Abstract
The burgeoning problem of malaria in the developing world and the relentless march of drug resistance demand that we continue to seek new chemotherapeutic strategies. Given the enormous expense of developing and marketing new chemical entities, we often rely on an increased understanding of the pharmacology of older drugs and judicious use of drug combinations. Development is being driven primarily by public–private partnerships from academic investigations. Two such agents are the antifolate combination Lapdap™, already licensed and soon to be combined with artesunate, and isoquine, a novel isoquinoline, about to enter clinical trials. Other drug combinations designed to minimize the spread of resistance are in the pipeline. Such developments are crucial as it becomes clear that existing drugs, even those used in combinations, may have limited lifetimes.
Keywords: Malaria, drug resistance, Lapdap, dapsone, chlorproguanil, artesunate, isoquine
Malaria is a worsening problem in the developing world. Snow and colleagues argue that there were 515 (range 300–660) million episodes of clinical Plasmodium falciparum malaria in 2002. These figures are up to 50% higher than those reported by the World Health Organization (WHO) and 200% higher for areas outside Africa [1]. The absence of an effective vaccine means that we continue to rely on drugs to manage a disease that has arguably been overshadowed by HIV and tuberculosis. Our failure to combat this surge in malaria cases can substantially be attributed to a frightening increase in multiple drug resistance. Chloroquine resistance is now common in every region where P. falciparum occurs [2]. Replacement of chloroquine with sulfadoxine–pyrimethamine provided a temporary respite, until this combination succumbed in South-east Asia, South America and, most recently, Africa. Clinical failure of mefloquine and atovaquone was reported soon after their introduction.
Fortunately, we have rarely been better placed to arrest this slide. Chemists, parasitologists, pharmacologists and clinicians have come together with the pharmaceutical industry and nongovernmental organizations in public–private partnerships to bring new chemical entities into clinical use. These compounds have often arisen from an increased understanding of the pharmacology of older drugs or judicious use of drug combinations [3]. While many projects have been initiated in recent years, few have made it to the finishing line or got within sight of it. We shall describe some of these here alongside the currently recommended strategies for malaria chemotherapy.
Two projects in which the University of Liverpool and Liverpool School of Tropical Medicine have been closely involved are Lapdap™/artesunate and isoquine. The former is a combination of chlorproguanil (an inhibitor of dihydrofolate reductase), dapsone (an inhibitor of dihydropteroate synthetase) and artesunate, one of the artemisinin family of antimalarial agents (Figures 1 and 2). The latter is a structural isomer of amodiaquine, whose use in malaria prophylaxis was curtailed because of an unacceptably high incidence of adverse drug reactions. Development of both drugs is being driven by a public–private partnership between the WHO and Glaxo SmithKline under the auspices of the Medicines for Malaria Venture (MMV).
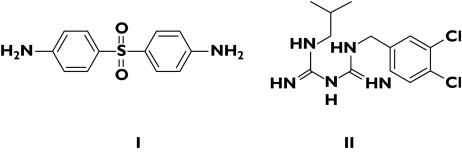
Figure 1.
Chlorproguanil (I) and dapsone (II)
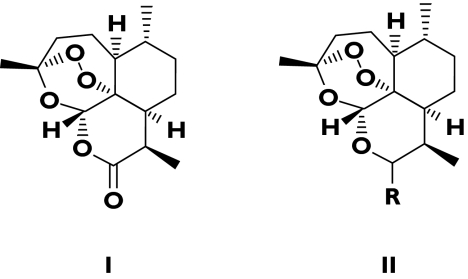
Figure 2.
Artemisinin (I) and the general structure of artemisinin derivatives (II). R = α OC (O) CH2CH2COONa = artesunate; R = β OMe = artemether
Chlorproguanil/dapsone/artesunate
Lapdap™, a combination of chlorproguanil and dapsone, was launched in 2003 as an effective and cheap antimalarial, principally to replace sulfadoxine–pyrimethamine in Africa. Chlorproguanil/dapsone/artesunate (CDA) is being developed in response to an initiative by the WHO in 2001 to implement artemisinin-based antimalarial therapies designed to slow the spread of drug resistance (see below). Clinical evaluation of CDA is proceeding steadily and Phase III trials were due in 2005. The selection of chlorproguanil and dapsone for an antifolate combination arose from observations of Watkins and colleagues [4]. They argued convincingly that drugs with longer terminal half-lives predisposed to the selection of drug-resistant parasites and that this was the reason for the relative failure of sulfadoxine–pyrimethamine (Fansidar™). The addition of artesunate allows the rapidly acting artemisinin derivative to reduce the parasite biomass sufficiently to allow the antifolate to clear away the remainder [5] and delay the emergence of resistance to the combination.
Isoquine
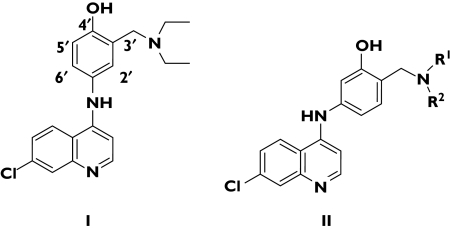
Amodiaquine (Figure 3) is a 4-aminoquinoline that may be useful in P. falciparum infections that are resistant to chloroquine [6]. An association of amodiaquine with hepatotoxicity and agranulocytosis when the drug was used for prophylaxis meant that its use was restricted to treatment of malaria. Amodiaquine is a Mannich base with a para-hydroxyanilino side-chain that bears some resemblance to paracetamol. This structure can undergo oxidation to a chemically reactive quinoneimine that can initiate both hepatotoxicity and myelotoxicity. Chemists at the University of Liverpool, through a simple exchange of the 3′-hydroxyl and the 4′-Mannich side-chain functions, created a new chemical entity, called isoquine (Figure 3). The new drug could not undergo bioactivation and was therefore unable to generate a chemically reactive structure. The toxicity of amodiaquine was by-passed but the antiparasitic activity was retained. Isoquine is scheduled to enter Phase I trials in March 2006.
Figure 3.
Chemical structure of amodiaquine (I) and the isoquine series (II) (R1 = R2 = alkyl)
Combination chemotherapy
Combination chemotherapy is well established in the treatment of tuberculosis, HIV disease and cancer. If drug resistance develops by spontaneous point mutation or gene amplification, the probability of resistance to two structurally unrelated drugs with different modes of action is a product of the two mutation frequencies. Since these frequencies are very low in malaria parasites, it is unlikely that a parasite could exist that is spontaneously resistant to two unrelated drugs [5, 7]. For three drugs, the likelihood of a spontaneously resistant viable mutant is the product of the three mutation frequencies. Sulfadoxine and pyrimethamine were originally used in combination with mefloquine when it was introduced into Thailand in 1984. This was done specifically to delay the development of resistance to mefloquine. While theoretically sound, this strategy failed in practice, because P. falciparum in Thailand in 1984 was already highly resistant to both sulfadoxine and pyrimethamine. Furthermore, when circulating concentrations of mefloquine became subtherapeutic, both pyrimethamine and sulfadoxine had disappeared because of their more rapid systemic clearance. Thus, pyrimethamine/sulfadoxine provided no protection for mefloquine.
So, for antimalarial drug combinations to be effective, either the pharmacokinetics of the two or three components must be well matched or the parasite biomass must be reduced sufficiently by one of the drug components, so that the chances of mutation to the other, more slowly eliminated drug are greatly reduced [4]. This is the rationale underlying the combination of artemisinin derivatives with antifolate drugs (see above), aminoquinolines, mefloquine and lumefantrine. Artemisinin derivatives are the most active of the available antimalarial compounds and reduce parasite biomass by about 104 per asexual cycle [8]. Treatment with two cycles over 3 days produces a 108-fold reduction in biomass, leaving a maximum of 105 parasites for the other antimalarial drug to clear. This considerably reduces both the exposure of the parasite population to the other drug and the chance of an escape-resistant mutant arising [5]. So, there is a strong case for no longer using single antimalarial drugs but always using a combination of an artemisinin derivative with an existing or newly introduced antimalarial compound. In areas of low malaria transmission this may be a very effective control measure, although such artemisinin-based combinations are not the only ones that are useful in different geographical circumstances [9].
Co-Artem™: the first fixed ratio combination of antimalarials
Artemether–lumefantrine (coartemether or Co-Artem™) is the first fixed combination of an artemisinin derivative and a second unrelated antimalarial compound. Lumefantrine (formerly benflumetol; Figure 4) is an arylamino alcohol in the same general group as mefloquine and halofantrine. It was discovered in the Peoples’ Republic of China and has been used there for several years. Lumefantrine is active against all human malaria parasites, including multidrug resistant P. falciparum. Artemether–lumefantrine has been used mainly in an adult oral dose of 80/480 mg given at 0, 8, 24 and 48 h. This has given satisfactory cure rates in semi-immune subjects, but in non-immune subjects it has proved inferior to artesunate–mefloquine. Pharmacokinetic–pharmacodynamic (PK–PD) studies have shown that the principal pharmacokinetic determinant of cure was the area under the plasma lumefantrine concentration–time curve (AUC) or its surrogate, the day 7 plasma concentration of lumefantrine on day 7 – concentrations > 500 ng ml−1 are associated with cure rates of > 90%[10]. Lumefantrine absorption (like that of atovaquone and halofantrine) critically depends on coadministration with fats and so plasma concentrations vary markedly between patients. To increase the AUC, and thus the cure rate, a six-dose regimen (adult dose 80/480 mg at 0, 8, 24, 36, 48 and 60 h) was evaluated. This proved highly effective and was remarkably well tolerated but it raised issues of adherence to therapy. Against multidrug-resistant P. falciparum malaria the six-dose regimen of artemether–lumefantrine was as effective as and better tolerated than artesunate–mefloquine [11].
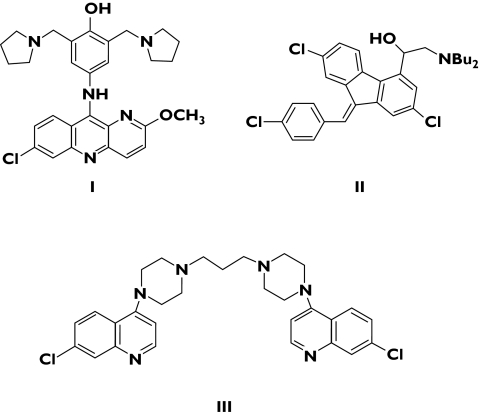
Figure 4.
Chemical structures of pyronaridine (I) lumefantrine (II) and piperaquine (III)
Artemether–lumefantrine is becoming increasingly available in tropical countries, despite its cost. The rapid and reliable therapeutic response, the high degree of efficacy and the theoretical mutual protection provided by each of the drugs against resistance selection makes combinations such as this attractive treatments for malaria. However, a note of caution was sounded by Sisowath [12] and colleagues, who have demonstrated that although parasites that carry the 86N form of the pfmdr gene are killed by Co-Artem™, they can tolerate residual drug concentrations that allow them to re-infect individuals more rapidly after treatment, compared with parasites that carry the 86Y form of the gene. Hastings and Ward [13] have argued that a large increase in drug tolerance induced through mutation may mean that the long-term future of Co-Artem™ is bleak and that combination therapies with better-matched pharmacokinetics should be sought, probably by using a partner drug with a shorter half-life than lumefantrine.
Alternative combinations
Pyronaridine artesunate (PANDA)
Pyronaridine (Figure 4) has been used for nearly 20 years to treat malaria in China. MMV are funding a project to develop a fixed-ratio combination of pyronaridine and artesunate for the treatment of acute uncomplicated malaria. While some concern has been expressed on account of the 1,4-aminophenol structural alert for hepatotoxicity, pyronaridine contains alkyl amino side-chains at both the 3′ and 5′ positions of the aminophenol ring. Although metabolic oxidation to a quinoneimine occurs with this drug, the presence of an additional 5′ substituent could prevent reactions with sulphur-containing nucleophiles [14]. A Phase I study was completed in 2004 and enrolment for a clinical Phase II study began in August 2005, with completion expected in the second quarter of 2006.
Dihydroartemisinin–piperaquine (Artekin™)
Piperaquine (Figure 4) is a bisquinoline antimalarial drug developed in the 1960s by Shanghai Pharmaceutical Industry Research (China) and Rhone Poulenc (France). Piperaquine has been combined with dihydroartemisinin (as Artekin™) to provide a cheap and reasonably well-tolerated, short-course treatment for P. falciparum, with a high cure rate against drug-resistant parasites. Artekin™ has been extensively used in controlled clinical trials in South-East Asia, with good results against chloroquine-resistant parasites. However, piperaquine has a long half-life (23 days), which is markedly different from that of dihydroartemisinin (1 h); this discrepancy has raised concerns that such a combination might rapidly attract resistance [15].
Conclusions
Converting our knowledge of malaria parasite biology into new antimalarial drugs is a relatively slow process. Drug development strategies that are based on known parasitic targets and that build on our current understanding of the clinical pharmacology of antimalarial compounds are more likely to yield effective and cheap antimalarial drug therapies.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–18. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 3.Biagini G, O’Neill PM, Bray PG, Ward SA. Current drug development portfolio for antimalarial therapies. Curr Opin Pharmacol. 2005;5:473–8. doi: 10.1016/j.coph.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Watkins WM, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine–sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Trans R Soc Trop Med Hyg. 1993;87:75–8. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 5.White NJ. Antimalarial drug resistance and combination chemotherapy. Phil Trans R Soc Lond B. 1999;354:739–49. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins WM, Sixsmith DG, Spencer HC, Boriga DA, Kariuki DM, Kipingor T, Koech DK. Effectiveness of amodiaquine as treatment for chloroquine-resistant Plasmodium falciparum infections in Kenya. Lancet. 1984;1:357–9. doi: 10.1016/s0140-6736(84)90410-0. [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–22. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt G, Shanks GD, Phintuyothin P. Prognostic significance of rises in parasitaemia during treatment of falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:359–60. doi: 10.1016/0035-9203(92)90217-z. [DOI] [PubMed] [Google Scholar]
- 9.Kremsner PG, Krishna S. Antimalarial cocktails – tropical flavours of the month. Lancet. 2002;360:1998–9. doi: 10.1016/s0140-6736(02)12032-0. [DOI] [PubMed] [Google Scholar]
- 10.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. The pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2002;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, Slight T, Looareesuwan S, White NJ, Nosten F. Randomised comparison of artemether–benflumetol and artesunate–mefloquine in the treatment of multi-drug resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–9. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisowath C, Strömberg J, Mårtensson A, Msellem M, Obondo C, Bjorkman A, Gil JP. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether–lumefantrine (Coartem) J Infect Dis. 2005;191:1014–7. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 13.Hastings IM, Ward SA. Coartem (artemether–lumefantrine) in Africa: the beginning of the end? J Infect Dis. 2005;192:1303–4. doi: 10.1086/432554. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Son J, Chung SJ, Lee ES, Kim DH. In vitro and in vivo metabolism of pyronaridine characterized by low-energy collision-induced dissociation mass spectrometry with electrospray ionization. J Mass Spectrom. 2004;39:1036–43. doi: 10.1002/jms.663. [DOI] [PubMed] [Google Scholar]
- 15.Hastings IM, Watkins WM, White NJ. The evolution of drug resistant malaria: the role of drug elimination half-life. Phil Trans R Soc Lond B. 2002;357:505–19. doi: 10.1098/rstb.2001.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]