Abstract
Oxidative stress has been recognized as a key mechanism in the development of vascular damage, particularly atherosclerosis. In spite of substantial experimental evidence demonstrating reversal of endothelial dysfunction and prevention of atherosclerosis in experimental settings, no benefits have been observed in large clinical trials in which antioxidants have been given in high-risk patients for the prevention of cardiovascular events. Evaluation of the clinical relevance of the oxidative modification hypothesis requires identification of potential molecular targets of antioxidant interventions and effective antioxidant agents. Future research should necessarily consider quantification of interindividual variations in oxidative stress using appropriate biomarkers.
Keywords: Oxidative stress, antioxidants, vascular damage
Oxidative stress has been recognized as a key mechanism in the development of vascular damage, particularly atherosclerosis, but whether it is a suitable target for pharmacological intervention is still debated. The data available to date show conflicting results, which are being clarified by research efforts. In fact, while vasoprotective effects of antioxidant compounds are apparent in experimental settings [1, 2], such effects have not been confirmed in large clinical studies of cardiovascular events in high-risk patients taking antioxidant vitamins [3–6], with some significant exceptions [7, 8]. However, there are several difficulties in directly transferring the results of mechanistic studies, on which the oxidative hypothesis is based, to the clinical setting. While the theory itself may be considered inadequate to explain the complexity of the atherosclerotic process, potential molecular targets of antioxidant interventions and antioxidant agents with proven efficacy have yet to be identified. Moreover, neither interindividual variations in the degrees of oxidative stress nor clinical conditions in which antioxidant treatment could be appropriately tested have so far been defined.
It was originally hypothesized that oxidative modification of low-density lipoproteins (LDL) in the intima of large arteries was a necessary requirement for the promotion of lipid accumulation, cellular recruitment and vascular inflammation that characterize atherosclerotic lesions [9]. Later, the oxidative hypothesis became more complex when oxidative modification of different substrates was found to be responsible for vascular damage and generation of reactive oxygen species (ROS) in vascular cells was determined to be part of the signalling system engaged by receptor-mediated cell stimuli [10]. Experimental evidence suggests that both vascular and nonvascular enzymes have a role in the generation of reactive oxygen species, and that NAD(P)H oxidase in particular plays a major role in their generation [10]. In addition, lipoxygenases, xanthine oxidase, a dysfunctional mitochondrial respiratory chain, and uncoupled nitric oxide synthase are sources of oxidative stress [11–13]. Increased generation of superoxide anions also results in reduced bioactivity of nitric oxide and the formation of cytotoxic peroxynitrite, responsible for increased platelet aggregability and vasoconstriction [11–13]. Thus, a complex condition emerges in which different oxidative products and reactive species are generated, and this may well explain the difficulties that have been encountered in testing the pharmacological potential of individual antioxidant agents in humans.
It has been observed that augmented oxidative stress and subclinical inflammation coexist in clinical conditions that are associated with increased cardiovascular risk [14, 15]; this association amplifies the signals that lead to vascular damage. Proinflammatory cytokines, angiotensin II and advanced glycation end-products generate intracellular ROS responsible for the activation of redox-sensitve transcription factors and phenotypical changes in endothelial cells, including expression of adhesion molecules and tissue factor and the release of proinflammatory cytokines that characterize endothelial dysfunction [16]. Thus, cardiovascular risk factors and mediators of inflammation may prove to be more suitable targets for intervention than oxidative stress itself [17].
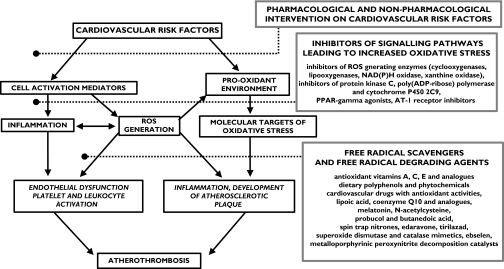
As Steinberg and Witztum have discussed [18], antioxidants might be effective in inhibiting the initial stages of human atherosclerosis and yet be ineffective, or much less effective, in reducing plaque instability and rupture. If this were so, it might be necessary to find some way to assess the early stages of lesion development (perhaps by high-resolution ultrasound or magnetic resonance imaging) rather than relying on the conventional late clinical endpoints. Undoubtedly, if the development of early lesions were successfully inhibited, there would eventually be a reduction in the frequency of clinical events, but in that case clinical trials would need to extend beyond the standard 5 years. Further research is needed to identify the biochemical steps in which oxidative injury or ROS signalling is implicated and which antioxidants may therefore be effective in inhibiting vascular damage (Figure 1).
Figure 1.
The role of oxidative stress in the process of atherothrombosis and potential therapeutic strategies
When antioxidant compounds, in most cases vitamins C and E, were tested in different dosages in large prospective interventional studies in high-risk patients, beneficial effects on cardiovascular mortality and morbidity were observed only in specific subgroups of subjects [7]. These included patients with end-stage renal insufficiency, who have increased oxidative stress [19]. In fact, experimental evidence suggests that increased oxidative stress is a prerequisite for effective pharmacological intervention with antioxidants [20]. This clearly indicates that future research should be aimed at identifying, using appropriate biomarkers, which clinical condition and which individual patients would be most likely to benefit from treatment with antioxidants.
Vitamins C and E have been used in clinical and experimental studies, mostly because they are both safe and readily available; however, other compounds, possibly equally devoid of toxicity, might prove to be more effective in the prevention of cardiovascular events (Figure 1). Modulation of enzyme activity to reduce free radical production has already been tested as a possible approach to the reduction of oxidative stress. Phytochemicals have been the object of extensive research and several natural compounds can reverse endothelial dysfunction and platelet activation [21]. Some cardiovascular drugs may also have antioxidant properties, as has been shown for some calcium channel blockers and β-adrenoceptor antagonists [22]. Several synthetic compounds, including free radical scavengers and free radical degrading agents, are currently under investigation in different clinical settings, including diabetes mellitus and acute ischaemic stroke, in which neuroprotective effects have been demonstrated [23].
In conclusion, the revised oxidative modification hypothesis still requires substantiation. This will entail evaluation of the potential effectiveness of antioxidant agents in cardiovascular medicine through extensive experimental and clinical research.
References
- 1.Koga T, Kwan P, Zubik L, Ameho C, Smith D, Meydani M. Vitamin E supplementation suppresses macrophage accumulation and endothelial cell expression of adhesion molecules in the aorta of hypercholesterolemic rabbits. Atherosclerosis. 2004;176:265–72. doi: 10.1016/j.atherosclerosis.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Foxall T, Blumberg JB, Meydani M. Vitamin E inhibits low-density lipoprotein-induced adhesion of monocytes to human aortic endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 1997;17:429–36. doi: 10.1161/01.atv.17.3.429. [DOI] [PubMed] [Google Scholar]
- 3.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 4.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 6.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 7.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–8. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 8.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 9.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–33. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 11.Stocker R, Keaney JJ. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 12.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 13.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435:502–6. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 14.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–14. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–7. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 16.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part II. Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R, Cipollone F, Filardo FP, Zimarino M, Bernini W, Lazzerini G, Bucciarelli T, Falco A, Marchesani P, Muraro R, Mezzetti A, Ciabattoni G. Low-density lipoprotein level reduction by the 3-hydroxy-3-methylglutaryl coenzyme-A inhibitor simvastatin is accompanied by a related reduction of F2-isoprostane formation in hypercholesterolemic subjects: no further effect of vitamin E. Circulation. 2002;106:2543–9. doi: 10.1161/01.cir.0000038500.43292.d7. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg D, Witztum J. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–11. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 19.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 20.Patrignani P, Panara MR, Tacconelli S, Seta F, Bucciarelli T, Ciabattoni G, Alessandrini P, Mezzetti A, Santini G, Sciulli MG, Cipollone F, Davi G, Gallina P, Bon GB, Patrono C. Effects of vitamin E supplementation on F(2)-isoprostane and thromboxane biosynthesis in healthy cigarette smokers. Circulation. 2000;102:539–45. doi: 10.1161/01.cir.102.5.539. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81(1 Suppl):292S–7S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 22.Taddei S, Virdis A, Ghiadoni L, Magagna A, Pasini AF, Garbin U, Cominacini L, Salvetti A. Effect of calcium antagonist or beta blockade treatment on nitric oxide-dependent vasodilation and oxidative stress in essential hypertensive patients. J Hypertens. 2001;19:1379–86. doi: 10.1097/00004872-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39:429–43. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]