Abstract
Aims
To determine the effect of gender and the genetic polymorphisms of CYP2J2, CYP3A4, CYP3A5 and MDR1 on the urinary excretion of the H1 antihistamine ebastine in healthy subjects.
Methods
Eighty-nine Caucasians were studied. The presence of polymorphisms in genes known to be involved in ebastine metabolism and transport (CYP2J2*2,*3,*4,*6,*7, CYP3A4*1B, CYP3A5*3, *6 and MDR1(ABCB1)C3435T) was assessed by means of PCR-restriction fragment length polymorphism and sequencing methods. Genotype was correlated with the urinary excretion of the main ebastine metabolites (desalkylebastine and carebastine) under basal conditions and after administration of grapefruit juice.
Results
Women excreted statistically greater amounts of desalkylebastine in urine (mean ± SD (95% confidence intervals, 95% CI), 23.0 ± 19.5 (18.1, 27.9) µmol) than men (12.4 ± 11.0 (7.9, 16.9)), (mean difference: 10.6 (2.4, 18.7), P < 0.005). The CYP2J2, CYP3A4 and CYP3A5 analysed polymorphisms did not greatly affect ebastine metabolite excretion. The MDR1C3435T polymorphism was found to affect both the urinary excretion of the active metabolite carebastine (32.3 ± 18.3 (23.1, 41.4), 22.8 ± 14.7 (18.6, 27.0) and 21.5 ± 15.3 (14.7, 28.3) for CC, CT and TT carriers, respectively; P < 0.05) and the grapefruit juice-induced inhibition of its transport/formation (mean fold-decrease ± SD (95% CI), 1.5 ± 0.8 (1.0, 2.0), 1.1 ± 0.9 (0.7, 1.4) and 0.9 ± 0.4 (0.6, 1.2) for CC, CT and TT carriers, respectively; P = 0.01).
Conclusions
Gender and the presence of the MDR1C3435T polymorphism both influence the excretion of ebastine metabolites in urine.
Keywords: ebastine, gender, polymorphism
Introduction
Ebastine (4-diphenylmethoxy-1-[3-(4-terbutylbenzoyl)-propyl] piperidine) is a long-acting new generation H1-receptor antagonist, which binds preferentially to peripheral H1 receptors in vivo and shows no apparent sedative properties over the therapeutic dose range [1].
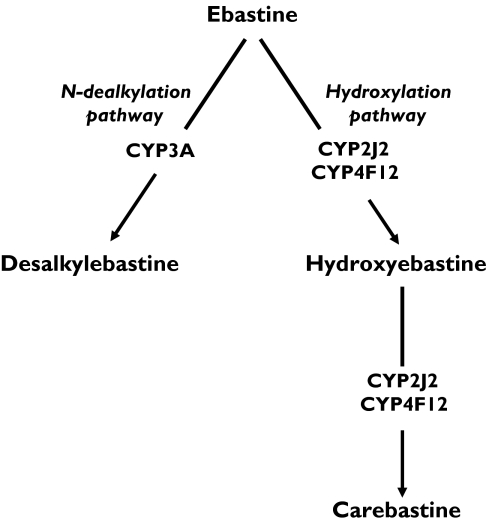
After oral administration, ebastine undergoes extensive first-pass metabolism, displaying pharmacokinetics that show marked interindividual variability [2]. The drug is rapidly and almost completely oxidized to carebastine, which is the major detectable metabolite and is believed to be responsible for the pharmacological effects of the drug [3]. Two inactive metabolites, hydroxyebastine, and desalkylebastine, are also formed [3–5] (Figure 1). Studies in human liver microsomes have shown that cytochrome P450 (CYP) 3A was the main ebastine dealkylase [6]. These studies were later extended to human intestinal microsomes, and the results showed that CYP2J2, and to a lesser extent CYP4F12, were the enzymes responsible for ebastine hydroxylation leading to the sequential formation of hydroxyebastine and carebastine [7] (Figure 1).
Figure 1.
Pathways of ebastine metabolism
All the genes involved in ebastine metabolism are known to possess allelic variants. The activity of the CYP3A sub-family is the sum of that of CYP3A4 and CYP3A5. These genes possess many single nucleotide polymorphisms (SNPs) (for a regularly updated review see http://www.imm.ki.se/CYPalleles), but only CYP3A4*1B and CYP3A5*3 have a significant frequency in Caucasians. The gene coding for CYP2J2, another important enzyme in ebastine metabolism, is also polymorphic and several SNPs (CYP2J2*2, CYP2J2*3, CYP2J2*4, CYP2J2*6 and CYP2J2*7) have recently been found to affect its ability to metabolize arachidonic acid [8]. However, in contrast to the large database on CYP3A4 and CYP3A5 polymorphisms, the in vivo importance of CYP2J2 allelic variants and their frequencies in different populations have not been established.
In addition to being extensively metabolized by isoforms of CYP, ebastine and its metabolites are also substrates of the MDR1 (also known as ABCB1)-encoded efflux transporter P-glycoprotein (P-gp) [9]. Among other locations, P-gp is found in the brush border surface of enterocytes and the apical surface of proximal tubular cells in kidneys, where it may contribute to the elimination of ebastine and to the excretion of its metabolites, respectively. There are a number of allelic variants of the MDR1 gene and one of them, the MDR1C3435T polymorphism in exon 26, has been associated with a decrease in P-gp expression in both intestine [10] and kidney [11]. However, other reports have failed to find alterations in the expression or functionality of P-gp in carriers of this polymorphism [12, 13].
The aim of the present work was to determine whether gender, grapefruit juice and any of the polymorphisms thought to determine ebastine biotranformation and transport, affect the urinary excretion of ebastine.
Methods
Subjects
The population consisted of 89 healthy Spanish Caucasians (63 women and 26 men) who were aware of the purpose of the study and gave oral and written informed consent for participation. Among the men, there were 22 nonsmokers and four smokers with an average consumption of 11 ± 4 cigarettes per day. Among the women, there were 48 nonsmokers and 15 smokers with an average consumption of 14 ± 6 cigarettes per day. Mean ± SD age of the men was 20.7 ± 1.9 years (range 18–24) and their mean body weight was 75.4 ± 10.5 kg (range 55–105). The age of the women averaged 20.1 ± 2.0 years (range 19–22) and their mean body weight was 58.5 ± 8.0 kg (range, 47–88). All subjects reported only occasional alcohol consumption and were not taking any medication at the time of the study, except six nonsmoking and three smoking women who were using oral contraceptives. All subjects were healthy as assessed by a thorough physical examination.
The study was approved by the Human Research Ethics Committee of the ‘Infanta Cristina’ University Hospital (Badajoz, Spain) and was conducted in accordance with the Declaration of Helsinki and its subsequent revisions.
Study protocol
A single oral dose of 20 mg ebastine (Ebastel Forte®, Almirall Prodesfarma, Barcelona, Spain) along with a standardized breakfast was administered to each subject in the morning (day 1). Urine was collected for the next 24 h in refrigerated containers, and total urine volumes were recorded. The samples were divided into aliquots and stored at −80 °C until analysis.
Five days after this study, a subgroup of 61 subjects (15 men) took 1500 ml of grapefruit juice divided between two consecutive days (days 5 and 6, three 250 ml cups per day). On the morning of day 7 they were administered a further dose of 20 mg ebastine along with 250 ml of grapefruit juice. Subsequent urine sampling was carried out as described for the first phase of study.
To prevent variation in the potency of inhibition between different batches of juice, we used at least a five-fold greater quantity of juice than that reported to cause a 50% decrease in intestinal CYP3A4 expression [14]. The grapefruit juice used in this study was obtained from a local supermarket and was chilled and made from concentrate. The juice had been produced from grapefruit (Citrus paradisi) and its brand name was Juver Alimentación S.A. (Murcia, Spain).
Genotyping
A 7 ml blood sample was drawn from each subject on day 1 of the study and immediately stored at −80 °C until genotype analysis. Genomic DNA was isolated from peripheral blood leucocytes using a Qiagen blood midi kit (Qiagen Inc., Chatsworth, CA, USA).
Previously sequenced DNA samples were used as negative and positive controls to rule out possible genotyping errors. Likewise, duplicate analysis of index samples was performed and the results were confirmed by sequencing (ABI3700 DNA Analyzer; Perkin-Elmer/Applied Biosystems, Stabvida Co., Oeiras, Portugal).
CYP2J2
Analysis of wild-type and allelic variants was achieved by means of a PCR-restriction fragment length polymorphism (PCR-RFLP) method developed in our laboratory. First, the exonic and proximal promoter regions containing known mutations were amplified by using primers and PCR conditions previously described [8] with one exception. Thus, in order to create an adequate restriction site for CYP2J2*3 detection (exon 3, 14,532C > T), we designed a new pair of primers for exon 3 amplification with a mismatch in the forward oligo [5′-GGTTTAGGAAAGAAGAGCTTAG AGGG-3′ (mismatch in boldface type), 5′-CTGTCC AATGAACAAATGGGC-3′] yielding a PCR product of 135 base pairs (bp). The following PCR conditions were used to amplify this fragment: 3 min at 94 °C (1 cycle); 30 s at 94 °C, 57 s at 30 s and 30 s at 72 °C (30 cycles); 7 min at 72 °C (1 cycle).
The amplification products for the different alleles of interest were then digested with restriction enzymes [TseI, HgaI, MfeI, Tsp509I and BfaI (New England Biolabs, Beverly, MA, USA) for CYP2J2*2, CYP2J2*3, CYP2J2*4, CYP2J2*6 and CYP2J2*7, respectively], using conditions recommended by the manufacturer. The resulting fragments were analysed in an agarose gel.
To our knowledge, this is the first time that a PCR-RFLP technique has been used for CYP2J2 genotyping.
CYP3A4
We used a PCR-RFLP method developed by Cavalli et al.[15] with some minor modifications in order to detect the CYP3A4*1B allele. The following PCR conditions were used: 7 min at 95 °C (1 cycle), 30 s at 94 °C, 15 s at 64 °C and 30 s at 72 min (40 cycles) and a final extension step of 7 min at 72 °C. The PCR products were submitted to MboII cleavage (New England Biolabs) in a total reaction volume of 10 µl and the digestion products were run for 2 h on a 3% agarose gel.
CYP3A5
The frequency of the CYP3A5*3 allele was determined by a previously described PCR-RFLP method [16]. In brief, 293 bp amplicons were incubated with SspI endonuclease restriction enzyme (New England Biolabs) at 37 °C for 2 h in a total volume of 10 µl. The resulting fragments were subsequently analysed on a 4% agarose gel.
We also screened the study population for the presence of the CYP3A5*6 allele by using a method described elsewhere [16]. The rationale for determining such an uncommon allele in Caucasians is that frequencies of CYP3A5 variant alleles have not been determined in Spanish subjects, even though our population has previously shown some anomalies in some CYP SNPs [17]. Moreover, CYP3A5*6 is present in up to 4% of Hispanics [18].
MDR1C3435T
The presence of the 3435T mutant allele was detected by using PCR conditions described previously [19]. PCR products were then subjected to direct sequencing (ABI3700 DNA Analyzer; Perkin-Elmer/Applied Biosystems).
Analysis of ebastine metabolites in urine
Urine samples (2 ml) were prepared using the method by Matsuda et al. [20] with minor modifications. Briefly, samples extracted with methanol-acetonitrile were centrifuged and the supernatant loaded to a preconditioned solid-phase extraction column. Compounds were eluted with a methanol-phosphate buffer solution, which was evaporated to dryness under nitrogen. The residue was then reconstituted in 100 µl of mobile phase and a 30 µl aliquot of the solution was analysed.
HPLC-mass spectrometry was used to determine ebastine metabolite concentrations. The chromatography consisted of an Ultrasphere ODS (750 mm × 4.6 mm i.d., 5 µm particle size; Beckman Coulter, Fullerton, CA, USA) reversed-phase column, eluted isocratically with a mobile phase containing acetonitrile : 0.012 m ammonium acetate buffer (52 : 48, v : v). The mobile phase was filtered and degassed ultrasonically for 15 min. The flow-rate was 0.5 ml min−1. The eluted compounds were detected by positive electrospray ionization. The selected ion monitoring (SIM) mode was used for quantification using the protonated molecular ions of each analyte.
To establish the appropriate SIM conditions, we collected full-scan mass spectra of ebastine metabolites and flunarizine (used as internal standard) in the range of 220–550 atomic mass units at different fragmentor voltages (range 55–180 V). On the basis of the observed fragmentation, the fragmentor was set at 55 V for SIM detection of desalkylebastine and 105 V for detection of carebastine and internal standard.
Urine standard curves ranged from 30–10 000 ng ml−1 for each compound.
The intraday accuracy of the analytes ranged from 93% to 108% and the coefficient of variation from 2–11%. The interday accuracy varied from 85% to 113%, with the coefficient of variation from 2 to 12%. The smallest determinable concentration was 0.3 ng ml−1 for all the compounds.
Statistical analysis
Results from the different genotypes and genders were compared using the nonparametric Wilcoxon or Kruskal–Wallis tests, depending on the distribution of the data. Multiple comparisons between groups (using the Bonferroni method) were also carried out. For the grapefruit juice inhibition study, the Wilcoxon signed rank test was used to analyse differences between matched pairs. The unpaired t-test and the chi-square test were used to compare differences between demographic data. Analysis of covariance (ancova) was carried out after logarithmic conversion of the data, to assess collectively the effect of two categorical variables (gender and genotype) on the variability of ebastine metabolite concentrations (JMP program package, version 5.0.1, SAS Institute, Cary, NC, USA). A power analysis was not carried out as this was a pilot study and there were no prior data on which to base these calculations.
A P value less than 0.05 was regarded as statistically significant.
Results
The genotyping analysis carried out on CYP2J2, CYP3A4, CYP3A5 and MDR1 genes was successful in all 89 Caucasian subjects studied. None of the individuals was a carrier of the CYP2J2*2, CYP2J2*3, CYP2J2*4, CYP2J2*6 or CYP3A5*6 variants. The frequencies of the remaining mutant alleles analysed (Table 1) showed no significant deviations from Hardy–Weinberg equilibrium (χ2, P values: 0.46, 0.792; 0.15, 0.927; 1.61, 0.445 and 0.951, 0.621 for CYP2J2*7, CYP3A4*1B, CYP3A5*3 and MDR1C3435T, respectively) and were in agreement with previously reported data in Caucasians [8, 16, 21].
Table 1.
Allelic frequencies for CYP2J2, CYP3A and MDRIC3435T and number of subjects possessing each genotype
| Genotype | Observed (%) | Mutant allele frequency (%) |
|---|---|---|
| CYP2J2*7 | q = 6.74 | |
| *1/*1 | 77 (86.5) | |
| *1/*7 | 12 (13.5) | |
| *7/*7 | 0 (0) | |
| CYP3A4*1B | q = 3.93 | |
| *1 A/*1 A | 82 (92.1) | |
| *1 A/*1B | 7 (7.9) | |
| *1B/*1B | 0 (0) | |
| CYP3A5*3 | q = 89.88 | |
| *1/*1 | 2 (2.2) | |
| *1/*3 | 14 (15.7) | |
| *3/*3 | 73 (82.0) | |
| MDR1C3435T | q = 52.24 | |
| CC | 18 (20.2) | |
| CT | 49 (55.1) | |
| TT | 22 (24.7) |
Urine concentrations of desalkylebastine and carebastine showed large interindividual variability in the population. Ninety-five per cent of the desalkylebastine data (excluding the highest outliers) displayed a 60-fold range (0.9–54.9 µmol), which was skewed towards lower values. Data for carebastine exhibited lower variability, with 93% of the data varying by 19-fold (2.4–44.8 µmol), which also skewed to the lower values.
Women excreted statistically significant higher amounts of desalkylebastine (mean ± SD (95% CI) 23.0 ± 19.5 µmol (18.1, 27.9)) than men (12.4 ± 11.0 (7.9, 16.9)) (mean difference 10.6 (2.4, 18.7), P < 0.005; Table 2). The same trend for amounts of carebastine was observed, but differences did not reach statistical significance (mean difference: 7.6 (0.4, 14.9), P = 0.07; Table 2). Differences in age (P = 0.78) and smoking status (χ2 = 0.35, P = 0.55) between men and women were not statistically significant. As body weight was significantly different between men and women (P = 0.001), we introduced body weight as a correction factor. After correction for body weight, gender-related discrepancies were decreased for desalkylebastine excretion (mean difference: 22.9 (0.4, 46.7), P < 0.05), although they were still statistically significant. However, no differences were found after correction for body weight of the amounts of carebastine excreted in the urine (8.7 (−14.4, 31.7), P = 0.79).
Table 2.
Influence of smoking and gender on the excretion of ebastine, measured as µmols of metabolites in urine, in Caucasian healthy subjects. Values are mean ± SD (95% CI)
| Nonsmoking | Smoking | Total | |
|---|---|---|---|
| Men | |||
| n | 22 | 4 | 26 |
| Desalkylebastine | 11.8 ± 11.0 (6.9, 16.6) | 15.8 ± 12.3 (−3.8, 35.3) | 12.4 ± 11.0 (7.9, 16.9) |
| Carebastine | 19.4 ± 10.0 (14.9, 23.8) | 17.0 ± 12.0 (−2.7, 36.1) | 19.0 ± 10.1 (14.9, 23.1) |
| Women | |||
| n | 48 | 15 | 63 |
| Desalkylebastine | 25.5 ± 21.5 (19.2, 31.7) | 15.1 ± 7.4 (11.0, 19.2) | 23.0 ± 19.6*** (18.1, 27.9) |
| Carebastine | 25.8 ± 16.1 (21.1, 30.5) | 29.2 ± 21.3 (17.4, 41.0) | 26.6 ± 17.4 (22.2, 31.0) |
n, number of individuals; SD, standard deviation; 95% CI, 95% confidence interval
P < 0.005 vs. men.
No significant differences in metabolite excretion were observed after comparing smoking and nonsmoking subjects, either considering the population as a whole (data not shown) or after stratification for gender (Table 2).
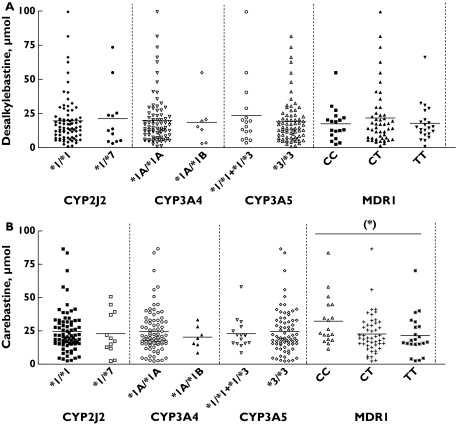
There were no apparent associations between desalkylebastine or carebastine excretion and the presence of the CYP3A4*1B or CYP2J2*7 variant alleles (Table 3, Figure 2). The analysis of the CYP3A5*3 polymorphism was carried out assuming that individuals carrying only one active allele (*1/*3) are able to express functional enzyme in amounts comparable with wild type homozygotes [22]. Thus both genotypes were grouped for statistical purposes. Subjects with at least one active allele exhibited modestly higher desalkylebastine excretion in urine than CYP3A5*3/*3 carriers (Table 3, Figure 2), although the differences were not statistically significant. Multiple-comparison analysis for all the CYP genotypes studied revealed no associations with the amount of desalkylebastine excreted in urine.
Table 3.
Urinary desalkylebastine and carebastine values (µmol) (mean ± SD (95% CI)) according to CYP2J2*7, CYP3A4*1B, CYP3A5*3 and MDR1C3435T genotypes
| Genotype | n | Desalkylebastine | Carebastine |
|---|---|---|---|
| CYP2J2*7 | |||
| *1/*1 | 77 | 19.7 ± 17.6 (15.7, 23.7) | 24.6 ± 16.0 (20.1, 28.2) |
| *1/*7 | 12 | 21.2 ± 21.9 (7.3, 35,2) | 22.9 ± 16.2 (12.6,33.2) |
| CYP3A4*1B | |||
| *1 A/*1 A | 82 | 20.0 ± 18.2 (16.0, 24.0) | 24.7 ± 16.5 (21.0, 28.3) |
| *1 A/*1B | 7 | 18.7 ± 17.5 (2.5, 34.8) | 21.2 ± 7.1 (14.6, 27.8) |
| CYP3A5*3 | |||
| *1/*1 +*1/*3 | 16 | 23.6 ± 24.3 (10.6, 36.6) | 23.4 ± 11.0 (17.6, 29.3) |
| *3/*3 | 73 | 19.1 ± 16.5 (15.2, 22.9) | 24.6 ± 16.9 (20.7, 28.6) |
| MDR1C3435T | |||
| CC | 18 | 17.5 ± 12.4 (11.3, 23.6) | 32.3 ± 18.3* (23.2, 41.4) |
| CT | 49 | 21.7 ± 21.4 (15.6, 27.8) | 22.8 ± 14.7 (18.6, 27.0) |
| TT | 22 | 17.9 ± 13.7 (11.8, 23.9) | 21.5 ± 15.3 (14.7, 28.3) |
n, number of individuals;
P < 0.05 vs. the other C3435T genotypes.
Figure 2.
Genotype–phenotype associations between the amounts of desalkylebastine (A) and carebastine (B) excreted in the urine and the CYP2J2*7, CYP3A4*1B, CYP3A5*3 and MDR1C3435T polymorphisms. (*) P < 0.05
When the MDR1C3435T polymorphism was analysed, no differences were observed in desalkylebastine urine excretion between the three different genotypes. However, multiple-comparison analysis of the three MDR1 genotypic groups showed that there was a statistically significant difference between CC vs. CT (P < 0.05) and TT (P < 0.05) carriers with regard to carebastine excretion in urine (Table 3, Figure 2).
To determine whether gender could have biased the observed effect of C3435T polymorphism, ancova analysis was performed to include both variables in a statistical model. Based on this analysis, the CC genotype is the factor that most likely contributes to the differences in carebastine excretion (P = 0.01), whereas the effect of gender did not reach statistical significance (P = 0.06).
Administration of grapefruit juice to a subgroup of 61 individuals resulted in an approximately 25% statistically significant decrease in desalkylebastine excretion in urine (mean ± SD (CI), 23.9 ± 20.0 (18.8, 29.0) µmol vs. 18.7 ± 15.7 (14.6, 22.7); mean difference: −5.25 (−1.23, −9.27); P = 0.01).
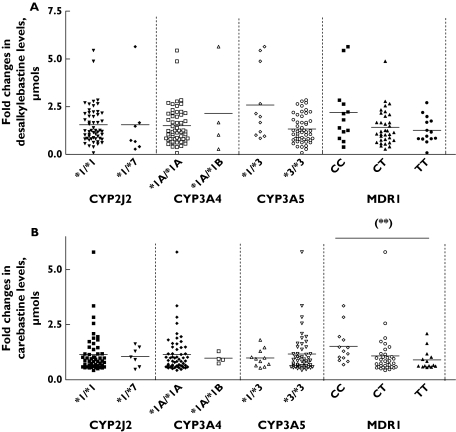
An analysis of the different CYP3A genotypes revealed that CYP3A5*1/*3 carriers showed two-fold higher decreases in desalkylebastine excretion (mean ± SD (95% CI), 2.6 ± 1.9 (1.3, 3.8)) than CYP3A5*3/*3 carriers (1.3 ± 0.7 (1.1, 1.5); χ2 = 4.6, P < 0.05) (Figure 3). However, when multiple-comparison analysis was carried out, this association was not statistically significant (P = 0.09).
Figure 3.
Genotype–phenotype associations between fold-changes (day 1/day 7) in the amounts of desalkylebastine excreted in the urine and CYP2J2*7, CYP3A4*1B, CYP3A5*3 and MDR1C3435T polymorphisms following administration of grapefruit juice. (**) P = 0.01
ancova analysis was performed to assess the role of the CYP3A5 genotype while controlling for the effect of gender. The whole model showed a significant contribution to the observed changes in the degree of impairment of metabolite excretion (R2 = 0.13; P = 0.01). The CYP3A5*1/*3 genotype was more strongly related to such variability (P < 0.01) than gender (P = 0.23). Thus the presence of at least one active CYP3A5 allele may have an effect on the degree of grapefruit juice-induced inhibition of ebastine desalkylation independent of gender.
In contrast to the results for desalkylebastine, the urinary excretion of the CYP2J2-generated metabolite, carebastine, was not substantially altered after grapefruit juice administration (mean ± SD (95% CI), 23.0 ± 14.3 (19.4, 26.7) µmol vs. 23.7 ± 13.7 (20.1, 27.2); mean difference −0.61 (−2.98, 4.21); P = 0.73). However, when subjects were stratified according to MDR1C34345T genotype, homozygous wild types (but not the other genotypes) showed a significant decrease in carebastine urinary excretion after grapefruit juice (mean fold-decrease ± SD (95% CI), 1.5 ± 0.8 (1.0, 2.0), 1.1 ± 0.9 (0.7, 1.4) and 0.9 ± 0.4 (0.6, 1.2) for CC, CT and TT carriers, respectively; χ2 = 9.0, P = 0.01) (Figure 3). Multiple-comparison testing revealed statistically significant differences between CC vs. CT (P < 0.05) and TT (P < 0.05) genotypes. ancova analysis including gender as a variable again showed a significant effect of the whole statistical model on the inhibition of carebastine elimination (r2 = 0.15; P < 0.05). However, gender did not affect the degree of inhibition (P = 0.24), whereas the MDR1 CC genotype contributed significantly to the differences in the extent of the impairment of carebastine elimination by grapefruit juice (P = 0.01).
Discussion
Ebastine undergoes extensive intestinal first pass metabolism, and subjects with severely impaired hepatic function do not apparently show differences in their metabolic profile of the drug [25]. The main enzymes responsible for ebastine metabolism, CYP3A and CYP2J2, are present in intestine [7, 26, 27], where their activity could be contributing to the first pass metabolism of ebastine and to the large interindividual variability observed [7]. Our findings indicated that urine excretion of the CYP3A-generated metabolite, desalkylebastine, displayed a three-fold higher variability than that of carebastine, which is consistent with the large variation observed in the urinary excretion of other substrates metabolized by CYP3A [29, 30].
Our findings showed that women excreted more of the CYP3A-generated metabolite, desalkylebastine, than men. Previous studies have shown that CYP3A activity in female subjects is higher than in males [31, 32]. Furthermore, gender has been related to the extension of impairment in driving performance after consumption of second-generation antihistamines [33]. However, a previous study did not detect gender-related differences in the pharmacokinetics of ebastine, although the authors did not measure CYP3A-generated metabolites [2].
We did not find an association between the presence of the CYP2J2*7 and CYP3A4*1B alleles and the excretion of ebastine metabolites in urine, which is consistent with the observations that CYP2J2*7 is not an exonic mutation and the in vivo effect of CYP3A4*1B on enzyme activity remains to be consistently demonstrated. On the other hand, CYP3A5*1 carriers were found to display a modestly higher excretion of desalkylebastine than individuals with the CYP3A5*3/*3 genotype, suggesting that along with CYP3A4, CYP3A5 could also be involved in ebastine metabolism.
Ebastine and its metabolites are also substrates of P-gp [9, 28], an efflux transporter found in the apical surface of enterocytes, where it may reduce the drug’s disposition. Our results indicate that MDR1C3435T polymorphism is associated with an impaired elimination of the active metabolite of ebastine, carebastine. The observation of lower excretion of carebastine values being displayed by T allele carriers could be the result of a decrease in the efflux transport of the drug from the enterocytes to the intestinal lumen, as this polymorphism has been associated with decreased expression of P-gp in the gut [10]. However, and according to previous reports, this finding could also be the consequence of decreased expression and transport capacity of the P-gp variant located in the luminal brush border membrane of the proximal tubule cells in the kidney [11]. These results are consistent with data in experimental animals suggesting that carebastine is a substrate of P-gp [28], albeit with lower affinity than the parent drug [9].
To determine whether inhibition of the intestinal first pass effect of ebastine had consequences for the urinary excretion of the drug, we administered grapefruit juice prior to the administration of ebastine. Components of this beverage have been shown to both decrease intestinal CYP3A4 expression and inhibit P-gp-mediated activity [23, 24]. Grapefruit juice produced a decrease in the excretion of urinary desalkylebastine, which is consistent with inhibition of CYP3A activity. Furthermore and irrespective of gender, CYP3A5*1 carriers showed two-fold higher decrease in metabolite excretion values after grapefruit juice intake compared with the CYP3A5*3/*3 genotype. Thus subjects who express CYP3A5 could be more susceptible to the influence of enzyme inhibitors, which might have clinical relevance. For instance, carriers of the CYP3A5*1 allele have higher dosage requirements for tacrolimus [34]. If these subjects were administered a CYP3A inhibitor, their drug plasma concentrations may increase to a hazardous level.
A gene-dose effect was evident regarding the magnitude of inhibition of carebastine elimination by grapefruit juice between the different MDR1C3435T genotypes. This effect may be a direct consequence of inhibition of P-gp in kidney as well as in the intestine. It has been shown that the components of grapefruit juice are able to decrease the expression and activity of P-gp in human renal cell lines [35].
Some of the limitations of this study are those typical of a Caucasian population, namely the low number of subjects carrying the CYP3A4*1B and CYP3A5*1 alleles. Another limitation could be that only one MDR1 variant allele, although with demonstrated functional differences in activity in the kidney [11], and not haplotypes of this gene were assessed. In the same manner, single and multiple-dose pharmacokinetic studies to determine how well carebastine metabolite urine excretion reflects plasma concentration could better clarify the influence of MDR1 pharmacogenetics on ebastine disposition.
In summary, we have shown that gender is an element to be considered in ebastine elimination, especially with regard to the CYP3A-mediated metabolism of the drug. In addition, whereas polymorphisms in CYP3A4, CYP3A5 and CYP2J2 genes did not greatly affect the amounts of ebastine metabolites excreted in urine, those of the non-CYP3A-generated active metabolite carebastine were shown to be lower in individuals carrying the mutant MDR1 C3435T allele, suggesting decrease of P-gp activity in the gut and/or renal tubular cells. Moreover, this MDR1 polymorphism is related to the degree of grapefruit juice-induced inhibition of the urinary excretion of ebastine metabolites into urine. Additional studies should confirm whether variations in these urinary measures of ebastine disposition might be indicative of clinical outcomes such as drowsiness or potential cardiotoxicity.
Acknowledgments
We thank Mr Luis Lozano for technical help in blood sampling and DNA extraction and Professor Miguel Gonzalez for helpful statistical advice. We also thank Almirall Laboratories (Almirall Prodesfarma SA, Barcelona, Spain) for kindly providing ebastine and metabolites for analysis.
This study was supported in part by grants 02/0406 and 03/1432 from Fondo de Investigacion Sanitaria, Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid (Spain) and Fondo Social Europeo; and grant SCSS0502 and SCSS0549 from Junta de Extremadura, Consejeria de Sanidad y Consumo, Merida (Spain).
References
- 1.Wiseman LR, Faulds D. Ebastine: a review of its pharmacological properties and clinical efficacy in the treatment of allergic disorders. Drugs. 1996;51:260–77. doi: 10.2165/00003495-199651020-00006. [DOI] [PubMed] [Google Scholar]
- 2.Rohatagi S, Gillen M, Aubeneau M, Jan C, Pandit B, Jensen BK, Rhodes G. Effect of age and gender on the pharmacokinetics of ebastine after single and repeated dosing in healthy subjects. Int J Clin Pharmacol Ther. 2001;39:126–34. doi: 10.5414/cpp39126. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi T, Hashizume T, Matsuda M, Sakashita M, Fujii T, Sekine Y, Nakashima M, Uematsu T. Pharmacokinetics of the H1-receptor antagonist ebastine and its active metabolite carebastine in healthy subjects. Arzneimittelforschung. 1994;44:59–64. [PubMed] [Google Scholar]
- 4.Fujii T, Matsumoto S, Amejima H, Hatoyama T, Nakao M, Kagemoto A, Tanaka K, Miyazaki H. Absorption, distribution, metabolism and excretion of [14C]ebastine after a single administration in rats. Arzneimittelforschung. 1994;44:527–38. [PubMed] [Google Scholar]
- 5.Matsuda M, Sakashita M, Mizuki Y, Yamaguchi T, Fujii T, Sekine Y. Comparative pharmacokinetics of the histamine H1-receptor antagonist ebastine and its active metabolite carebastine in rats, guinea pigs, dogs and monkeys. Arzneimittelforschung. 1994;44:55–9. [PubMed] [Google Scholar]
- 6.Hashizume T, Mise M, Terauchi Y, O L, Fujii T, Miyazaki H, Inaba T. N-Dealkylation and hydroxylation of ebastine by human liver cytochrome P450. Drug Metab Dispos. 1998;26:566–71. [PubMed] [Google Scholar]
- 7.Hashizume T, Imaoka S, Mise M, Terauchi Y, Fujii T, Miyazaki H, Kamataki T, Funae Y. Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes. J Pharmacol Exp Ther. 2002;300:298–304. doi: 10.1124/jpet.300.1.298. [DOI] [PubMed] [Google Scholar]
- 8.King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, Spiecker M, Liao JK, Mohrenweiser H, Zeldin DC. Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol. 2002;61:840–52. doi: 10.1124/mol.61.4.840. [DOI] [PubMed] [Google Scholar]
- 9.Imamura Y, Shimizu K, Yamashita F, Yamaoka K, Takakura Y, Hashida M. Transport characteristics of ebastine and its metabolites across human intestinal epithelial Caco-2 cell monolayers. Biol Pharm Bull. 2001;24:930–4. doi: 10.1248/bpb.24.930. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegsmund M, Brinkmann U, Schaffeler E, Weirich G, Schwab M, Eichelbaum M, Fritz P, Burk O, Decker J, Alken P, Rothenpieler U, Kerb R, Hoffmeyer S, Brauch H. Association of the P-glycoprotein transporter MDR1 (C3435T) polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol. 2002;13:1847–54. doi: 10.1097/01.asn.0000019412.87412.bc. [DOI] [PubMed] [Google Scholar]
- 12.Siegmund W, Ludwig K, Giessmann T, Dazert P, Schroeder E, Sperker B, Warzok R, Kroemer HK, Cascorbi I. The effects of the human MDR1 genotype on the expression of duodenal P-glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther. 2002;72:572–83. doi: 10.1067/mcp.2002.127739. [DOI] [PubMed] [Google Scholar]
- 13.Gerloff T, Schaefer M, Johne A, Oselin K, Meisel C, Cascorbi I, Roots I. MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br J Clin Pharmacol. 2002;54:610–6. doi: 10.1046/j.1365-2125.2002.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, He K, Lown KS, Woster PM, Rahman A, Thummel KE, Fisher JM, Hollenberg PF, Watkins PB. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25:1228–33. [PubMed] [Google Scholar]
- 15.Cavalli SA, Hirata MH, Hirata RD. Detection of MboII polymorphism at the 5′ promoter region of CYP3A4. Clin Chem. 2001;47:348–51. [PubMed] [Google Scholar]
- 16.van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48:1668–71. [PubMed] [Google Scholar]
- 17.Agundez JA, Martinez C, Ledesma MC, Ladona MG, Ladero JM, Benitez J. Genetic basis for differences in debrisoquin polymorphism between a Spanish and other white populations. Clin Pharmacol Ther. 1994;55:412–7. doi: 10.1038/clpt.1994.50. [DOI] [PubMed] [Google Scholar]
- 18.Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics. 2004;5:243–72. doi: 10.1517/phgs.5.3.243.29833. [DOI] [PubMed] [Google Scholar]
- 19.von Ahsen N, Richter M, Grupp C, Ringe B, Oellerich M, Armstrong VW. No influence of the MDR-1 C3435T polymorphism or a CYP3A4 promoter polymorphism (CYP3A4–V allele) on dose-adjusted cyclosporin A trough concentrations or rejection incidence in stable renal transplant recipients. Clin Chem. 2001;47:1048–52. [PubMed] [Google Scholar]
- 20.Matsuda M, Mizuki Y, Terauchi Y. Simultaneous determination of the histamine H1-receptor antagonist ebastine and its two metabolites, carebastine and hydroxyebastine, in human plasma using high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;757:173–9. doi: 10.1016/s0378-4347(00)00494-1. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Martin E, Martinez C, Pizarro RM, Garcia-Gamito FJ, Gullsten H, Raunio H, Agundez JA. CYP3A4 variant alleles in white individuals with low CYP3A4 enzyme activity. Clin Pharmacol Ther. 2002;71:196–204. doi: 10.1067/mcp.2002.121371. [DOI] [PubMed] [Google Scholar]
- 22.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 23.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997. pp. 2545–53. [DOI] [PMC free article] [PubMed]
- 24.Honda Y, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br J Pharmacol. 2004;143:856–64. doi: 10.1038/sj.bjp.0706008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasseter KC, Dilzer SC, Vargas R, Waldman S, Noveck RJ. Pharmacokinetics and safety of ebastine in patients with impaired hepatic function compared with healthy volunteers: a phase I open-label study. Clin Pharmacokinet. 2004;43:121–9. doi: 10.2165/00003088-200443020-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hashizume T, Mise M, Matsumoto S, Terauchi Y, Fujii T, Imaoka S, Funae Y, Kamataki T, Miyazaki H. A novel cytochrome P450 enzyme responsible for the metabolism of ebastine in monkey small intestine. Drug Metab Dispos. 2001;29:798–805. [PubMed] [Google Scholar]
- 27.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Tamai I, Kido Y, Yamashita J, Sai Y, Tsuji A. Blood–brain barrier transport of H1-antagonist ebastine and its metabolite carebastine. J Drug Target. 2000;8:383–93. doi: 10.3109/10611860008997914. [DOI] [PubMed] [Google Scholar]
- 29.McCune JS, Lindley C, Decker JL, Williamson KM, Meadowcroft AM, Graff D, Sawyer WT, Blough DK, Pieper JA. Lack of gender differences and large intrasubject variability in cytochrome P450 activity measured by phenotyping with dextromethorphan. J Clin Pharmacol. 2001;41:723–31. doi: 10.1177/00912700122010627. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki K, Inagaki M, Kataoka T, Sekido I, Gill MA, Nishida M. A wide interindividual variability of urinary 6beta-hydroxycortisol to free cortisol in 487 healthy Japanese subjects in near basal condition. Ther Drug Monit. 2002;24:722–7. doi: 10.1097/00007691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Zhu B, Liu ZQ, Chen GL, Chen XP, Ou-Yang DS, Wang LS, Huang SL, Tan ZR, Zhou HH. The distribution and gender difference of CYP3A activity in Chinese subjects. Br J Clin Pharmacol. 2003;55:264–9. doi: 10.1046/j.1365-2125.2003.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–21. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 33.Verster JC, Volkerts ER. Antihistamines and driving ability: evidence from on-the-road driving studies during normal traffic. Ann Allergy Asthma Immunol. 2004;92:294–303. doi: 10.1016/S1081-1206(10)61566-9. quiz 303–5, 355. [DOI] [PubMed] [Google Scholar]
- 34.Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–54. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Romiti N, Tramonti G, Donati A, Chieli E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004;76:293–302. doi: 10.1016/j.lfs.2004.06.015. [DOI] [PubMed] [Google Scholar]