Abstract
Aims
St John’s wort (SJW) decreases the blood concentration of ciclosporin A (CsA), which may result in allograft rejection. In addition, the time course of this interaction is not parallel with the administration of SJW. We aimed to develop a pharmacokinetic model to predict the time profile of blood CsA concentrations during and after the intake of SJW.
Methods
We developed a pharmacokinetic model incorporating turnover of detoxicating proteins, with the assumption that the amount of detoxicating proteins is in inverse proportion to the ratio of trough blood concentration to daily dose (C/D ratio) of CsA. First, we collected time profiles of blood CsA during and after the intake of SJW from the literature. Next, we analysed the relationship between D/C ratio and the daily dose of SJW at steady state. Subsequently, the developed model was simultaneously fitted to the time profiles of C/D ratios by using a nonlinear least-squares method to obtain model parameters.
Results
The model analysis revealed that the induction of the detoxicating proteins by SJW was saturable with an elimination rate constant of the detoxicating proteins (ke) of 4.72 month−1. Elimination half-life of the detoxicating proteins calculated from the ke value was 4.4 days, suggesting that the dose of CsA should be carefully monitored for up to 2 weeks after the cessation of SJW intake.
Conclusions
The present model may provide additional information for use in identifying optimal dosage regimens of CsA during and after the intake of SJW to prevent an adverse drug interaction between CsA and SJW.
Keywords: ciclosporin A, dosage, drug interaction, pharmacokinetic model, St John’s wort
Introduction
St John’s wort (Hypericum perforatum, SJW) is effective in the treatment of mild to moderate depression [1]. However, an SJW-associated decrease in the blood concentration of a variety of drugs, such as theophylline and ciclosporin A (CsA), has been reported [2]. The interaction between SJW and theophylline is attributable to the induction of the primary metabolic enzyme of theophylline, cytochrome P450 (CYP) 1A2, by an ingredient of SJW, hypericin [2]. On the other hand, the interaction between SJW and CsA has been attributed to the activation of the nuclear receptor, pregnane X receptor (PXR), by another ingredient of SJW, hyperforin, which induces both CYP3A4 and multidrug resistance 1 (MDR1) [3]. It seems likely that the induction and de-induction of detoxicating proteins such as CYP3A4 and MDR1 would involve a certain time-lag.
CsA is one of the key drugs for post-transplant patients. It has been reported in Germany that 7.3% of inpatients were self-medicated with SJW [4]. Before or after transplantation, patients tend to feel anxious and may take supplements with antidepressive activity, such as SJW. Indeed, there are many reports of an interaction between CsA and SJW [5–12]. In most cases, the blood concentration : dose ratio of CsA fell gradually after the start of SJW and recovered gradually after cessation. However, there is no pharmacokinetic model, based on the induction and de-induction of the detoxicating proteins by SJW, to provide optimal dosage regimens of CsA for avoiding the interaction between CsA and SJW.
The purpose of this study was to analyse retrospectively the clinical cases of an interaction between SJW and CsA and to develop a pharmacokinetic model based on the turnover of the detoxicating proteins to predict the time profile of this interaction.
Methods
Collection of cases
We collected case reports of the interaction that describes the time profile of the blood concentration of CsA, dose of CsA and the amount of SJW intake from the literature and calculated the time course of the trough blood concentration : dose ratio (C/D ratio) of CsA. Details of the cases are shown in Table 1.
Table 1.
Details of nine cases included in this study
| Age (years) | Gender | Transplanted organ | Co-medications | Reference | |
|---|---|---|---|---|---|
| Case 1 | 61 | Male | Heart | Azathioprine, corticosteroid | [5] |
| Case 2 | 63 | Not cited | Heart | Azathioprine, corticosteroid | [5] |
| Case 3 | 29 | Female | Kidney, pancreas | Prednisone, clonidine | [6] |
| Case 4 | 55 | Female | Kidney | Benzbromarone, betaxolol, amlodipine, pravastatin, magnesium | [7] |
| Case 5 | 44 | Female | Kidney | Mycophenoloate mofetil, prednisone | [8] |
| Case 6 | 63 | Male | Liver | Acetyldigoxin | [9] |
| Case 7 | 55 | Female | Kidney | Allopurinol, verapamil, enalapril, furosemide, cisapride, zinc, alpha-tocopherol, alpha-lipoic acid | [10] |
| Case 8 | 58 | Male | Kidney | Azathioprine, prednisone | [11] |
| Case 9 | Not cited | Not cited | Kidney | Not cited | [12] |
Analysis of the dose–response relationship of SJW for the induction of the detoxicating proteins
We calculated the inverse of the C/D ratio (D/C ratio) of CsA at steady state during the period of taking SJW (SJW period) and the SJW-free (control) period for each case. The latest blood sampling time during the SJW period was assumed to be at steady state, and was employed to calculate the D/C ratios for the SJW period (D/C (SJW)) in all cases. The mean D/C ratio of the control period for each case was assigned as the control D/C ratio (D/C (control)) except for one case, in which the latest D/C ratio during the control period was employed as D/C (control) because the control period was preceded by an SJW period. We analysed the relationship between the ratio of D/C (SJW) to D/C (control) (extent of decrease in C/D ratio) and the daily dose of SJW in order to survey the characteristics of the dose–response relationship of SJW for the induction of detoxicating proteins.
Development of the pharmacokinetic model
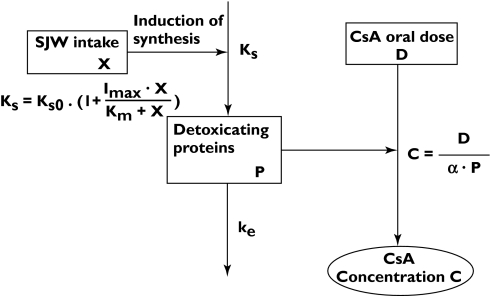
The interaction between SJW and CsA was modelled based on the following assumptions (Figure 1). The amount of the detoxicating proteins (P), a hypothetical value that is assumed to reflect the amount of CYP3A4 and/or MDR1, determines the blood CsA concentration. The indirect response model with zero-order synthesis and first-order elimination has been commonly used to explain the indirect pharmacological responses [13]. Therefore, we applied the indirect model to explain the change in the amount of P by SJW, i.e. P is synthesized with a zero-order rate constant, Ks (AU/month), and disappears with a first-order rate constant, ke. The mass balance equation for P is given as follows:
Figure 1.
Pharmacokinetic and pharmacodynamic model of the interaction between SJW and CsA. X is the daily intake of SJW (mg day−1), Ks is the rate constant of synthesis of detoxicating proteins (AU/month), ke is the elimination rate constant of the detoxicating proteins (/month), P is the amount of the detoxicating proteins (AU), D is the daily dose of CsA (mg day−1), C is CsA trough blood concentration (ng ml−1), Ks0 is the rate constant of detoxicating proteins in the absence of SJW (AU/month), Imax is the maximal induction potency of SJW for detoxicating proteins, Km is the dose of SJW required to induce half-maximal induction (mg day−1), and α is a constant ((ng ml−1)/(mg day−1)/AU)
| (1) |
The intake of SJW is considered to increase Ks. The analysis in the previous section demonstrated that the extent of decrease in the C/D ratio of CsA is saturable and SJW dose-dependent. Therefore, Ks can be described by equation 2:
| (2) |
where Ks0, X, Imax and Km represent a zero-order synthesis rate constant of P in the absence of SJW (AU/month), the daily dose of SJW (mg day−1), the maximal induction potency of SJW for P and the dose of SJW required to induce half-maximal induction (mg day−1), respectively. In each case, the C/D ratio was assumed to be in inverse proportion to P for each patient. The relationship between C and D can be represented by equation 3:
| (3) |
where C, D and α represent the trough blood concentration of CsA (ng ml−1), the daily dose of CsA (mg day−1) and a constant ((mg day−1)/(ng ml−1)/AU), respectively. Equation 3 can be rewritten as follows:
| (3′) |
Substituting equation 3′ into equation 1 gives equation 4:
| (4) |
Substituting equation 2 into equation 4 gives equation 4′:
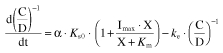 |
(4′) |
Model analysis
Equation 4′ was simultaneously fitted to the time profiles of C/D ratio for all the cases, taking the dose profiles of SJW as input functions, by using a nonlinear least-squares method (MLAB, Civilized Software Inc., MD, USA) to obtain common pharmacokinetic parameters, Imax, Km and ke, and an individual parameter for each case, α · Ks0. The Km value was modelled based on a log-normal distribution.
Results
Analysis of the dose–response relationship of SJW for the induction of the detoxicating proteins
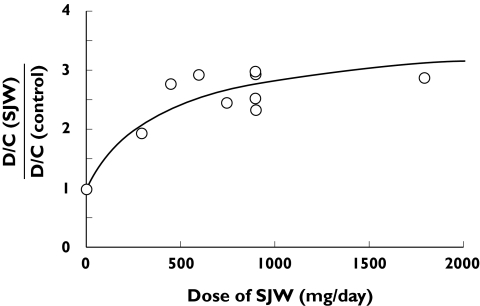
The increase in the steady-state D/C ratio of CsA by SJW was dose-dependent and described by saturable Michaelis-Menten kinetics, suggesting that the induction of detoxicating proteins by SJW is saturable (Figure 2).
Figure 2.
Relationship between the dose of SJW and D/C ratio of CsA at the steady state. Dose-to-trough blood concentration ratio (D/C ratio) of CsA in the SJW period and SJW-free period at the steady state was calculated in nine cases and plotted. The latest blood sampling time during the SJW period was employed to calculate the D/C ratio for the SJW period. The mean D/C ratio of the SJW-free (control) period for each case (case 1–4, 6–9) was assigned as the D/C (control) except for case 5, in which the latest D/C ratio during the SJW free period was employed as D/C (control). The line represents the parameter obtained by this analysis and points represent the ratio of D/C (SJW) to D/C (control) calculated in each case
Model analysis
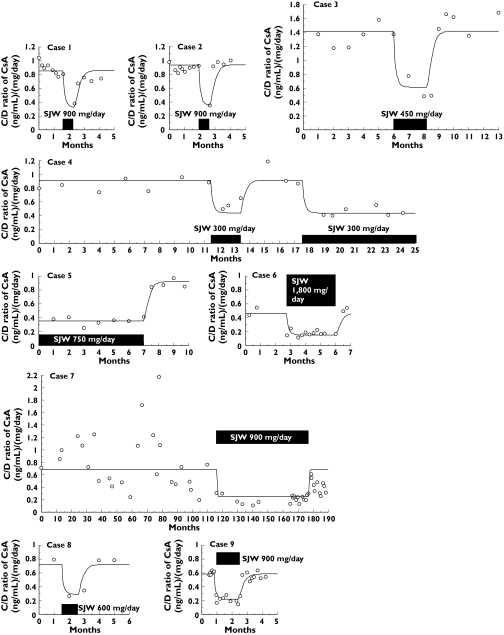
As a result of model analysis, Imax, Km and ke were calculated to be 2.61, 428 (mg day−1) and 4.72 (/month), respectively. Moreover the individual parameter values α · Ks0, ranged from 3.33 to 10.0 ((mg day−1)/(ng ml−1)/month) (Table 2). The developed model could adequately explain the observed time profile of the C/D ratio in each case (Figure 3).
Table 2.
Pharmacokinetic and pharmacodynamic parameters obtained by the model analysis for the interaction between SJW and CsA
| Imax | 2.61 ± 1.80 | |
| Km (mg day−1) | 428 (100.2–1828)* | |
| ke (month−1) | 4.72 ± 1.42 | |
| α · Ks0 ((mg day−1)/ (ng ml−1)/month) | Case 1 | 5.53 ± 1.42 |
| Case 2 | 5.05 ± 1.62 | |
| Case 3 | 3.33 ± 1.03 | |
| Case 4 | 5.20 ± 1.61 | |
| Case 5 | 5.09 ± 1.69 | |
| Case 6 | 10.0 ± 3.75 | |
| Case 7 | 6.58 ± 2.31 | |
| Case 8 | 8.10 ± 2.65 | |
| Case 9 | 6.92 ± 2.11 |
Estimate ± SD.
range Imax: maximal induction potency of SJW for P.Km: the dose of SJW required for half-maximal induction (mg day−1). ke: the elimination rate constant of the detoxicating proteins (/month). α: a constant ((mg day−1)/(ng ml−1)/AU). Ks0: the synthesis rate constant of detoxicating proteins in the absence of SJW (AU/month).
Figure 3.
The falls of CsA blood concentration due to intake of SJW [5–12] along with the fitting lines obtained with the developed model. The time profiles of the trough blood concentration : dose ratio (C/D ratio) of CsA were calculated in nine cases. The C/D ratios of CsA were fitted with the developed model. The sum of squares normalized by dividing by the number of samples in case1 to case 9 as an indication of goodness of fit were 0.00856, 0.0118, 0.0462, 0.0120, 0.00275, 0.00985, 0.126, 0.0143 and 0.00366, respectively. Point, box and line represent C/D ratio, the period of SJW intake and the fitting line, respectively
Discussion
We have reported a pharmacokinetic model to explain the mechanism-based inhibition of CYP3A4 by grapefruit juice in which the turnover of CYP3A4 protein was incorporated. The model provided the dosing-interval dependency of the extent of interaction based on the time-dependent changes of the active CYP3A4 content [14]. With regard to the induction of detoxicating protein(s), model analysis based on the turnover of protein(s) has not been carried out. CsA concentration is decreased as a result of the induction of detoxicating proteins by SJW intake. Therefore, we employed the C/D ratio as an indicator of CsA metabolism. In this study, we developed a model to explain the induction of the detoxicating proteins by SJW, and established that the model could adequately explain the time course of the C/D ratio of CsA (Figure 3).
However, the estimation of Km value for the induction was not definite enough. A possible explanation for this was that the preparations were not standardized, so that there may be variations in the bioavailability, contents of ingredients and so on among products.
The elimination rate constant of the detoxicating proteins, ke, was calculated to be 4.72 month−1, so that the elimination half-life was 4.4 days. This result suggests that a period of 2–3 weeks may be required for normalization of the level of the detoxicating proteins after the cessation of SJW intake. Therefore, the dose of CsA should be carefully monitored and modified as necessary for at least 2 weeks after the start or cessation of SJW intake.
Although the present model incorporated the induction of detoxicating proteins by SJW, the nature of these proteins is unspecified in this study. The major contributing proteins are thought to be CYP3A4 and/or MDR1 in the intestine and/or liver, but contributions from other proteins or from the same proteins in other organs cannot be excluded. However, it is not feasible at present to analyse quantitatively the contributions of plural proteins in various organs to the metabolism and excretion of CsA, so we considered them collectively, and this approach seemed to be adequate for the present purpose.
The present model is based on the turnover of the detoxicating proteins, and parameters other than α · Ks0 are drug-independent. As the interaction between SJW and CsA is well known and their co-administration is usually avoided in the clinical setting, it may be not feasible to evaluate further whether the present model can be applied to the clinical cases. Therefore it might be worth investigating whether the kinetics of induction and de-induction of the detoxicating proteins obtained in this study can be applied to analyse other interactions between SJW and different drugs for which the kinetics are regulated by CYP3A4 or MDR1.
By applying the developed model, the optimal dose of CsA after the cessation of SJW intake can be calculated from equation 5:
| (5) |
where T (day), t (day) and D0 represent the duration of SJW intake, the period after the cessation of SJW intake and the dose of CsA in the SJW-free period, respectively. Bauer et al. have reported that they were obliged to increase the dose of CsA from 2.7 to 4.2 mg day−1 kg−1 to keep CsA blood concentration in the therapeutic range during the intake of SJW for 10 days [15]. They carefully controlled the dose of CsA to maintain a therapeutic concentration during the SJW period and found that the dose of CsA reached the steady state about 2 weeks after the start of SJW in most cases. This finding is consistent with our conclusion that dose of CsA should be modified for at least 2 weeks after the start of SJW intake. Substitution of Bauer’s parameters, including D0 (2.7 mg day−1 kg−1), T (14 days), t (0 day) and X (600 mg day−1), into equation 5 yields the D value of 6.0 mg day−1 kg−1. Taking into consideration the fact that they allowed a range of 70–150 ng ml−1 for CsA concentration and that they may have minimized the modification of the dose, the D value calculated from equation 5 is comparable with the mean actual dose (4.2 mg day−1 kg−1). To estimate the dose more accurately, the model parameters should be defined by more clinical reports.
In conclusion, we have developed a pharmacokinetic model to describe the induction and de-induction of detoxicating proteins by SJW. Although the obtained parameters remain to be verified further by other case reports and studies, the present model may provide additional information to estimate the optimal dosage regimen of CsA during and after the intake of SJW to prevent adverse drug interaction between CsA and SJW.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (category B) from the Japan Society for the Promotion of Science (JSPS) (Yasufumi Sawada, ♯16390043).
References
- 1.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression: an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253–8. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nebel A, Schneider BJ, Baker RK, Kroll DJ. Potential metabolic interaction between St. John’s wort and theophylline. Ann Pharmacother. 1999;33:502. doi: 10.1345/aph.18252. [DOI] [PubMed] [Google Scholar]
- 3.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Facklam M, Rieger K, Riedel KD, Burhenne J, Walter-Sack I, Haefeli WE. Undeclared exposure to St. John’s wort in hospitalized patients. Br J Clin Pharmacol. 2004;58:437–41. doi: 10.1111/j.1365-2125.2004.02169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruschitzka F, Meier PJ, Turina M, Lüscher TF, Noll G. Acute heart transplant rejection due to Saint John’s wort. Lancet. 2000;355:548–9. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 6.Barone GW, Gurley BJ, Ketel BL, Lightfoot ML, Abul-Ezz SR. Drug interaction between St. John’s wort and cyclosporine. Ann Pharmacother. 2000;34:1013–6. doi: 10.1345/aph.10088. [DOI] [PubMed] [Google Scholar]
- 7.Mai I, Krüger H, Budde K, Johne A, Brockmöller J, Neumayer HH, Roots I. Hazardous pharmacokinetic interaction of Saint John’s wort (Hypericum perforatum) with the immunosuppressant cyclosporin. Int J Clin Pharmacol Ther. 2000;38:500–2. doi: 10.5414/cpp38500. [DOI] [PubMed] [Google Scholar]
- 8.Barone GW, Gurley BJ, Ketel BL, Abul-Ezz SR. Herbal supplements: a potential for drug interactions in transplant recipients. Transplantation. 2001;71:239–41. doi: 10.1097/00007890-200101270-00012. [DOI] [PubMed] [Google Scholar]
- 9.Karliova M, Treichel U, Malagò M, Frilling A, Gerken G, Broelsch CE. Interaction of Hypericum perforatum (St. John’s wort) with cyclosporin A metabolism in a patient after liver transplantation. J Hepatol. 2000;33:853–5. doi: 10.1016/s0168-8278(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 10.Beer AM, Ostermann T. St. John’s wort: interaction with cyclosporine increases risk of rejection for the kidney transplant and raises daily cost of medication. Med Klin (Munich) 2001;96:480–4. doi: 10.1007/pl00002231. [DOI] [PubMed] [Google Scholar]
- 11.Moschella C, Jaber BL. Interaction between cyclosporine and Hypericum perforatum (St. John’s wort) after organ transplantation. Am J Kidney Dis. 2001;38:1105–7. doi: 10.1053/ajkd.2001.28617. [DOI] [PubMed] [Google Scholar]
- 12.Breidenbach T, Kliem V, Burg M, Radermacher J, Hoffmann MW, Klempnauer J. Profound drop of cyclosporin A whole blood trough levels caused by St. John’s wort (Hypericum perforatum) Transplantation. 2000;69:2229–30. doi: 10.1097/00007890-200005270-00052. [DOI] [PubMed] [Google Scholar]
- 13.Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–78. doi: 10.1007/BF01061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takanaga H, Ohnishi A, Matso H, Murakami H, Sata H, Kuroda K, Urae A, Higuchi S, Sawada Y. Pharmacokinetic analysis of felodipine–grapefruit juice interaction based on an irreversible enzyme inhibition model. Br J Clin Pharmacol. 2000;49:49–58. doi: 10.1046/j.1365-2125.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer S, Störmer E, Johne A, Krüger H, Budde K, Neumayer HH, Roots I, Mai I. Alterations in cyclosporin A pharmacokinetics and metabolism during treatment with St John’s wort in renal transplant patients. Br J Clin Pharmacol. 2003;55:203–11. doi: 10.1046/j.1365-2125.2003.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]