all hospitalization summaries including an ICD-10th code related to a potential ADR were selected. From the French Pharmacovigilance Database, all serious ADRs which occurred during the study period and were reported by physicians working in the University Hospital were collected. After identification of common cases, the capture–recapture method was applied in order to estimate the real number of ADRs occurring during the first semester of 2001.
Results
From PMSI, we identified 274 different hospital stays related to an ADR. Out of 241 reports selected from the French Pharmacovigilance Database, we retained 151 ADRs for analysis. Fifty-two ADRs were common in the two databases, giving an estimated number of serious ADRs of 796 [95% confidence interval (CI) 638, 954], corresponding to 2.9% of inpatients (95% CI 2.3, 3.5).
Conclusions
This study shows the lack of exhaustiveness of ADR reporting whatever the sources of data and underlines the interest of merging data from different databases to identify fully the real impact of ADR in hospitals.
Keywords: capture-recapture, adverse drug reactions, spontaneous reporting, computerized database, hospitalization
Introduction
The review by Lazarou et al. estimates that the rate of serious adverse drug reactions (ADR) in North America is 6.7%[95% confidence interval (CI) 5.2, 8.2] of hospitalized patients with a rate of fatal ADR of 0.3% (95% CI 0.2, 0.4), making ADRs a serious public health issue [1]. In France, although ADRs represent an important source of morbidity among inpatients, their extent remains largely unknown. Only two nationwide studies were performed in 1997 and 1998 to investigate ADR frequency in public hospitals [2, 3]. These studies found a prevalence rate of ADRs of 10.3% (95% CI 8.7, 11.9) that were serious in 33% of cases, and estimated that 3.19% (95% CI 2.37, 4.01) of inpatients were admitted because of an ADR.
Until now, the basis of drug safety monitoring has largely remained spontaneous ADR reporting. This method is, nevertheless, limited mainly by under-reporting, which does not allow medical care assessment of ADRs’ real impact. Most hospitals identify an ADR by spontaneous or stimulated reporting, leading to a systematically underestimated frequency of ADRs. Chart review identifies considerably more ADRs, but it is not sufficiently cost effective for routine use. Another approach is computerized detection. The principle is to look for signals suggesting the possible presence of an ADR from hospital information systems. The databases most often used are pharmacy and laboratory databases, but also medical administrative databases, with in particular data about diagnoses and therapeutic interventions [4–8]. The querying of computerized databases is interesting for generating pharmacovigilance signals, but alone they do not permit estimation of the real frequency of ADRs. In fact, no source of ADR identification (spontaneous reporting or computerized detection) is really exhaustive. Nevertheless, simultaneous use of these sources should improve detection of ADRs and thus provide better knowledge of the impact of hospital ADRs [9, 10].
The capture–recapture method allows estimation of the total number of events or the total size of a population by crossing several information sources. This method was developed in ecology for censuses of wildlife populations: animals were captured, marked, released and subject to recapture.
The aim of this study was to estimate the real frequency of serious ADRs in a French university hospital using the capture–recapture method applied to two sources of information: the Programme de Medicalization des Systemes d’Information (PMSI) database and the French Pharmacovigilance Database.
Materials and methods
The study was conducted in one teaching hospital of 2818 beds, with a total number of hospital admissions of around 160 000 per year, covering a population of more than 900 000 inhabitants in Toulouse, South-west France. We limited the study to patients hospitalized in medical wards in the hospital, including hospital admission dates from 1 January 2001 and hospital discharge until 30 June 2001.
Data sources
We used data collected in the PMSI database for hospital management and data spontaneously reported to the Regional Pharmacovigilance Centre and recorded, as well in the French Pharmacovigilance Database after assessment and validation of causality.
The Regional Pharmacovigilance Centre is located in the Department of Clinical Pharmacology of the Toulouse University Hospital and is charged by law with assessing drug safety in the hospital. Prescribers have to report ‘serious’ ADRs (i.e. resulting in death, requiring patient hospitalization or prolongation of existing hospitalization, resulting in permanent disability or incapacity, or life-threatening) and/or ‘unexpected’ ADRs (not labelled in the Summary Product Characteristics) to their regional Pharmacovigilance Centre. For each spontaneous report, data concerning patient, drug exposure and event are collected to assess drug causality. All reports are registered anonymously in the French Pharmacovigilance Database. However, original medical records including patient identity are retained in the Pharmacovigilance Centre, for patient follow-up inside and outside of the hospital.
The PMSI is the French system for case-mixed classification for the management of hospitals. A standardized medical outcome summary is filled in for each hospital stay. This summary contains administrative data (name, gender, birthdate and dates of hospital admission/discharge) and main clinical data (diagnoses and medical or surgical procedures coded following the ICD 10th classification).
Case definition
We studied ‘serious’ ADRs with a date of occurrence or diagnosis during the first semester 2001 (1 January to 30 June) and cared for in a medical ward of the University Hospital of Toulouse. An ADR is a noxious and unintended event which occurs at doses generally used in humans for prophylaxis, diagnosis, therapy or modification of physiological functions. We excluded drug-related headache since it was not possible to ascertain precisely the date of onset. We also excluded aplastic anaemia occurring in the context of bone-marrow transplants, events related to inadequate use of drugs (poisoning, intentional overdose, addiction), complications of radiotherapy or of any medical technical act (such as puncture) and hospitalizations for drug allergy check-up.
We included all medical wards except two (an endocrinology unit and an emergency unit), for which computerized discharge reports were not systematically available; psychiatric and paediatric wards were also not included, because medical data were not exhaustively recorded at the time of the study, because of hospital relocation.
Selection of cases and identification of common cases
We selected ICD 10th diagnoses codes describing a possible drug-related event (Table 1). From the PMSI database, we listed, during the study period and among participating wards, all discharge summaries including a selected ICD-10 code. We examined all corresponding discharge reports to check the validity of the ADR, to verify the criteria of case definition and to identify the nature and seriousness of ADRs and the drug involved. We assumed that ‘serious’ ADRs were always mentioned in discharge report, unlike nonserious ADRs. If the same ADR for a patient was reported in different summaries, it was counted as only one record. ADRs were classified according to the World Health Organization-Adverse Reaction Terminology (WHO-ART) classification.
Table 1.
List of ICD 10th diagnosis codes used for selection of cases from Programme de Medicalization des Systemes d’Information, the French system of case-mix classification of hospital care
| A80.0 | Acute paralytic poliomyelitis, vaccine-associated |
| D52.1 | Drug-induced folate deficiency anaemia |
| D59.0 | Drug-induced autoimmune haemolytic anaemia |
| D59.2 | Drug-induced non-autoimmune haemolytic anaemia |
| D61.1 | Drug-induced aplastic anaemia |
| D64.2 | Secondary sideroblastic anaemia due to drugs and toxins |
| D70 | Agranulocytosis |
| E03.2 | Hypothyroidism due to medicaments and other exogenous substances |
| E06.4 | Drug-induced thyroiditis |
| E16.0 | Drug-induced hypoglycaemia without coma |
| E23.1 | Drug-induced hypopituitarism |
| E24.2 | Drug–induced Cushing’s syndrome |
| E27.3 | Drug-induced adrenocortical insufficiency |
| E66.1 | Drug-induced obesity |
| F11 | Mental and behavioural disorders due to use of opioids |
| F13 | Mental and behavioural disorders due to use of sedatives or hypnotics |
| F15 | Mental and behavioural disorders due to use of other stimulants, including caffeine |
| F16 | Mental and behavioural disorders due to use of hallucinogens |
| F19 | Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances |
| G04.0 | Acute disseminated encephalitis |
| G21.0 | Malignant neuroleptic syndrome |
| G21.1 | Other drug-induced secondary parkinsonism |
| G24.0 | Drug-induced dystonia |
| G25.1 | Drug-induced tremor |
| G25.3 | Myoclonus |
| G25.4 | Drug-induced chorea |
| G25.6 | Drug-induced tics and other tics of organic origin |
| G40.5 | Special epileptic syndromes |
| G44.4 | Drug-induced headache, not elsewhere classified |
| G62.0 | Drug-induced polyneuropathy |
| G71.1 | Myotonic disorders |
| G72.0 | Drug-induced myopathy |
| G95.8 | Other specified diseases of spinal cord |
| H26.3 | Drug-induced cataract |
| H40.6 | Glaucoma secondary to drugs |
| I42.7 | Cardiomyopathy due to drugs and other external agents |
| I95.2 | Hypotension due to drugs |
| J70.2 | Acute drug-induced interstitial lung disorders |
| J70.3 | Chronic drug-induced interstitial lung disorders |
| J70.4 | Drug-induced interstitial lung disorders, unspecified |
| K71 | Toxic liver disease |
| L10.5 | Drug-induced pemphigus |
| L23.3 | Allergic contact dermatitis due to drugs in contact with skin |
| L24.4 | Irritant contact dermatitis due to drugs in contact with skin |
| L25.1 | Unspecified contact dermatitis due to drugs in contact with skin |
| L27.0 | Generalized skin eruption due to drugs and medicaments |
| L27.1 | Localized skin eruption due to drugs and medicaments |
| L43.2 | Lichenoid drug reaction |
| L56.0 | Drug phototoxic response |
| L56.1 | Drug photo-allergic response |
| L64.0 | Drug-induced androgenic alopecia |
| M02.2 | Postimmunization arthropathy |
| M10.2 | Drug-induced gout |
| M32.0 | Drug-induced systemic lupus erythematosus |
| M34.2 | Systemic sclerosis induced by drugs and chemicals |
| M80.4 | Drug-induced osteoporosis with pathological fracture |
| M81.4 | Drug-induced osteoporosis |
| M87.1 | Osteonecrosis due to drugs |
| N14.0 | Analgesic nephropathy |
| N14.1 | Nephropathy induced by other drugs, medicaments and biological substances |
| N14.2 | Nephropathy induced by unspecified drug, medicament or biological substance |
| T88.0 | Infection following immunization |
| T88.1 | Other complications following immunization, not elsewhere classified |
| T88.2 | Shock due to anaesthesia |
| T88.3 | Malignant hyperthermia due to anaesthesia |
| T88.5 | Other complications of anaesthesia |
| T88.6 | Anaphylactic shock due to adverse effect of correct drug or medicament properly administered |
| T88.7 | Unspecified adverse effect of drug or medicament |
| Y40–Y59 | Drugs, medicaments and biological substances causing adverse effects in therapeutic use |
| Y88.0 | Sequelae of adverse effects caused by drugs, medicaments and biological substances in therapeutic use |
| Z03.6 | Observation for suspected toxic effect from ingested substance |
From the French Pharmacovigilance Database, we identified all ‘serious’ ADRs that occurred or were diagnosed during the first semester 2001 (1 January to 30 June) and were reported by health professionals from the Toulouse University Hospital during the year 2001. We examined all corresponding spontaneous reporting forms in order to verify the case definition and to exclude ADRs not requiring hospitalization in a selected ward during the study period. We also collected demographic data about the patient and ADR characteristics to identify common cases between the two data sources. We confirmed periods of hospital stay by crossing data with dates of hospital admission and discharge recorded in the PMSI database.
Common cases were identified between the two sources using demographic data (first name, last name and birth date), characteristics of ADR, involved drugs and dates of hospital stay.
Capture–recapture method
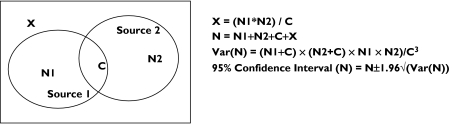
The capture–recapture method is used to provide population estimates from two or more incomplete sources of information [11]. It allows refinement of frequency estimations and ascertaining the exhaustiveness of monitoring systems. Its principle consists of combining data provided by several sources coming from the same population. After identification of matches between sources, the capture–recapture method allows estimation of the number non-identified cases by any of the sources. Thus, the total number of cases in population and sensitivity of each source can be deduced. Assuming that N1 + C is the number of ADRs reported by the PMSI database (the first source of information), N2 + C the number of ADRs reported by the pharmacovigilance database (the second source), with C the number of common cases between the two sources, the number X of non-identified cases, the total number N of cases, its variance and its confidence interval can be estimated (Figure 1).
Figure 1.
Distribution of cases in two data sources
Results
From the PMSI database, hospitalizations were identified according to the dates of hospital stay and preselected ICD-10 codes. After reviewing discharge reports, we excluded: 22 hospitalizations reporting ADRs identified before 2001, 141 reporting nonserious ADRs, 33 reporting aplastic anaemia in context of bone-marrow transplantation, 50 corresponding to allergy check-up, 34 reporting drug-related headache, 12 reporting inadequate use of drugs, 28 for which discharge reports were not available and two corresponding to complications of radiotherapy. Finally, according to inclusion criteria, we retained 274 different ADRs, corresponding to 261 inpatients (some people presenting two or more different serious ADRs during the 6-month period).
From the French Pharmacovigilance Database, we found 241 ‘serious’ ADRs identified during the first semester 2001 and reported by the medical staff of the University Hospital of Toulouse to the Regional Pharmacovigilance Centre. We excluded 53 reports because of nonselected wards or inadequate hospitalization periods. We also excluded 14 reports not requiring hospitalization and three reporting inadequate use of drugs or complications of technical medical procedures. For 13 reports, mistakes were noticed in data capture (ADR occurred before 2001 or was not reported by the University Hospital of Toulouse). Three ADRs were recorded twice and for four, information about hospitalization was not available. Finally, we retained 151 ADRs.
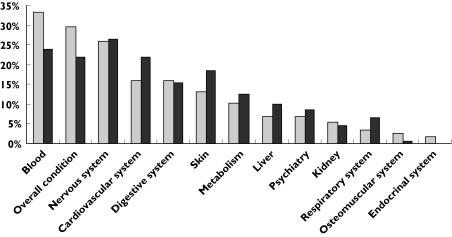
Figure 2 presents the distribution of ADRs by target organs according to the ‘soc-term’ of the WHO-ART classification. We grouped together terms related to the haematological system, terms related to the cardiovascular system and terms related to the nervous system. One ADR could concern several systems. Differences were seen in the type of ADR identified from each database. Physicians tended not to report well-established reactions to their Regional Pharmacovigilance Centre, e.g. haematological reactions related to chemotherapy were noticed in the PMSI database but were rarely reported to the pharmacovigilance system.
Figure 2.
Distribution of adverse drug reactions selected from the Programme de Medicalization des Systemes d’Information and Pharmacovigilance databases by target organs according to the World Health Organization-Adverse Reaction Terminology classification. PMSI ( ), PV (
), PV ( )
)
After matching ADRs from the two databases, we identified 52 common cases. According to the capture–recapture method, the estimated total number of ‘serious’ ADRs was 796 (95% CI 638, 954). Taking into account the effect of age did not greatly modify this estimate (Table 2). After stratification for age, the total number was 852, a slightly higher value than the raw estimate but remaining within its 95% CI.
Table 2.
Capture–recapture estimates of the number of adverse drug reactions (ADRs)
| Number of observed ADRs | Estimate of total number of ADRs | ||||
|---|---|---|---|---|---|
| PMSI | PV | Matches | N | 95% CI | |
| Unstratified analysis | |||||
| All | 274 | 151 | 52 | 796 | 638, 954 |
| Analysis by age group, years | |||||
| 16–39 | 62 | 26 | 14 | 115 | 79, 151 |
| 40–64 | 87 | 48 | 16 | 261 | 167, 355 |
| 65–79 | 65 | 38 | 8 | 309 | 131, 487 |
| >80 | 60 | 39 | 14 | 167 | 106, 229 |
PMSI, Programme de Medicalization des Systemes d’Information; PV, French Pharmacovigilance database.
During the first semester of 2001, 27 426 patients were hospitalized at least once in study wards and the total number of hospital admissions was 39 441. The frequency of ADRs among patients was 2.9% (95% CI 2.3, 3.5), corresponding to 2.0% of admissions (95% CI 1.6, 2.4).
Discussion
Following ecologists and demographers, epidemiologists have used the capture–recapture method to estimate the prevalence or incidence of diseases in humans. They have applied it to different epidemiological areas, including drug abuse, viral epidemic, injuries … In the field of pharmacovigilance, there has been less use of the capture–recapture method [12–14]. As far as we know, there is no study in France using this method to assess the impact of ADRs related to hospitalization.
The capture–recapture method represents a helpful tool for estimating frequency when several sources of information are available and can be matched. Its application is easy and the principles of calculation are simple. However, the accuracy and the appropriateness of results in epidemiology can be discussed for their methodological aspects [15, 16]. In fact, six conditions which must be satisfied to obtain accurate estimates are discussed in this study: all cases identified by each source are real cases; sources concern the same population, the same period and the same geographical area; the study population is closed; all matches and only true matches are identified; sources are independent; there is equal ‘catchability’ within sources.
The first condition implies that all cases identified by each source are real cases, i.e. genuine, serious ADRs. The review of discharge reports and spontaneous reporting forms allowed the checking of cases according to the case definition established for this study. Nevertheless, in some cases selected from the PMSI, it was sometimes difficult, from the discharge report, to be precise concerning the date of occurrence and/or the seriousness of the reaction.
The identification of cases in two steps (short-listing by computer requests followed by review of records) allowed refocusing the two sources on the same population, the same period and the same geographical area. To take into account delayed reports and to ensure equal catchability of cases in the Pharmacovigilance Database according to the date of occurrence, we extended the search to the whole year 2001.
Concerning the identification of matches, as the databases did not share a unique identifier, we identified common cases according to characteristics of patients, type of ADR, drugs involved and period of hospital stay. These criteria were sufficiently accurate to allow adequate identification of matches.
A further condition is that sources are independent. When three sources or more are available, the dependency can be assessed and taken into account using, in particular, log linear modelling. In the case of two sources, the dependency is mainly approached qualitatively by examining methods of data collection. In this study, despite different methods of data collection among the two sources, ADRs are often notified by the same physician who filled in the discharge report. A positive dependency would exist if, for example, an ADR notified to the regional Pharmacovigilance Centre, because it is particularly serious and/or unexpected, is more likely to be mentioned in the discharge summary. A positive dependency would underestimate the results.
ADRs could not have the same probability of being identified by each source: ADRs related to a new drug or unexpected ADRs are more likely to be reported to a pharmacovigilance structure. Since the PMSI has been established for care management, notification to the PMSI depends on the cost of care. In addition, procedures for coding data could differ between wards and some specific coding of ADRs in the ICD-10, such as drug-related aplastic anaemia, might facilitate reporting of these ADRs. These differences concerning heterogeneity between the two sources could lead to an overestimation of the total number of ADRs.
Despite these limitations, our estimate is in accordance with literature in this field [1–3]. During this 6-month period, 2.9% of inpatients presented a ‘serious’ ADR at admission or during their stay. The French network of Regional Centres of Pharmacovigilance has estimated the prevalence of ADRs in hospitalized patients at 10.3%, with ‘serious’ ADRs in 33% of cases [2].
These results highlight the lack of exhaustiveness of hospital and pharmacovigilance databases in identifying hospital-related ADRs. We found about twice as many ADRs by interrogating the PMSI database as were reported spontaneously, when only one-third of reports from the pharmacovigilance database were also identified in the administrative database. Previous studies comparing spontaneous reporting and screening ICD codes also found that more ADRs were identified by ICD codes than were spontaneously reported to a pharmacovigilance structure [4, 17]. Moreover, differences were seen in the profile of ADRs detected, with reactions with antitumoral agents, in particular, being identified more often from ICD codes. The two methods of detection bring additive information.
Conclusion
This study shows the lack of exhaustiveness of ADR recording whatever the sources of data – computerized medical record databases or traditional system of spontaneous reporting. Merging data from different databases could improve the detection of ADRs. Application of the capture–recapture method to hospital and pharmacovigilance databases could contribute to an estimate of the real impact of ADRs in hospital. However, more investigations concerning its validity and coding adjustments are necessary before its application in routine practice.
References
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Imbs JL, Pouyanne P, Haramburu F, Welsch M, Decker N, Blayac JP, Begaud B. Iatrogenic medication: estimation of its prevalence in French public hospitals. Regional Centers of Pharmacovigilance. Therapie. 1999;54:21–7. [PubMed] [Google Scholar]
- 3.Pouyanne P, Haramburu F, Imbs JL, Begaud B French Pharmacovigilance Centres. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000;320:1036. doi: 10.1136/bmj.320.7241.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox AR, Anton C, Goh CHF, Easter M, Langford NJ, Ferner RE. Adverse drug reactions in patients admitted to hospital identified by discharge ICD-10 codes and by spontaneous reports. Br J Clin Pharmacol. 2001;52:337–9. doi: 10.1046/j.0306-5251.2001.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003;10:115–28. doi: 10.1197/jamia.M1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couffignal AL, Lapeyre-Mestre M, Bonhomme C, Bugat R, Montastruc JL. Adverse effects of anticancer drugs: a propos of a pharmacovigilance study at a specialized oncology institution. Therapie. 2000;55:635–41. [PubMed] [Google Scholar]
- 7.Bagheri H, Michel F, Lapeyre-Mestre M, Lagier E, Cambus JP, Valdiguie P, Montastruc JL. Detection and incidence of drug-induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol. 2000;50:479–84. doi: 10.1046/j.1365-2125.2000.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugue A, Bagheri H, Lapeyre-Mestre M, Tournamille JF, Sailler L, Dedieu G, Salvayre R, Thouvenot JP, Massip P, Montastruc JL. Detection and incidence of muscular adverse drug reactions: a prospective analysis from laboratory signals. Eur J Clin Pharmacol. 2004;60:285–92. doi: 10.1007/s00228-004-0760-1. [DOI] [PubMed] [Google Scholar]
- 9.Bäckström M, Mjörndal T, Dahlqvist R. Under-reporting of serious adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2004;13:483–7. doi: 10.1002/pds.962. [DOI] [PubMed] [Google Scholar]
- 10.Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT, Hahn EG, Levy M, Brune K, Geisslinger G. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000;22:161–8. doi: 10.2165/00002018-200022020-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gallay A, Nardonel A, Vaillant V, Desenclos JC. The capture–recapture applied to epidemiology: principles, limits and application. Rev Epidemiol Sante Publique. 2002;50:219–32. [PubMed] [Google Scholar]
- 12.Jonville-Bera AP, Autret E. Can the sub-notification of drug adverse effects by capture/recapture method be evaluated? Therapie. 1996;51:169–75. [PubMed] [Google Scholar]
- 13.Vaccine Adverse Event Reporting System Team. Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT. Enhancing vaccine safety surveillance: a capture–recapture analysis of intussusception after rotavirus vaccination. Am J Epidemiol. 2001;154:1006. doi: 10.1093/aje/154.11.1006. [DOI] [PubMed] [Google Scholar]
- 14.Seeger JD, Schumock GT, Kong SX. Estimating the rate of adverse drug reactions with capture–recapture analysis. Am J Health Syst Pharm. 1996;53:178–81. doi: 10.1093/ajhp/53.2.178. [DOI] [PubMed] [Google Scholar]
- 15.Cormack RM. Problems with using capture–recapture in epidemiology: an example of a measles epidemic. J Clin Epidemiol. 1999;52:909–14. doi: 10.1016/s0895-4356(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis SN, Lowe PJ, Avery A, Levene S, Cormack RM. Children are not goldfish: mark/recapture techniques and their application to injury data. Inj Prev. 2000;6:46–50. doi: 10.1136/ip.6.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapeyre-Mestre M, Gary J, Machelard-Roumagnac M, Bonhomme C, Bugat R, Montastruc JL. Incidence and cost of adverse drug reactions in a French cancer institute. Eur J Clin Pharmacol. 1997;53:19–22. doi: 10.1007/s002280050331. [DOI] [PubMed] [Google Scholar]