Abstract
Aims
Diabetic dyslipidaemia with decreased high-density lipoprotein-cholesterol (HDL-C) concentration plays a key role in enhanced atherosclerosis. The antioxidant effect of HDL is due to the influence of human paraoxonase 1 (PON1) and several authors have described decreased activity of this enzyme in Type 2 diabetics and subjects with metabolic syndrome. The goal of this study was to examine the effect of daily ciprofibrate on serum PON1 and lipoprotein concentrations in patients with metabolic syndrome.
Methods
Fifty-one patients with metabolic syndrome were enrolled into the study. We examined the effect of 100 mg day−1 ciprofibrate treatment on lipid concentrations, oxidized low-density lipoprotein (LDL), PON1 concentrations and activity. We also investigated the calculated size of LDL-cholesterol (LDL-C).
Results
During the 3-month study, it was observed that following treatment with ciprofibrate, the serum triglyceride concentration decreased significantly (from 2.76 ± 0.9 mmol l−1 to 2.27 ± 1.6 mmol l−1; −18%; P < 0.001), while HDL-C increased significantly (from 0.95 ± 0.2 mmol l−1 to 1.2 ± 0.3 mmol l−1; 26%; P < 0.001). The oxidatively modified LDL-C concentration decreased significantly (from 137 ± 19 U l−1 to 117 ± 20 U l−1; P < 0.001), while HDL-associated apolipoprotein A1 significantly increased (from 1.35 ± 0.2 g l−1 to 1.75 ± 0.3 g l−1; P < 0.001). The LDL-C/LDL-apoB ratio, which reflects the size of LDL, increased significantly (from 0.96 ± 0.05 to 1.05 ± 0.06; P < 0.05). Serum PON1 activity was significantly elevated (from 108 ± 34 U l−1 to 129 ± 31 U l−1; P < 0.05), while standardized values for HDL-C remained significantly unchanged (PON1/HDL-C) (from 114 ± 21 to 107 ± 20; NS).
Conclusion
Three months of treatment with ciprofibrate favourably affected the lipid profile, increased LDL resistance to oxidation and improved antioxidant status by increasing serum paraoxonase activity in these patients.
Keywords: ciprofibrate, hyperlipidaemia, metabolic syndrome, paraoxonase
Introduction
Metabolic syndrome is a combination of metabolic abnormalities including glucose intolerance or Type 2 diabetes mellitus, obesity, dyslipdaemia and high blood pressure. Abnormal glucose metabolism in Type 2 diabetes and metabolic syndrome develops over years, during which period patients are at high risk for atherogenesis and cardiovascular diseases. Disturbed lipid metabolism is the early manifestation of insulin resistance. Peripherial cells are resistant to insulin, which results in the disturbance of glucose uptake. The abnormal cellular glucose uptake leads to hyperglycaemia and hyperinsulinaemia. Loss of insulin’s peripherial antilipolytic action in adipocytes results in an increase in free fatty acid (FFA) concentrations [1]. Persisting hyperinsulinaemia and increased FFA concentrations enhance very low density lipoprotein (VLDL) production in the liver [2], and decrease lipoprotein lipase activity resulting in hypertriglyceridaemia, moderate hypercholesterolaemia and decreased high-density lipoprotein-cholesterol (HDL-C) concentrations. Changes in lipoprotein metabolism also lead to the modification in composition of lipid components [3]. Since hypertriglyceridaemia is the dominant lipid abnormality in metabolic syndrome, data regarding the role of triglyceride in atherogenesis are especially interesting. Clinical studies show that hypertriglyceridaemia enhances the atherogenetic process [4–6]. The increase in the proportion of oxidation-sensitive small, dense low-density lipoprotein (LDL), with a corresponding decrease in the concentration of HDL-C, may together play a significant role in the atherosclerosis observed in hypertriglyceridaemia [7].
Human paraoxonase 1 (PON1), a HDL-associated enzyme, is able to prevent LDL oxidation in vitro and in vivo[8, 9]. The genetic polymorphism at codon 192 (Gln/Arg) is principally responsible for the changes in enzyme activity; however, polymorphism at codon 55 (Leu/Met) has been shown to be an independent risk factor for cardiovascular diseases in patients with Type 2 diabetes [10, 11]. The PON1-192 activity polymorphism is substrate dependent. Paraoxon is hydrolysed faster by Arg alloenzyme, while both Arg and Gln alloenzymes hydrolyse phenylacetate at the same rate. Mackness et al. found decreased PON1 activity in patients with Type 1 diabetes and familial hypercholesterolaemia [12]. Both polymorphisms have been identified as independent risk factors for cardiovascular disease in diabetic and nondiabetic patients [13–15]. Others could not verify these results [16].
Fibrates are one of the commonly used lipid-lowering drugs. Their most important clinical effects are a marked reduction in serum triglycerides and an increase in HDL-C [17, 18]. In previous studies using gemfibrozil and micronized fenofibrate, we found, in addition to a more favourable lipid profile, that these drugs have an advantageous effect on HDL-associated antioxidant PON1 activity [19, 20].
Several clinical trials of fibrates proved the earlier role of fibrates in the management of obese, insulin-resistant and diabetic patients, features characteristic of the metabolic syndrome [21]. Serum PON1 activity was found to be significantly reduced in subjects with metabolic syndrome [22]. The goal of the present study was to investigate changes in lipid profile and HDL-associated PON1 concentration and activity in metabolic syndrome using ciprofibrate.
Methods
Study design and patients
Thirty patients with metabolic syndrome were enrolled in an open, prospective, self-control study. Inclusion criteria were abdominal obesity [body mass index (BMI) > 27 kg m−2], hypertension (treated or recognized hypertension, RR > 140/90 mmHg), dyslipidaemia (triglyceride > 1.7 mmol l−1, cholesterol > 5.2 mmol l−1, HDL-C < 0.9 mmol l−1 in men, < 1.17 mmol l−1 in women), glucose intolerance (treated diabetes mellitus, haemoglobin A1c < 7.5%, or decreased glucose tolerance in oral glucose tolerance test).
Patients with hepatic disorders, endocrine or renal disorders (serum creatinine concentration > 130 µmol l−1), coronary heart disease, alcoholism, drug dependence, gallstones, malignancy, pregnancy or lactation, or treated with anticoagulant or lipid-lowering therapy were excluded from the study. Only nonsmoking patients were recruited. Patients’ physical activity and diet did not change during the study period. After 5 weeks on the National Cholesterol Education Program (NCEP) step 1 diet, patients received ciprofibrate (Lipanor®) 100 mg day−1 for 3 months. Examinations were performed at the beginning of the study and at the end of the third month (physical examination, BMI, electrocardiogram, laboratory tests). The main markers of infection [C-reactive protein (CRP), leucocyte number, sedimentation rate, blood smear], and lipoprotein(a) [Lp(a)] were normal.
Patients gave written, informed consent to participate, and the study was performed according to the requirements of the Ethics Committee of the Medical and Health Science Centre, University of Debrecen.
Blood sampling
After overnight fasting, 10 ml venous blood was drawn. Haemoglobin, haematocrit, white blood cell count, sedimentation rate, liver enzymes, urea, creatinine, creatinine kinase, fibrinogen, CRP, bilirubin, uric acid, serum glucose, cholesterol, HDL-C, LDL-C, apolipoprotein AI (apoAI), apolipoprotein B-100 (apoB-100), triglyceride concentrations and serum paraoxonase activity and concentration were measured. The lipid parameters were determined from fresh sera. Sera for paraoxonase activity measurements were kept at − 20 °C before analysis.
Lipid measurements
Serum cholesterol and triglyceride were assayed using a Boehringer Mannheim GmbH Diagnostic enzyme kit, while HDL-C was investigated by the phospho-tungstic-magnesium precipitation method. The LDL-C fraction was calculated indirectly using the Friedewald equation. Apolipoprotein examination was performed by the immuno-nephelometric assay in which the Orion Diagnostic kit (Orion Diagnostica, Turku, Finland) was used.
Estimation of LDL size
The ratio of LDL-C/LDL-apolipoprotein B was calculated, where LDL-apolipoprotein B (LDLB) was obtained as follows: LDLB = apoB − 0.09Chol + 0.09HDL − 0.08Triglyceride.
This ratio reflects the size of LDL. Normal values range from 1.2 to 2.3 (90% percentile); the preponderance of small dense particles was postulated when the ratio fell below 1.2 [23].
Analysis of paraoxonase activity
PON1 activity was determined using paraoxon (O,O-diethyl-O-p-nitrophenylphosphate; Sigma Chemical Co., St. Louis, MO, USA) as substrate, and measuring the increase in the absorbance at 412 nm due to formation of 4-nitrophenol. Activity was measured by adding 50 µl serum to 1 ml Tris–HCl buffer (100 mmol l−1, pH 8.0) containing 2 mmol l−1 CaCl2 and 5.5 mmol l−1 paraoxon. The rate of generation of 4-nitrophenol was determined at 412 nm, 25 °C, using a Hewlett-Packard (Hewlett-Packard Company, Palo Alto, CA, USA) 8453 UV-visible spectrophotometer. Enzyme activity was calculated from the molar extinction coefficient 17 100 m−1 cm−1. One unit of PON1 activity is defined as 1 µmol of 4-nitrophenol formed per minute under the assay conditions mentioned above [24].
Arylesterase assay
Arylesterase activity was measured spectrophotometrically. The assay contained 1 mm phenylacetate in 20 mm Tris–HCl pH 8.0. The reaction was started by the addition of the serum and then the increase in absorbance at 270 nm was read. Blanks were included to correct for the spontaneous hydrolysis of phenylacetate. Enzyme activity was calculated using the molar extinction coefficient 1310 m−1 cm−1. Arylesterase activity is expressed in U ml−1; 1 U is defined as 1 µmol phenylacetate hydrolysed per minute.
Paraoxonase phenotype distribution
The phenotypic distribution of PON1 was determined by the dual substrate method [25]. The ratio of the hydrolysis of paraoxon in the presence of 1 m NaCl (salt-stimulated PON1 activity) to the hydrolysis of phenylacetate was used to assign individuals to one of the three possible (AA, AB, BB) phenotypes.
ELISA for plasma oxidized LDL concentration
Serum oxidized LDL (oxLDL) concentration was determined by enzyme-linked immunosorbent assay (WAK-chemie Medical GmBH, Bad Soden, Germany). Human plasma containing oxLDL was coated onto microtitre strips covered by monoclonal mouse antioxLDL antibody. The coated strips were immersed in buffer containing antilipoprotein B antibody to detect bound oxLDL. After removal of unbound conjugates by washing, tetramethylbenzidine (TMB) was added as a chromogenic substrate. The concentration of oxLDL was quantified by an enzyme-catalysed colour change detectable spectrophotometrically. Plasma concentration of oxLDL was determined by reference to a standard curve constructed with purified oxLDL.
Measure of PON1 plasma concentration
The plasma PON1 concentration was determined by ELISA. (WAK-Chemie Medical GmBH). Microtitre plate wells were coated with rat HDL (100 µl 10 µg ml−1 in 50 mm carbonate/bicarbonate buffer pH 9.6) overnight at room temperature. After removing the coating buffer, the remaining absorption sites were blocked (1 h at 37 °C) with 200 µl blocking buffer [50 mm carbonate/bicarbonate pH 9.6, containing 1% (w/v) bovine serum albumin (BSA)]. After washing with washing buffer [10 mm sodium phosphate buffer pH 7.4, 140 mm NaCl, 2.7 mm KCl, 0.02% NaN3, containing 0.1% (w/v) BSA], 100 µl of diluted test serum (1 : 250 with PBS pH 7.4) and 100 µl of antiparaoxonase antibody were added and incubated for 3 h at 37 °C. After removing the antibody–serum mix, the wells were washed with washing buffer and incubated with alkaline phosphatase-conjugated goat antimouse IgG (100 µl of 1 : 500 dilution, 2 h, 37 °C). After washing, 50 µl of substrate solution (p-nitrophenyl phosphate in 10% diethanolamine pH 9.8, containing 0.05 mm MgCl2 and 0.02% sodium azide) was added and the plate was incubated for 30 min at 37 °C. The reaction was stopped with 50 µl of 1 m NaOH, and the absorbent at 405 nm was measured with a microplate reader (Anthos Labtec, Wien, Austria). A calibration curve was used with purified PON1. The linear range of the assay was 0.17–1.36 µg PON ml−1. The intra-assay coefficient of variation was 3.2%.
Statistical analysis
Statistical analysis was performed using the SAS for Windows 6.12 computer program (SAS Inc., Cary, NC, USA). Data are presented by descriptive analysis (mean ± SD). Comparisons between groups (before and after treatment) were performed by the paired t-test and anova. Significance was accepted at the P < 0.05 level.
Results
Laboratory parameters of the patients before and after the 3-month treatment with ciprofibrate 100 mg day−1 are shown in Table 1. After ciprofibrate administration the serum triglyceride concentration was reduced significantly by 17.9%. The HDL-C concentration was significantly increased (26%) after therapy (0.95 ± 0.2 vs. 1.2 ± 0.3 mmol l−1; P < 0.001). The ApoAI concentration was significantly elevated by 30% (1.35 ± 0.2 vs. 1.75 ± 0.3 g l−1; P < 0.001). There was no difference between serum cholesterol and LDL-C concentrations before and after treatment. The reduction in apoB (8.3%) and Lp(a) concentrations (2.5%) was not significant. The serum fibrinogen concentration was significantly reduced by 7.6% following ciprofibrate therapy (4.12 ± 0.73 vs. 3.81 ± 0.97 g l−1; P < 0.01).
Table 1.
Demographic characterisctics and laboratory parameters of the study population before and after ciprofibrate treatment
| Before treatment | After treatment | Percent change | |
|---|---|---|---|
| Number of patients | 51 | 51 | – |
| Age (years) | 51.9 ± 8.1 | 51.9 ± 8.1 | – |
| Body weight (kg) | 110 ± 6.9 | 106.4 ± 5.3 | −3.3 |
| Body mass index (kg m−2) | 40.13 ± 2.7 | 39.2 ± 2.4 | −2.3 |
| Glucose (mm) | 6.94 ± 1.15 | 6.87 ± 1.24 | −1.0 |
| Haemoglobin A1c (%) | 7.19 ± 0.94 | 6.76 ± 0.58 | −6.0 |
| Insulin (mU l−1) | 23.37 ± 16.3 | 21.48 ± 11.5 | −8.1 |
| Fibrinogen (g l−1) | 4.12 ± 0.73 | 3.72 ± 0.97* | −9.7 |
| Cholesterol (mmol l−1) | 5.58 ± 0.74 | 5.58 ± 0.82 | 0 |
| LDL-cholesterol (mmol l−1) | 3.36 ± 0.48 | 3.42 ± 0.67 | +1.78 |
| Triglyceride (mmol l−1) | 2.76 ± 0.8 | 2.27 ± 0.6** | −17.7 |
| HDL-cholesterol (mmol l−1) | 0.95 ± 0.21 | 1.2 ± 0.33** | +26.3 |
| Apolipoprotein A1 (g l−1) | 1.35 ± 0.2 | 1.75 ± 0.3** | +29.6 |
| Apolipoprotein B (g l−1) | 1.7 ± 0.45 | 1.56 ± 0.43 | −8.2 |
| Lipoprotein(a) (mg l−1) | 311.9 ± 250 | 304.2 ± 260 | −2.5 |
Values are mean ± SD
P < 0.001
P < 0.01
OxLDL is an important factor in the development and progression of atherosclerotic lesions. After ciprofibrate treatment the oxLDL-c concentration was significantly decreased (137 ± 19 U l−1vs. 117 ± 20 U l−1, P < 0.001). Small, dense LDL has been reported to be more susceptible to oxidation. We estimated the LDL-C/LDL-apoB ratio, which reflects the size of LDL. The LDL-C/LDLB ratio was significantly increased (from 0.96 ± 0.05 to 1.05 ± 0.06; P < 0.05) (Table 2).
Table 2.
Antioxidant and oxidative parameters of the study population
| Before treatment | After treatment | |
|---|---|---|
| Paraoxonase activity (U l−1) | 108 ± 34 | 129 ± 31* |
| Paraoxonase concentration (µg ml−1) | 57.32 ± 7.17 | 52.82 ± 8.55 |
| Paraoxonase specific activity (U mg−1) | 1.88 ± 0.68 | 2.44 ± 0.83* |
| Paraoxonase/HDL-cholesterol | 114 ± 39 | 107 ± 28 |
| Paraoxonase/apolipoprotein A1 | 80 ± 21 | 73.7 ± 18 |
| Arylesterase activity (U ml−1) | 98 ± 24 | 89 ± 30 |
| Oxidized LDL (U l−1) | 137.4 ± 20.5 | 117.6 ± 18.3** |
| LDL-C/LDL-B | 0.96 ± 0.05 | 1.05 ± 0.06* |
Values are mean ± SD
P < 0.001
P < 0.05
HDL-associated PON1 activity was significantly increased after ciprofibrate therapy (108 ± 34 U l−1vs. 129 ± 31 U l−1, P < 0.05). The serum PON1 concentration did not change significantly (57.32 ± 7.17 vs. 52.82 ± 8.55 mg ml−1).
PON1 specific activity was significantly lower before than after ciprofibrate therapy (1.88 ± 0.68 vs. 2.44 ± 0.83 U mg−1, P < 0.05) (Table 2).
Since PON1 is associated with HDL, we examined if the increase in PON1 activity could be explained by the higher HDL-C concentration. The standardized enzyme activity value for HDL-C (PON1/HDL-C) was calculated. After ciprofibrate treatment, the HDL-C concentration was significantly increased (P < 0.001). There was no significant change in PON1/HDL-C ratio after therapy (114 ± 21 vs. 107 ± 20). Similarly, there was no significant change in PON1/apoAI ratio after ciprofibrate treatment (80 ± 19 vs. 73 ± 24) (Table 2).
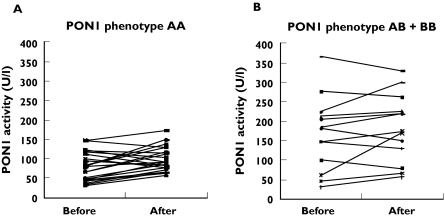
Most of the patients showed the low-activity AA (68.9%), 27.6% of the patients the immediate-activity AB and 3.9% of the patients the high-activity BB phenotype. The individual responses of patients were not related to their phenotypes (Figure 1). In 78.4% of all patients an increase and in 21.6% a reduction of paraoxonase activity was found. In the AA group these ratios were 80% and 20%, in the AB group 78.6% and 21.4%, respectively. There were only two patients with the BB phenotype, which was not enough for a statistical analysis.
Figure 1.
The individual responses to the ciprofibrate treatment according to PON1 phenotype
Discussion
Metabolic syndrome is characterized by obesity, insulin resistance, hypertension and dyslipidaemia. In patients with metabolic syndrome, increased triglyceride and decreased HDL can often be observed. Each of these factors increases the fibrinogen production of the liver. Beneficial effect of micronised fenofibrate and ciprofibrate on fibrinogen concentration have been examined in several studies, in which changes in fibrinogen concentration were 15% and 20.1%, respectively [26, 27]. In agreement with these findings, in our study a significant fibrinogen reduction of 7.6% was found. Since there was no significant change in body weight during the study period, that possible cause of fibrinogen concentration reduction can be ignored.
The effect of fibrates on lipids and lipoproteins has been observed in triglyceride- and cholesterol-rich particles. They perform this action via reduction of FFA concentration as substrate of triglyceride formation and increased lipolysis of triglyceride-rich lipoprotein [28]. Blane et al. detected that fenofibrate caused a reduction of 40–60% in triglyceride concentration in type IIb hyperlipoproteinaemia [29]. In this study, we found that the triglyceride concentration was decreased by 17.8% after 100 mg day−1 ciprofibrate treatment. Other authors have reported a reduction of 43.5% in triglyceride concentration in dyslipidaemic patients after 8 weeks of therapy [30]. Blane et al. demonstrated that 300 mg fenofibrate administration reduced cholesterol concentration by 10–30%[29]; however, Kornitzer et al. described a reduction of 20.5% in cholesterol concentration after 200 mg micronized fenofibrate treatment [31]. Fu et al. reported that ciprofibrate decreased hepatic apoB mRNA editing and altered the pattern of hepatic lipoprotein secretion towards VLDL in LDL receptor-deficient mice [32]. We found a nonsignificant reduction in the cholesterol concentration and the apoB concentration was decreased by 8.3%. A previous study claimed a more significant reduction in cholesterol (13.2%) and apoB concentration (18.6%) and described the HDL-C concentration as increased by 20.8%[31]. Guerin et al. found that ciprofibrate 100 mg day−1 induced a decrease in LDL-C (17%) and apoB100 (25%) and an elevation in plasma total HDL-C (13%) and HDL-3 (22%) in patients with type IIb hyperlipidaemia [33]. In our study, the HDL-C (26%) and apoAI (30%) were significantly increased after 100 mg day−1 ciprofibrate therapy (P < 0.001). A reduction in HDL-C concentration can be detected in the metabolic syndrome. This can be explained in part by increased HDL catabolism and in part by decreased formation. The effect of ciprofibrate in increasing HDL-C is manifested via the peroxisoma proliferator-activated receptor alpha (PPARα) receptor, which results in increased lipoprotein lipase activity and apoAI formation. PPARα is expressed mainly in hepatocytes and directly regulates genes involved in fatty acid uptake and ω-oxidation and has also been shown to downregulate apolipoprotein CIII, which inhibits triglyceride hydrolysis by lipoprotein lipase [34]. Antioxidant effect plays an important role in the antiatherogenetic action of HDL. PON1 has the strongest antioxidant effect among the HDL-associated enzymes. Previous studies have demonstrated the reduced PON1 activity in patients with diabetes mellitus, so it could be responsible for increased oxidation of LDL and atherogenesis [24, 35]. Previously, Sentíet al. found reduced serum PON1 activity and lipid peroxide concentrations in patients with metabolic syndrome compared with unaffected subjects. They could exclude the influence of the PON1-192 polymorphism [22]. The effect of fibrates on PON1 activity is ambiguous. Durrington et al. have reported that 8 weeks’ bezafibrate and gemfibrozil therapy did not influence the activity of paraoxonase in type IIb hyperlipidaemic patients [36]. Tsimihodimos et al. proved that 3 months of micronized fenofibrate administration did not affect serum PON1 and arylestherase activity in type IIa, IIb and IV dyslipidaemic patients [37]. Turay et al. have investigated the effect of ciprofibrate on PON1 activity in patients with familial combined hyperlipoproteinaemia. They found a nonsignificant decrease in PON1 activity [38]. Previously, we found that, following a 3-month treatment period, gemfibrozil was able to increase the activity of paraoxonase in patients with hypertriglyceridaemia [19]. Three months’ gemfibrozil treatment was found to be effective on PON1 activity in Type 2 diabetic patients with associated hypertriglyceridaemia [39]. Gouédard et al. proved in an in vitro study that fenofibrate resulted in an increase of PON1 activity and mRNA concentrations in Hu H7 human hepatoma cell culture and slightly increased the promoter activity [40]. In this study the PON1 concentration did not change, but PON1-specific activity rose significantly after ciprofibrate treatment. Ciprofibrate administration to patients caused an increase of 26% in HDL-C concentrations and 30% in apoAI concentrations and contributed to the significant increase of paraoxonase activity. Arylesterase activity, which shows principally the mass of the enzyme, changed nonsignificantly after ciprofibrate therapy. Similarly, serum paraoxonase concentration did not changed significantly. We examined the standardized PON1 activity of HDL and apoAI (PON1/HDL, PON1/apoAI), which were not significantly changed following treatment, showing that the increase in enzyme activity was probably due to the lipoprotein particle modification. We found that ciprofibrate exerts a favourable PON1 activity similar to micronized fenofibrate and gemfibrozil [18]. Although several genes involved in the lipid metabolism and influenced by fibrates are PPARα target genes, Gouédard et al. have proved that, in the case of the PON1 gene, other mechanisms, such as LXR pathway, seem to be involved [40].
Most of the patients possessed the low-activity AA phenotype, which is unfavourable clinically with regard to atherogenesis. Data on phenotype distribution of the present study are unfavourable compared with our previous observations in Hungarian populations [39].
Mackness et al. have demonstrated that PON1 inhibits the Cu2+-induced LDL oxidation in vitro[8]. We examined whether the increased antioxidant effect could exert an influence on the concentration of oxLDL in vivo. We found that oxLDL was significantly decreased after ciprofibrate treatment. Navab et al. have reported that PON1 removes oxidized phospholipids in LDL and prevents oxLDL-induced effects in endothelial cells [41]. Increased HDL-C was shown to have a negative correlation with ischaemic heart disease, considerably inhibiting the formation of oxLDL and progression of atherosclerosis by the increased antioxidant effect [35]. The insulin resistance syndrome is associated with increased VLDL concentration and elevated triglyceride transfer into LDL, leading to the enhanced hydrolysis of LDL triglyceride content and resulting in a smaller, denser, lipid-depleted LDL particle with enhanced susceptibility to oxidation [42]. Previously, Guerin et al. proved that ciprofibrate treatment could qualitatively normalize the dense LDL profile by specifically reducing plasma concentrations of dense LDL subfractions in subjects with type IIb hyperlipidaemia. Our findings are consistent with the above-mentioned observations [43]. We found that, before treatment, the estimated ratio of LDL-C/LDLB, which reflects the size of LDL, was < 1.2. This means a preponderance of small dense particles. After ciprofibrate treatment, this ratio increased significantly, reflecting a reduction in the number small, dense particles. In parallel, the specific activity of HDL-bound antioxidant PON1 increased. Together, they may result in a decrease in oxLDL concentrations.
In conclusion, the results of this study demonstrate that ciprofibrate, with its favourable effect on HDL remodelling, apoA1, triglyceride and PON1 antioxidative properties, could favourably influence the high risk for cardiovascular events. Some limitations of the study must be recognized. The lack of a control group is probably a weak point, although several relevant data can be obtain from this self-control designed study. Beside the measurement of PON1 activity and concentration, genotyping of the two most common PON1 polymorhisms would have additional importance. However, the phenotype distribution can be estimated by use of the dual substrate method.
Data of the present study demonstrate that ciprofibrate therapy in the usual dosage may not suffice to reduce effectively all the lipid components, and a combination statin/fibrate therapy would be necessary to control all lipid abnormalities.
Acknowledgments
This study was supported by a grant from Health Scientific Council, Hungary (ETT 503/2003).
References
- 1.Assmann G, Schulte H. Relation of high density lipoprotein cholesterol and triglycerides to atherosclerotic coronary artery disease (the PROCAM experience) Am J Cardiol. 1992;70:733–7. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 2.Hadden DR, Montgomery DA, Skelly RJ, Trimble R, Weaver JA. Maturity onset diabetes mellitus response to intensive dietary management. BMJ. 1975;3:276–8. doi: 10.1136/bmj.3.5978.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis B, Mancini M, Mattlock M. Plasma triglyceride and fatty acid metabolism in diabetes mellitus. Eur J Clin Invest. 1972;2:445–8. doi: 10.1111/j.1365-2362.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 4.Howard BJ. Lipoprotein metabolism in diabetes mellitus. J Lipid Res. 1987;28:613–28. [PubMed] [Google Scholar]
- 5.Bainton D, Miller NE, Bolton CH, Yarnell JQ, Sweetnam PM, Baker IA, Lewis B, Elwood PC. Plasma triglyceride and high density lipoprotein cholesterol as predictors of ischaemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Br Heart J. 1992;68:60–6. doi: 10.1136/hrt.68.7.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–7. doi: 10.1016/0002-8703(86)90296-6. [DOI] [PubMed] [Google Scholar]
- 7.Watson KE, Horowitz BN, Matson G. Lipid abnormalities in insulin resistant states. Rev Cardiovasc Med. 2003;4:228–36. [PubMed] [Google Scholar]
- 8.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–4. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 9.Mackness MI, Durrington PN. High density lipoprotein, its enzymes and their potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–53. doi: 10.1016/0021-9150(94)05524-m. [DOI] [PubMed] [Google Scholar]
- 10.Nevin DN, Zambon A, Furlong CE, Richter RJ, Humbert R, Hokanson JE, Brunzell JD. Paraoxonase genotypes, lipoprotein lipase activity, and HDL. Arterioscler Thromb Vasc Biol. 1996;16:1243–9. doi: 10.1161/01.atv.16.10.1243. [DOI] [PubMed] [Google Scholar]
- 11.Garin MC, James RW, Dussoix P, Blanche H, Passa P, Froguel P, Ruiz J. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest. 1997;99:62–6. doi: 10.1172/JCI119134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackness MI, Harty D, Bhatnagar D, Winocour PH, Arrol S, Ishola M, Durrington PN. Serum paraoxonase activity in familial hypercholesterolemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991;86:193–9. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- 13.Odawara M, Tachi Y, Yamashita K. Paraoxonase polymorphism (Gln192-Arg) is associated with coronary heart disease in Japanese noninsulin dependent diabetes mellitus. J Clin Endocrin Metab. 1997;82:2257–60. doi: 10.1210/jcem.82.7.4096. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz J, Blanche H, James RW, Garin MC, Vaisse C, Charpentier G, Cohen N, Morabia A, Passa P, Froguel P. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet. 1995;346:869–72. doi: 10.1016/s0140-6736(95)92709-3. [DOI] [PubMed] [Google Scholar]
- 15.Malin R, Jarvinen O, Sisto T, Koivula T, Lehtimäki T. Paraoxonase producing PON1 gene M/L55 polymorphism is related to autopsy-verified artery-wall atherosclerosis. Atherosclerosis. 2001;157:301–7. doi: 10.1016/s0021-9150(00)00728-0. [DOI] [PubMed] [Google Scholar]
- 16.Antikainen M, Murtomaki S, Syvanne M. The Gln-Arg191 polymorphism of the human paraoxonase gene (HUMPONA) is not associated the risk of coronary artery disease in Finns. J Clin Invest. 1996;98:883–5. doi: 10.1172/JCI118869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guichard JP, Prades Sauron RL. A comparison of the bioavailability of standard or micronized formulations of fenofibrate. Curr Ther Res. 1993;54:610. [Google Scholar]
- 18.Schierf G, Shwat M, Feuerborn E. Biliary and plasma lipids and lipid-lowering chemotherapy. Studies with clofibrate, fenofibrate and etofibrate in healthy volunteers. Atherosclerosis. 1980;36:323–9. [Google Scholar]
- 19.Paragh Gy Balogh Z, Seres I, Harangi M, Boda J, Kovács P. Effect of gemfibrozil on HDL-associated serum paraoxonase activity and lipoprotein profile in patients with hyperlipidaemia. Clin Drug Invest. 2000;19:277–82. [Google Scholar]
- 20.Paragh Gy, Seres I, Harangi M, Balogh Z, Illyés L, Boda J, Szilvássy Z, Kovács P. The effect of micronised fenofibrate on serum paraoxonase activity in patients with coronary heart disease. Diab Met. 2003;29:613–8. doi: 10.1016/s1262-3636(07)70077-0. [DOI] [PubMed] [Google Scholar]
- 21.Fazio S, Linton MF. The role of fibrates in managing hyperlipidemia: mechanisms of action and clinical efficacy. Curr Atheroscler Rep. 2004;6:148–57. doi: 10.1007/s11883-004-0104-8. [DOI] [PubMed] [Google Scholar]
- 22.Sentí M, Tomás M, Fitó M, Weinbrenner T, Covas MI, Sala J, Masiá R, Marrugat J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab. 2003;88:5422–6. doi: 10.1210/jc.2003-030648. [DOI] [PubMed] [Google Scholar]
- 23.Hattori Y, Suzuki M, Tsushima M, Yoshida M, Tokunaga Y, Wang Y, Zhao D, Takeuchi M, Hara Y, Ryomoto KI, Ikebuchi M, Kishioka H, Mannami T, Baba S, Harano Y. Development of approximate formula for LDL-chol, LDL-apo B and LDL-chol/LDL-apo B as indices of hyperapobetalipoproteinemia and small dense LDL. Atherosclerosis. 1998;138:289–99. doi: 10.1016/s0021-9150(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 24.Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1812–8. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 25.La Du BN, Eckerson HW. The polymorphic paraoxonase/arylesterase isozymes of human serum. Fed Proc. 1984;43:2338–41. [PubMed] [Google Scholar]
- 26.Genest J, Jr, Nguyen NH, Theroux P, Davignon J, Cohn JS. Effect of micronized fenofibrate on plasma lipoprotein levels and hemostatic parameters of hypertriglyceridemic patients with low levels of high-density lipoprotein cholesterol in the fed and fasted state. J Cardiovasc Pharmacol. 2000;35:164–72. doi: 10.1097/00005344-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Okopien B, Cwalina L, Lebek M, Kowalski J, Zielinski M, Wisniewska-Wanat M, Kalina Z, Herman ZS. Effects of fibrates on plasma prothrombotic activity in patients with type IIb dyslipidemia. Int J Clin Pharmacol Ther. 2001;39:551–7. doi: 10.5414/cpp39551. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Vega GL. Fibric acids: effects on lipids and lipoprotein metabolism. Am J Med. 1987;83:9–20. doi: 10.1016/0002-9343(87)90866-7. [DOI] [PubMed] [Google Scholar]
- 29.Blane GF. Review of European clinical experience with fenofibrate. Cardiology. 1989;76:1. doi: 10.1159/000174541. [DOI] [PubMed] [Google Scholar]
- 30.Aguilar-Salinas CA, Fanghanel-Salmon G, Meza E, Montes J, Gulias-Herrero A, Sanchez L, Monterrubio-Flores EA, Gonzalez-Valdez H, Gomez Perez FJ. Ciprofibrate versus gemfibrozil in the treatment of mixed hyperlipidemias: an open-label, multicenter study. Metabolism. 2001;50:729–33. doi: 10.1053/meta.2001.23308. [DOI] [PubMed] [Google Scholar]
- 31.Kornitzer M, Dramaix M, Vandenbrock MD, Everaert L, Gerlinger C. Efficacy and tolerance of 200 mg micronised fenofibrate administered over a 6-month period in hyperlipidaemic patients: an open Belgian multicenter study. Atherosclerosis. 1994;110:S49–S54. doi: 10.1016/0021-9150(94)05378-v. [DOI] [PubMed] [Google Scholar]
- 32.Fu T, Mukhopadhyay D, Davidson NO, Borensztajn J. The PPARalpha agonist ciprofibrate inhibits apolipoprotein B mRNA editing in LDL receptor-deficient mice: effects on plasma lipoproteins and the development of atherosclerotic lesions. J Biol Chem. 2004;279:28662–9. doi: 10.1074/jbc.M403271200. [DOI] [PubMed] [Google Scholar]
- 33.Guerin M, Le Goff W, Frisdal E, Schneider S, Milosavljevic D, Bruckert E, Chapman MJ. Action of ciprofibrate in type IIb hyperlipoproteinemia: modulation of the atherogenic lipoprotein phenotype and stimulation of high-density lipoprotein-mediated cellular cholesterol efflux. J Clin Endocrinol Metab. 2003;88:3738–46. doi: 10.1210/jc.2003-030191. [DOI] [PubMed] [Google Scholar]
- 34.Desager JP, Horsmans Y, Vandenplas C, Harvengt C. Pharmacodynamic activity of lipoprotein lipase and hepatic lipase, and pharmacokinetic parameters measured in normolipidemic subjects receiving ciprofibrate (100 or 200 mg/day) or micronised fenofibrate (200 mg/day) therapy for 23 days. Atherosclerosis. 1996;124:S65–73. doi: 10.1016/0021-9150(96)05859-5. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda Y, Suehiro T, Inoue M, Nakauchi Y, Morita T, Arii K, Ito H, Kumon Y, Hasimoto K. Serum paraoxonase activity and its relationship to diabetic complications in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:598–602. doi: 10.1016/s0026-0495(98)90246-3. [DOI] [PubMed] [Google Scholar]
- 36.Durrington PN, Mackness MI, Bhatnagar D, Julier K, Prais H, Arrol S, Morgan J, Wood GN. Effects of two different fibric acid derivates on lipoproteins, cholesteryl ester transfer, fibrinogen, plasminogen activator inhibitor and paraoxonase activity in type Iib hyperlipoproteinaemia. Atherosclerosis. 1998;138:217–25. doi: 10.1016/s0021-9150(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 37.Tsimihodimos V, Kakafika A, Tambaki AP, Bairaktari E, Chapman MJ, Elisaf M, Tselepis AD. Fenofibrate induces HDL-associated PAF-AH but attenuates enzyme activity associated with apoB-containing lipoproteins. J Lipid Res. 2003;44:927–34. doi: 10.1194/jlr.M200452-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Turay J, Grnaková V, Valka J. Changes in paraoxonase and apolipoprotein A-I, B, C-III and E in subjects with combined familial hyperlipoproteinaemia treated with ciprofibrate. Drug Exp Clin Res. 2000;26:83–8. [PubMed] [Google Scholar]
- 39.Balogh Z, Seres I, Harangi M, Kovács P, Kakuk G, Paragh G. Gemfibrozil increases paraoxonase activity in type 2 diabetic patients. A new hypothesis of beneficial action of fibrates? Diab Met. 2001;27:604–10. [PubMed] [Google Scholar]
- 40.Gouédard C, Koum-Besson N, Barouki R, Morel Y. Opposite regulation of the human paraoxonase-1 gene PON-1 by fenofibrate and statins. Mol Pharmacol. 2003;63:945–56. doi: 10.1124/mol.63.4.945. [DOI] [PubMed] [Google Scholar]
- 41.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H, Fogelman AM. Monocyte transmigration of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–46. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein paticles. J Clin Invest. 1993;92:141–6. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerin M, Le Goff W, Frisdal E, Schneider S, Milosavljevic D, Bruckert E, Chapman MJ. Action of ciprofibrate in type IIb hyperlipoproteinemia: modulation of the atherogenic lipoprotein phenotype and stimulation of high-density lipoprotein-mediated cellular cholesterol efflux. J Clin Endocrinol Metab. 2003;88:3738–46. doi: 10.1210/jc.2003-030191. [DOI] [PubMed] [Google Scholar]