Abstract
Aims
A non-invasive proposed method for measuring CYP3A activity is the urinary 6β-hydroxycortisol:cortisol ratio. This ratio has been used as an indicator of CYP3A induction and inhibition, with mixed results. This investigation evaluated the relationship between a validated, biomarker, intravenous midazolam clearance and the urinary cortisol ratio under constitutive conditions and with the influence of a moderate CYP3A inhibitor.
Methods
This was a sequential, cross-over study design. Intravenous midazolam 0.025 mg kg−1 was administered to 10 male and 10 female subjects once every 14 days for 4 months. Fluvoxamine 150 mg day−1 was given to all subjects during the last two visits. Total body clearance of midazolam and urinary 6β-hydroxycortisol:cortisol molar ratio were used as biomarkers of hepatic CYP3A activity.
Results
No significant correlations were found between these two markers (r2 < 0.5, P > 0.05). Larger interindividual and intra-individual variability in CYP3A activity was observed in 6β-hydroxycortisol:cortisol ratios compared with midazolam clearances. With fluvoxamine therapy, midazolam clearance values decreased approximately 1.5-fold and cortisol ratios decreased approximately 1.9-fold.
Conclusions
The high intra-individual variability of the urinary cortisol ratio, compared with midazolam, makes this a suboptimal CYP3A phenotyping tool.
Keywords: 6β-hydroxycortisol, 6β-hydroxycortisol/cortisol molar ratio, CYP3A, fluvoxamine, midazolam clearance, phenotyping
Introduction
The cytochrome P450 3A (CYP3A) subfamily of enzymes is responsible for 50–60% of all CYP drug metabolism [1]. Commonly used drugs metabolized by CYP3A include immunosuppressive agents such as cyclosporine, HMG Co-A reductase (3-hydroxy-3-methyl-glutaryl-CoA reductase) inhibitors, benzodiazepines, macrolide antibiotics, calcium channel blockers and protease inhibitors [2]. Interindividual variability in CYP3A activity is reported to be 10–60-fold [3, 4]. This heterogeneity may result in subtherapeutic and/or toxic drug concentrations from a standard dose of medication in a given patient population [5].
Sex differences in the pharmacokinetics and pharmacodynamics for a number of drugs have been reported. Some previous work suggests that CYP3A4 activity can be 20–40% higher in women than in men [6, 7], although the data are conflicting [8]. Other evidence suggesting a hormonal influence on CYP3A activity exists in pregnancy. 6β-hydroxycortisol:cortisol ratios have been found to increase 300% in the third trimester of pregnancy and return to baseline within 3 months after delivery [9].
Several methods have been used to measure CYP3A activity in vivo: the erythromycin breath test (ERMBT), plasma midazolam clearance, urinary 6β-hydroxycortisol:cortisol ratio, urinary dapsone metabolite ratio and 6β-hydroxycortisol clearance [10]. Of these, the most accepted CYP3A biomarkers are midazolam plasma clearance and the ERMBT [11]. However, only midazolam has been most appropriately validated as a CYP3A biomarker. In general, these biomarkers require invasive methods to administer the drug and/or collect the samples for analysis.
A proposed non-invasive method for measuring CYP3A activity is the urinary 6β-hydroxycortisol: cortisol ratio. Although some investigators have suggested this method is suboptimal for measuring CYP3A activity [12, 13], it is still currently being used by many [14–17]. This ratio has been used as an indicator of CYP3A induction [18, 19] and inhibition [20] with mixed results. However, this ratio has not been effectively used as an individual measurement of true CYP3A activity [21]. The urinary cortisol ratio has been directly compared to two other nonvalidated CYP3A biomarkers. No notable correlation has been found with either the ERMBT [19, 20, 22] or the dapsone hydroxylation index [11, 19]. These relationships may be confounded by alternative pathways of metabolism for dapsone (intestinal and hepatic CYP3A4, CYP2C9, CYP2E1) and transport by P-glycoprotein for erythromycin.
One suggested explanation for the lack of correlation of these CYP3A probes is the differences in their Biopharmaceutical Classification System (BCS) classes. The BCS categorizes compounds according to their solubility, permeability and transporter affinity; cortisol is most likely Class 1, dapsone is Class 2 and erythromycin is Class 3 [12]. Midazolam is similar to cortisol in that it is a Class 1 compound and is not a substrate for P-glycoprotein [23]. Therefore, of all CYP3A probes, the correlation between midazolam and cortisol may result in the best prediction of CYP3A activity. The current investigation examined the relationship between intravenous midazolam plasma clearance and the urinary cortisol ratio, both under constitutive conditions and with fluvoxamine (moderate CYP3A inhibitor) as part of a comprehensive phenotyping investigation. The influence of sex and menstrual cycle on the cortisol ratio was also examined. Finally, inter- and intra-individual variability of these biomarkers were compared.
Methods
The protocol was approved by the Institutional Review Board of Bassett Healthcare. Written informed consent was obtained from all subjects.
Study subjects
Ten male and 10 premenopausal female caucasian healthy nonsmoking volunteers were enrolled into the study. All subjects underwent a thorough history and physical examination, an electrocardiogram, blood chemistry assessment and urinalysis screening. Subjects were excluded if they were taking any concomitant medications. Subjects were also excluded if their serum creatinine was not within the normal range (for men 0.6–1.2 mg dl−1; for women 0.5–1.0 mg dl−1) or if their hepatic transaminases, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were >60 U l−1 (normal range for AST and ALT is 5–43 U l−1 and 5–60 U l−1, respectively). Women who were pregnant or using hormonal contraception were also excluded. Women were required to have regular, predictable menstrual cycles, defined by a 3-month menstrual diary demonstrating cycle lengths within 3 days of each other.
Study design
This was a sequential, cross-over study design. Eight phenotyping visits were performed for each subject, including six visits for baseline (constitutive) measurements and two visits for CYP3A inhibition measurements. During the last two study visits (7 and 8), oral fluvoxamine 150 mg daily, a moderate inhibitor of CYP3A, was taken by subjects in an effort to evaluate the discriminatory abilities of the biomarkers (4 weeks of fluvoxamine therapy). Male subjects were phenotyped once every 14 days for 4 months. Female subjects were phenotyped once during the midfollicular (days 5 through 8 of the cycle) and midluteal (days 17 through 20 of the cycle) phases of one menstrual cycle for four cycles.
The follicular phase of the menstrual cycle was determined by patient assessment of the onset of menses. Approximately 2 weeks after the start of menses, a luteinizing hormone surge facilitates ovulation and this surge signals the start of the luteal phase. Urinary surge in leutenizing hormone (LH) was determined through the utilization of home ovulation kits (One-step Clearplan Easy™). Female subjects were instructed to test first morning urine for qualitative LH 3 days before predicted midcycle (day 14 of the cycle) and to continue until a positive result was found to determine ovulation accurately.
Subjects were required to abstain from ingesting caffeine, grapefruit and grapefruit juice products, cruciferous vegetables (e.g. broccoli, cauliflower, cabbage, brussels sprouts and kale), charbroiled meats, and ethanol 3 days prior to each study visit.
Midazolam and 6β-hydroxycortisol:cortisol phenotyping procedures
Midazolam total body clearance and urine 6β-hydroxycortisol:cortisol molar ratio were used to evaluate hepatic CYP3A activity. On the morning of each study visit, subjects received a single dose of 0.025 mg kg−1 intravenous midazolam (Versed, provided by Hoffman-La Roche, Inc., Nutley, NJ, USA) administered over 60 s into an antecubital vein. Blood samples (15 ml) were obtained at 0, 5, 30, 60, 120, 240, 300 and 360 min after midazolam administration using an intravenous catheter placed in the opposite arm. The respiratory status of all subjects was monitored by vital sign measurement and pulse oximetry for the first hour after midazolam administration. Samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes, kept on ice and centrifuged within 2 h after collection at 1000 g and 4 °C for 15 min. Blood plasma was aliquoted and stored at −80 °C until analysis. Urine was collected for 24 h at each study visit in amber containers that were refrigerated between collections. After a final void, 24 h after the start of the collection, 15-ml aliquots of urine were combined with 20 mg ascorbic acid per ml to maintain pH < 4 and were frozen at −80 °C until analysis.
Analytical procedures
Samples for β-hydroxycortisol were analysed within 20 months of collection and those for midazolam within 6 months. Plasma midazolam concentrations were assayed at Prevalere Life Sciences, Inc., using an LC/MS/MS method and alprazolam as the internal standard. Briefly, 1 ml of plasma was de-proteinated and extracted with methanol using C18 solid-phase columns. Samples were evaporated to dryness and reconstituted with 50 µl of an 80 : 20 mixture of methanol and 5 mmol l−1 ammonium acetate solution.
Chromatographic separation was accomplished using a Waters symmetry C18 column and a mobile phase composed of an 80 : 20 mixture of methanol and 5 mmol l−1 ammonium acetate (80 : 20). A PE Sciex API III+lC/MS/MS system (Perkin-Elmer Sciex Instruments, Rochester, NH, USA) equipped with a Waters 616 pump, 600S controller (Waters Corporation, Milford, MA, USA) and a Waters 717+ autosampler was used in this analysis. Retention times for alprazolam and midazolam were 4 and 7.5 min, respectively. This assay was linear in the range of 0.25–100 ng ml−1 for each analyte of interest. Interassay precision of the method was ±9.9% or less at quality control sample concentrations of 0.75, 7.5 and 75 ng ml−1. Interassay accuracy for the same quality control samples ranged from 90.8 to 109.2% of nominal values.
Urine cortisol and 6β-hydroxycortisol were quantified by LC/MS/MS using D3 cortisol and 6β-hydroxyprednisolone as internal standards. Briefly, 0.5 ml of urine was neutralized with 50 µl ammonium hydroxide. Internal standards were added and the samples extracted with 500 µl 30% n-butanol in methyl tert-butyl ether. The organic layer was transferred and evaporated to dryness under nitrogen. The residue was then reconstituted with a hexane:methylene chloride:methanol (10 : 2 : 2) solution. For cortisol, chromatography was conducted using a Zorbax Silica (250 × 4.6 mm) column with a mobile phase composed of hexane, methylene chloride, methanol and acetic acid at a flow rate of 2 ml min−1. MS detection was performed on a PE-Sciex API III+ mass spectrometer (Perkin-Elmer Sciex Instruments). For 6β-hydroxycortisol, chromatography was conducted using a Spheresorb ODS2 (5 µm, 250 × 4.6 mm) column and a mobile phase gradient. Mobile phase A was composed of 1% acetic acid and 1% tert-butyl alcohol in water with acetonitrile (85 : 15). Mobile phase B was composed of 1% acetic acid in water with acetonitrile (1 : 2). Gradient mobile phase was delivered at a flow rate of 1 ml min−1 with 0–5 min of mobile phase A, 7 min of mobile phase B and followed by 9 min of mobile phase A.
Both cortisol and 6β-hydroxycortisol assays were linear from 0.1 to 100 ng ml−1. The interassay precision of the method was ±15% or less at quality control sample concentrations of 0.8, 8 and 80 ng ml−1. The interassay accuracy for the same quality control samples were between 95 and 105% of nominal values.
Materials
Midazolam standard was provided by Mr T. Mulligan and Dr J. Sepinwall (Roche Laboratories, Nutley, NJ, USA). Cortisol and 6β-hydroxycortisol were purchased from Steraloids Inc. (Wilton, NH, USA). D3 cortisol and 6β-hydroxyprednisolone were purchased from Steraloids (Newport, RI, USA). All other regents were of the highest purity commercially available (Sigma Chemical Co., St Louis, MO, USA).
Data analysis
Midazolam pharmacokinetic parameters were calculated using noncompartmental methods with the pharmacokinetic program TOPFIT 2.0 (Gustav Fischer Verlag, Stuttgart, Germany). The trapezoidal rule was used to calculate area under the curve (AUC) and systemic clearance was calculated by dividing the midazolam dose by the AUC0–∞. The urinary 6β-hydroxycortisol:cortisol molar ratio was defined as [6β-hydroxycortisol concentration] × 362.45 (MW of cortisol)/[cortisol concentration] × 378.45 (MW of 6β-hydroxycortisol).
Statistical analysis
Statistics were calculated with SPSS v.10.0 (SPSS Inc., Chicago, IL, USA). The Wilcoxon sign rank test was used to determine differences in midazolam clearance and urine cortisol molar ratios during the midfollicular and midluteal phases of the menstrual cycle. The Mann–Whitney test was used to determine differences in midazolam clearance and urine cortisol molar ratios between males and females. Spearman's rank correlation coefficient and linear regression models were used to assess the relationship between midazolam clearance and urine cortisol molar ratios. The significance level for all statistical analyses was α = 0.05. Data are reported as mean ± SD unless otherwise specified.
Results
All subjects completed all eight phenotyping visits. Subject demographic data are listed in Table 1. Statistically significant differences existed between men and women with respect to ideal body weight and serum creatinine.
Table 1.
Demographic data for study subjects
| Variable | Male | Female |
|---|---|---|
| N | 10 | 10 |
| Age (years) | 34.8 ± 7.9 | 38.2 ± 9.3 |
| Total body weight (kg) | 76.3 ± 13.3 | 79.5 ± 20.1 |
| Ideal body weight (kg) | 74.0 ± 6.8 kg | 58.5 ± 6.3* |
| Midazolam dose (mg) | 1.9 ± 0.3 | 2.0 ± 0.5 |
| Aspartate aminotransferase (U l−1) | 24.2 ± 4.8 | 24.0 ± 7.4 |
| Alanine aminotransferase (U l−1) | 30.1 ± 8.3 | 24.6 ± 9.2 |
| Creatinine clearance (ml min−1 1–1.73 m2)† | 99.4 ± 14.7 | 81.3 ± 19.7* |
| Length of menstrual cycle (days) | 27.2 ± 2.3 | |
| Follicular phase study day | 5.6 ± 1.4 | |
| Luteal phase study day | 18.3 ± 3.3 |
Data are given as mean±SD.
P < 0.05 compared with men.
Creatinine clearance was calculated using the Cockcroft and Gault method.
Midazolam phenotyping
A summary of the midazolam pharmacokinetic analyses has been previously reported [8]. There were no significant differences in midazolam clearance between midfollicular and midluteal menstrual cycle phases either at baseline (during the first 3 months; P = 0.58) or after treatment with fluvoxamine (visits 7 and 8; p = 0.11). Therefore, the midfollicular and midluteal phase midazolam clearance values were pooled together for visits 1–6 as baseline and visits 7–8 as post treatment for all subsequent analyses. Mean midazolam clearance values for each subject are listed in Table 2. No sex differences were found between the mean midazolam clearance at baseline or after treatment with fluvoxamine (P= 0.91 and 0.32, respectively).
Table 2.
Plasma midazolam clearance and urinary 6β-hydroxycortisol:cortisol ratio for CYP3A phenotyping in men and women before (baseline) and after 28 days of fluvoxamine (post treatment)
| Subject no. | Midazolam CL *† (ml min−1 kg−1) at baseline | Midazolam CL‡ (ml min−1 kg−1) post treatment | 6β-Hydroxycortisol: cortisol ratio* at baseline | 6β-Hydroxycortisol: cortisol ratio‡ post treatment |
|---|---|---|---|---|
| Female | ||||
| Mean | 8.1 ± 2.4 | 5.9 ± 2.7 | 12.6 ± 8.7 | 6.0 ± 4.0 |
| CV % | 29.1 | 45.4 | 69.3 | 67.2 |
| Male | ||||
| Mean | 7.6 ± 0.9 | 4.7 ± 0.9 | 7.3 ± 2.5 | 4.5 ± 1.5 |
| CV % | 12.3 | 18.5 | 34.5 | 32.1 |
| Overall | ||||
| Mean§ | 7.9 ± 1.8 | 5.3 ± 2.0 | 10.0 ± 6.8 | 5.3 ± 3.1 |
| CV % | 22.5 | 38.1 | 68.4 | 58.1 |
Data are given as mean± SD.
Values were pooled of visits 1–6 for each subject.
Previously published by Kashuba et al. [6].
Values were pooled of visits 7–8 for each subject.
Overall mean indicates the mean for all male and female subjects.
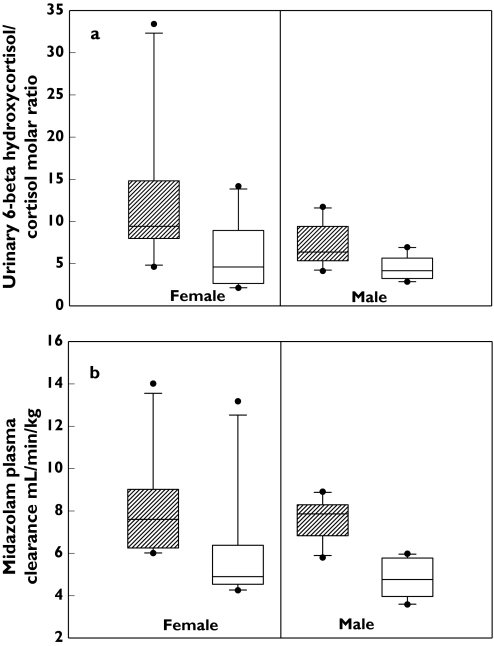
In all subjects, the point estimate for midazolam clearance decreased approximately 1.5-fold (7.86 ± 1.77 ml min−1 kg−1vs. 5.32 ± 2.03 ml min−1 kg−1; P < 0.05) after treatment with fluvoxamine. When stratified according to sex, the point estimate for midazolam clearance decreased by approximately 1.6-fold for men (7.61 ± 0.94 ml min−1 kg−1vs. 4.74 ± 0.88 ml min−1 kg−1; P < 0.05) and 1.3-fold for women (8.11 ± 2.36 ml min−1 kg−1vs. 5.9 ± 2.68 ml min−1 kg−1; P < 0.05) (Figure 1a).
Figure 1.
(a) Urinary 6β-hydroxycortisol:cortisol molar ratio at baseline and after treatment of fluvoxamine for men and women (the error bars indicate 5th and 95th percentile; circles indicate the outliers). (b) Midazolam plasma clearance at baseline and after treatment of fluvoxamine for men and women (the error bars indicate 5th and 95th percentile; circles indicate the outliers). Baseline ( ) post treatment (□)
) post treatment (□)
Urinary cortisol phenotyping
There were no significant differences in urinary 6β-hydroxycortisol:cortisol molar ratios between midfollicular and midluteal menstrual cycle phases either at baseline (P= 0.45) or after treatment with fluvoxamine (P= 0.33). Therefore the midfollicular and midluteal phase 6β-hydroxycortisol:cortisol molar ratios were pooled for visits 1–6 and visits 7–8 for all subsequent analyses. Mean urinary 6β-hydroxycortisol:cortisol molar ratio values for each subject are listed in Table 2. No statistically significant sex differences were found in the mean urinary 6β-hydroxycortisol:cortisol molar ratios at baseline (P= 0.09) or after treatment with fluvoxamine (P= 0.80) (Figure 1b).
In all subjects, the point estimate for urinary 6β-hydroxycortisol:cortisol molar ratio after treatment with fluvoxamine decreased approximately 1.9-fold (9.96 ± 6.82 vs. 5.26 ± 3.06; P < 0.05). When stratified according to sex, the point estimate for urinary 6β-hydroxycortisol:cortisol molar ratio decreased by approximately 1.6-fold for men (7.32 ± 2.52 vs. 4.50 ± 1.45; P < 0.05) and 2.0-fold for women (12.61 ± 8.73 vs. 6.02 ± 4.04; P < 0.05). This indicates moderate CYP3A inhibition activity of fluvoxamine.
Correlation between midazolam clearance and urinary cortisol ratio
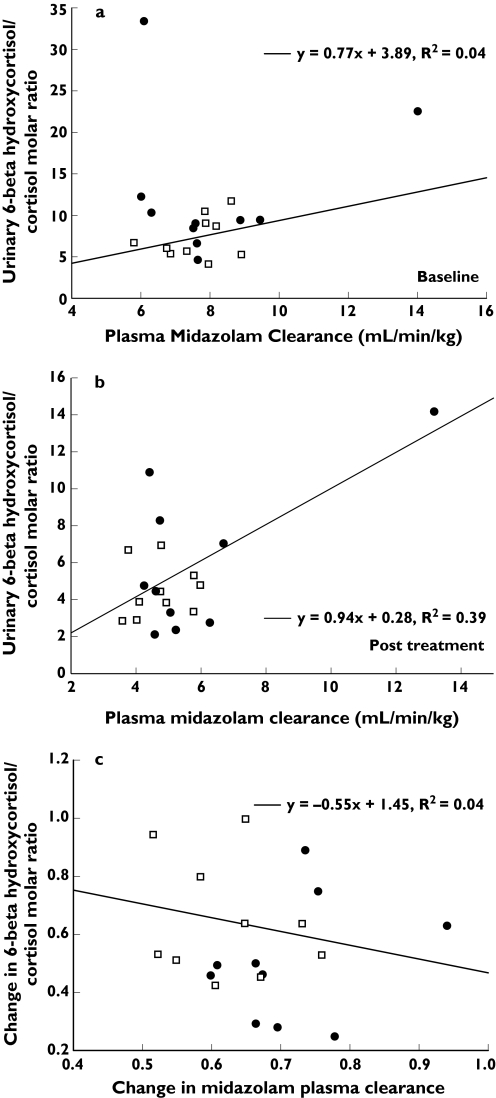
Figure 2a, b compares the results of the urinary 6β-hydroxycortisol:cortisol molar ratio with the corresponding midazolam clearance for each subject at baseline and after treatment with fluvoxamine. The Spearman's rank correlation coefficient test showed no significant correlations between these two markers either at baseline or after treatment for any subjects (r= 0.02, P = 0.92 and r = 0.17, P = 0.46, respectively). Stratification by sex did not improve the correlation (r= 0.06 at baseline and r = 0.12 after treatment for male and r = 0.22 at baseline and r = 0.32 after treatment for female with all P-values >0.05).
Figure 2.
(a) Correlations between plasma midazolam clearance and urinary 6β-hydroxycortisol:cortisol molar ratio for CYP3A phenotyping in all subjects at baseline (circles, women; squares, men). (b) Correlations between plasma midazolam clearance and urinary 6β-hydroxycortisol:cortisol molar ratio for CYP3A phenotyping in all subjects post treatment (circles, women; squares, men). (c) Correlations between the changes of plasma midazolam clearance and urinary 6β-hydroxycortisol:cortisol molar ratio (post treatment/baseline) for CYP3A phenotyping in all subjects (circles, women; squares, men)
Figure 2c shows the correlation between the change in midazolam CL and urinary cortisol ratio. The changes of these two biomarkers reflect the magnitude of inhibitory effects of fluvoxamine on CYP3A. There were also no significant correlations between the change in midazolam clearance and the change in cortisol ratio. Spearman's correlation coefficients were −0.15 for all subjects (0.210 for females and −0.19 for males; all P-values >0.05).
The urinary 6β-hydroxycortisol:cortisol molar ratio demonstrated higher interindividual variability [mean coefficient of variation (CV) of 68.4% at baseline and 58.1% post treatment] compared with midazolam clearance (CV of 22.5% at baseline and 38.1% post treatment) and higher intraindividual variability (CV of 26% at baseline and 40% post treatment) compared with plasma midazolam clearance (CV of 8% at baseline and 10% post treatment) (Table 2).
Discussion
There were no differences in CYP3A activity between the midfollicular and midluteal phases of the menstrual cycle as measured by either midazolam (data previously reported [8]) or cortisol phenotyping. This is in agreement with smaller investigations by Katz et al. [24] and Burstein et al. [25].
With our small sample size, we found no significant differences in CYP3A biomarkers between caucasian men and women. However, Inagaki et al. [26] demonstrated a twofold difference in 6β-hydroxycortisol: cortisol urinary ratios between 238 Japanese men and 249 Japanese women.
The urinary cortisol ratio has been proposed as a non-invasive marker for CYP3A induction activity. In subjects exposed to CYP3A inducers such as rifampin and phenobarbital, urinary cortisol ratios increase 5- and 2.5-fold, respectively [18, 22]. Eeckhoudt et al. [27] have compared midazolam clearance and 6β-hydroxycortisol:cortisol ratio before and after 6 days' treatment with rifampin (N = 8) and demonstrated a positive correlation between two biomarkers for CYP3A induction but no correlations before or after treatment of rifampin. However, in subjects exposed to CYP3A inhibitors, results are conflicting. For example, clarithromycin has shown a dose-dependent inhibitory effect on 6β-hydroxycortisol:cortisol ratios: 400 mg day−1 for 7 days reduced the urinary ratio by 47% and 800 mg day−1 for 7 days reduced the urinary ratio by 65%[28]. However, three HIV protease inhibitors known to be moderate to strong inhibitors of CYP3A activity (indinavir, ritonavir and amprenavir) did not significantly change the 6β-hydroxycortisol:cortisol ratio in 18 HIV-infected patients [10]. In the current study, we found a significant reduction of urinary 6β-hydroxycortisol:cortisol molar ratio (∼1.9-fold) and a significant reduction of midazolam clearance (∼1.5-fold) after 14 and 28 days of fluvoxamine (150 mg day−1), a moderate CYP3A inhibitor. However, the correlation of these two biomarkers did not reach statistical significance. This finding may be due in part to the use of a sensitive and specific LC/MS/MS method of quantifying cortisol and 6β-hydroxycortisol. Other endogenous steroid compounds can interfere with ultraviolet detection methods [29–31] or fluorescence detection methods [32] compared with mass spectroscopy detection. This lower specificity may have resulted in early reports of cortisol ratios being less sensitive for measuring changes in CYP3A activity under inhibitory conditions.
The cortisol ratio has previously been criticized for failing to predict measures with other presumed CYP3A biomarkers. Two studies failed to find a correlation between the ERMBT and the urinary 6β-hydroxycortisol:cortisol ratio [19, 20]. In contrast, Watkins [22] reported a significant correlation between these two tests (r= 0.59, P < 0.001) in a cohort of 47 men and women. Kinirons [19] found no correlation between cortisol 6β-hydroxylation and dapsone hydroxylation, a nonspecific marker of CYP3A activity (r = –0.13, P = 0.5). However, dapsone is metabolized by multiple CYP isozymes and is a nonspecific marker of CYP3A activity.
This study compared intravenous midazolam clearance, a hepatic CYP3A biomarker, with the urinary 6β-hydroxycortisol:cortisol ratio. We were unable to demonstrate a significant correlation between these two measures. Although both midazolam and cortisol fall into Class 1 based on BCS criteria [12], higher intra- and intersubject variability observed in the cortisol ratio may mask the possible correlation between these two biomarkers. This finding is in agreement with Furuta et al. [33], in that intra- (2.1- to 4.6-fold) and interindividual variabilities (approximately fivefold) in the cortisol renal clearance suggest that the urinary 6β-hydroxycortisol:cortisol ratio, a function of 6β-hydroxylation clearance and renal clearance of cortisol, does not always reflect the in vivo CYP3A activity. This was illustrated when the macrolide antibiotic clarithromycin was administered at a dose of 200 mg every 12 h for 6 days and did not reflect the inhibitory effects on CYP3A activity using the urinary 6β-hydroxycortisol:cortisol ratio.
The discordance of midazolam clearance and the urinary cortisol ratio may also be due in part to the fact that both CYP3A4 and CYP3A5 can catalyse cortisol 6β-hydroxylation [34]. Therefore, the urinary concentration of 6β-hydroxycortisol may reflect the combination of hepatic CYP3A4/5 activity and renal CYP3A5 activity, while the midazolam plasma clearance reflects hepatic CYP3A4/5 activity only. Alternatively, these compounds may bind differently to the active site of the drug-metabolizing isozymes, resulting in different rates of metabolism [35].
Using a non-invasive measure for screening inhibitory drug interactions is an attractive concept. Upon exposure to the moderate CYP3A inhibitor fluvoxamine, the urinary 6β-hydroxycortisol:cortisol ratio was reduced to a similar degree to midazolam clearance. However the high inter- and intra-individual variability of the urinary 6β-hydroxycortisol:cortisol ratio and its inability to reflect the change in midazolam clearance put its suitability for routine use into question.
Conflict of interest
A.D.M.K. has received support for scientific studies from Gilead and Abbott, and is a consultant to Boehringer Ingelheim and Roche. J.S.B. is a consultant to Janssen Ortho-McNeil and Merck. He has received support for scientific studies from Janssen Ortho-McNeil, Viropharma, Glaxo SmithKline and Merck. A.N.N. is a consultant for Merck-Schering Plough. She has received support for scientific studies from Glaxo SmithKline and Merck. At the time of investigation, Y-C.C. and S.K.G. were postdoctoral fellows at UNC and currently S.K.G. works for United Therapeutics and Y-C.C. works for Daiichi Asubio Pharmaceutical Inc.
Acknowledgments
This study was supported bt the E. Donnall Thomas Resident Research Program in Internal Medicine, the American College of Clinical Pharmacists' Wyeth-Ayerst Laboratories Women's Healthcare Research Award, RR00046 and AI54980 (A.D.M.K.).
References
- 1.Slaughter RL, Edwards DJ. Recent advances: the cytochrome P450 enzymes. Ann Pharmacother. 1995;29:619–24. doi: 10.1177/106002809502900612. [DOI] [PubMed] [Google Scholar]
- 2.Anzenbacher P, Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737–47. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Bacchi CE, Marsh CL, McVicar JP, Barr DM, Perkins JD, et al. Use of midazolam as a human cytochrome P4503A probe; II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Ther. 1994;27:557–66. [PubMed] [Google Scholar]
- 4.Forrester LM, Henderson CJ, Glancey MJ, Back DJ, Park BK, Ball SE, Kitteringham NR, McLaren AW, Miles JS, Skett P, et al. Relative expression of cytochrome P450 isozymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281:359–68. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–34. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 6.Bonate PL. Gender-related differences in xenobiotic metabolism. Clin Pharmacol. 1991;31:684–90. doi: 10.1002/j.1552-4604.1991.tb03760.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris RZ, Benet LZ, Schwart JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–39. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kashuba AD, Bertino JS, Jr, Rocci ML, Jr, Kulawy RW, Beck DJ, Nafziger AN. Quantification of 3-month intraindividual variability and the influence of sex and menstrual cycle phase on CYP3A activity as measured by phenotyping with intravenous midazolam. Clin Pharmacol Ther. 1998;64:269–77. doi: 10.1016/S0009-9236(98)90175-8. [DOI] [PubMed] [Google Scholar]
- 9.Ohkita C, Goto M. Increased 6β-hydroxycortisol excretion in pregnant women: implication of drug-metabolizing enzyme induction. DICP Ann Pharmacother. 1990;24:814–6. doi: 10.1177/106002809002400902. [DOI] [PubMed] [Google Scholar]
- 10.Gass RJA, Gal J, Fogle PW, Detmar D, Gerbr JG. Neither dapsone hydroxylation nor cortisol 6-beta-hydroxylation detects the inhibition of CYP3A4 by HIV-1 protease inhibitors. Eur J Clin Pharmacol. 1998;54:741–7. [Google Scholar]
- 11.Kovacs SJ, Martin DE, Evritt DE, Patterson SD, Jorkasky DK. Urinary excretion of 6-beta-hydroxycortisol as an in vivo marker for CYP3A induction: applications and recommendations. Clin Pharmacol Ther. 1998;63:617–22. doi: 10.1016/S0009-9236(98)90084-4. [DOI] [PubMed] [Google Scholar]
- 12.Benet LZ. There are no useful CYP3A probes that quantitatively predict the in vivo kinetics of other CYP3A substrates and no expectation that one will be found. Mol Interv. 2005;5:79–83. doi: 10.1124/mi.5.2.5. [DOI] [PubMed] [Google Scholar]
- 13.Masica AL, Mayo G, Wilkinson GR. In vivo comparisons of constitutive cytochrome P450 3A activity assessed by alprazolam, triazolam, and midazolam. Clin Pharmacol Ther. 2004;76:341–9. doi: 10.1016/j.clpt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Wenk M, Todesco L, Krahenbuhl S. Effect of St John's wort on the activities of CYP1A2, CYP3A4, CYP2D6, N-acetyltransferase 2, and xanthine oxidase in healthy males and females. Br J Clin Pharmacol. 2004;57:495–9. doi: 10.1111/j.1365-2125.2003.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–8. [PubMed] [Google Scholar]
- 16.Ushiama H, Echizen H, Nachi S, Ohnishi A. Dose-dependent inhibition of CYP3A activity by clarithromycin during Helicobacter pylori eradication therapy assessed by changes in plasma lansoprazole levels and partial cortisol clearance to 6β-hydroxycortisol. Clin Pharmacol Ther. 2002;72:33–43. doi: 10.1067/mcp.2002.125559. [DOI] [PubMed] [Google Scholar]
- 17.Boulence X, Ollier C. CYP3A induction: use and analysis of 6-β-hydroxycortisol/cortisol ratio after repeated administration of drug in clinic. Drug Metab Rev. 2005;37:28 (Abstract)–59. [Google Scholar]
- 18.Ged CR, Rouillon JM, Pichard L, Combalbert J, Bressot N, Bories P, Michel H, Beaune P, Maurel P. The increase in urinary excretion of 6-beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989;28:373–87. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinirons MT, O'Shea D, Downing TE, Fitzwilliam AT, Joellenbeck L, Groopman JD, Wilkinson GR, Wood AJ. Absence of correlations among three putative in vivo probes of human cytochrome P4503A activity in young healthy men. Clin Pharmacol Ther. 1993;54:621–9. doi: 10.1038/clpt.1993.199. [DOI] [PubMed] [Google Scholar]
- 20.Hunt CM, Watkins PB, Sanger P, Stave GM, Barlascini N, Watlington CO, Wright JT Jr, Guzelian PS. Heterogeneity of CPY3A isoforms metabolizing erythromycin and cortisol. Clin Pharmacol Ther. 1992;51:18–23. doi: 10.1038/clpt.1992.3. [DOI] [PubMed] [Google Scholar]
- 21.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Watkins PB, Turgeon DK, Saenger P, Lown KS, Kolars JC, Hamilton T, Fishman K, Guzelian PS, Voorhees JJ. Comparison of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992;52:265–73. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]
- 23.Kim RB, Wandel C, Leake B, Cvetkovic M, Fromm MF, Dempsey PJ, Roden MM, Belas F, Chaudhary AK, Roden DM, Wood AJ, Wilkinson GR. Interrelationship between inhibitors and substrates of human CYP3A and P-glycoprotein. Pharm Res. 1999;16:408–14. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- 24.Katz FH, Lipman MM, Frantz AG, Jailer JW. The physiologic significance of 6β-hydroxycortisol in human corticoid metabolism. J Clin Endocrinol. 1971;32:192–200. doi: 10.1210/jcem-22-1-71. [DOI] [PubMed] [Google Scholar]
- 25.Burstein AH, Reiss WG, Kantor E, Anderson GD. Cytochrome P450 3A4 activity in premenopausal and postmenopausal women based on 6β-hydroxycortisol:cortisol ratios. Pharmacotherapy. 1998;18:1271–6. [PubMed] [Google Scholar]
- 26.Inagaki K, Inagaki M, Kataoka T, Sekido I, Gill MA, Nishida M. A wide interindividual variability of urinary 6beta-hydroxycortisol to free cortisol in 487 healthy Japanese subjects in near basal condition. Ther Drug Monit. 2002;24:722–7. doi: 10.1097/00007691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Eeckhoudt SL, Desager JP, Robert AR, Leclercq I, Verbeeck RK, Horsmans Y. Midazolam and cortisol metabolism before and after CYP3A induction in humans. Int J Clin Pharmacol Ther. 2001;39:293–9. doi: 10.5414/cpp39293. [DOI] [PubMed] [Google Scholar]
- 28.Ushiama H, Echizen H, Nachi S, Ohnishi A. Dose-dependent inhibition of CYP3A activity by clarithromycin during Helicobactor pylori eradication therapy assessed by changes in plasma lansoprazole levels and partial cortisol clearance to 6β-hydroxycortisol. Clin Pharmacol Ther. 2002;72:33–43. doi: 10.1067/mcp.2002.125559. [DOI] [PubMed] [Google Scholar]
- 29.Bidart M, Lesgards G. Direct injection analysis of 6β-hydroxycortisol and cortisol in urine by HPLC-UV with online ISRP precolumn. J Liquid Chromatogr. 1995;18:725–38. [Google Scholar]
- 30.Lykkesfeldt J, Loft S, Poulsen HE. Simultaneous determination of urinary free cortisol and 6β-hydroxycortisol by high-performance liquid chromatography to measure human CYP3A activity. J Chromatogr B Biomed Appl. 1994;660:23–9. doi: 10.1016/0378-4347(94)00265-7. [DOI] [PubMed] [Google Scholar]
- 31.Bienvenu T, Rey E, Pons G, D'Athis P, Olive G. A simple non-invasive procedure for the investigation of cytochrome P450 IIIA-dependent enzymes in humans. Int J Clin Pharmacol Ther Toxicol. 1991;29:441–5. [PubMed] [Google Scholar]
- 32.Rogers JF, Rocci ML, Jr, Haughey DB, Bertino JS., Jr An evaluation of the suitability of intravenous midazolam as an in vivo marker for hepatic cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:153–8. doi: 10.1067/mcp.2003.23. [DOI] [PubMed] [Google Scholar]
- 33.Furuta T, Suzuki A, Mori C, Shibasaki H, Yokokawa A, Kasuya Y. Evidence for the validity of cortisol 6 beta-hydroxylation clearance as a new index for in vivo cytochrome P450 3A phenotyping in humans. Drug Metab Dispos. 2003;31:1283–7. doi: 10.1124/dmd.31.11.1283. [DOI] [PubMed] [Google Scholar]
- 34.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–84. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DVF, Eddershaw PJ, Goldfarb PS, Tarbit MH. Molecular modeling of CYP3A4 from an alignment with CYP102: identification of key interactions between putative active site residues and CYP3A-specific chemicals. Xenobiotica. 1996;26:1067–86. doi: 10.3109/00498259609167423. [DOI] [PubMed] [Google Scholar]