Abstract
Aims
Selective cyclooxygenase (COX)-2 inhibitors have recently been implicated as enhancing risk of myocardial infarction (MI). Nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) are also effective COX-2 inhibitors, so we investigated the hypothesis that they too increase risk of MI.
Methods
We conducted a case–control study with direct structured interview of cases and controls. Cases were all subjects (N = 205) with a first nonfatal MI who had no previously recognized cardiovascular disease. Community controls (N = 258) were randomly selected from the same practice as the index case. Hospital controls (N = 205) were those admitted at the same time as index cases for nonmyocardial conditions not influenced by NSAID use. The effects of aspirin, NSAIDs and previously recognized influences on MI were investigated by unconditional logistic regression analysis.
Results
NSAID use was associated with an increase risk of MI with an odds ratio of 1.77 (1.03, 3.03) vs. community controls and 2.61 (1.38, 4.95) vs. hospital controls. These values were 5.00 (1.18, 21.28) and 7.66 (0.87, 67.48), respectively, in aspirin users. Results were similar when naproxen was grouped with aspirin. Odds ratios for smoking and for use of antidiabetic medication were 3.91 (2.52, 6.04) and 3.92 (1.25, 12,33), respectively, vs. community controls.
Conclusions
Like nonselective NSAIDs, selective COX-2 inhibitors are associated with an increased risk of MI. The extent to which this reflects interference with aspirin warrants further investigation.
Keywords: aspirin, case–control, epidemiology, myocardial infarction, non-steroidal anti-inflammatory drugs, smoking
Introduction
Concerns about the cardiovascular effects of selective inhibitor cyclooxygenase (COX)-2 inhibitors [1–10], which led to the withdrawal of rofecoxib, have stimulated re-evaluation of strategies for the reduction of overall risk in patients requiring anti-inflammatory drug treatment [5]. It is important to establish whether nonsteroidal anti-inflammatory drugs (NSAIDs) which also inhibit COX-2, albeit nonselectively, share the same risks. One can speculate that likely mechanisms for an increased risk of myocardial infarction (MI) with rofecoxib could be inhibition of vascular prostacyclin synthesis or sustained hypertension, both properties of nonselective NSAIDs.
The APPROVe study, which led to the withdrawal of rofecoxib, reported an 80% increase in the number of MIs on rofecoxib compared with placebo [1]. There are no comparable large long-term placebo-controlled studies of nonselective NSAIDs. However, in phase IIB/III studies ibuprofen and diclofenac were associated with vascular event rates similar to or possibly higher than those seen with rofecoxib [11–13]. Epidemiological evidence has hitherto been inconclusive, but most studies show at least a trend to increased risk [10, 14–21]. The Kaiser Permanente study showed an increased risk of MI with rofecoxib but also with nonselective NSAIDs [12]. In addition, a number of cohort studies have found that protection against first or subsequent MI with aspirin may be lost in the presence of some NSAIDs [22–24]. Whilst these have been attributed to pharmocodynamic interference with aspirin by NSAIDs [25], they are equally compatible with the proposition of a more general prothrombotic influence of NSAIDs.
Given the widespread use of NSAIDs, even small increases in the risk of MI would have major public health implications. Because a number of authors are recommending a return to nonselective NSAIDs, with or without proton pump inhibitor (PPI) co-prescription, it is particularly important to establish whether NSAIDs share the cardiovascular risk, as this would make such advice inappropriate. Here, we therefore report results of a case–control study of NSAID use in patients experiencing a first MI. A particular feature of our study was a decision to study first episodes of MI in patients without a prior history of any evidence of cardiovascular disease. We reasoned that this would make our study more sensitive to disease-related differences, as confounding by treatment is substantial once a diagnosis has been established.
Methods
This was a prospectively conducted case–control study that was approved by the University Hospital Nottingham Ethics Committee. Cases consisted of all subjects admitted to Queen’s Medical Centre, Nottingham between November 1998 and December 1999 with a first nonfatal MI. Patients with previously recognized cardiovascular disease were excluded from the study. Each day, patients presenting with MI were identified by scrutiny of admission records and daily ward visits. All who were identified as fulfilling the requirements of the study were included. In order to be included, cases had to have a typical history, including chest pain and progressive evolution of typical ECG findings (which must at some time include ST elevation > 1 mm and/or development of new Q waves) and/or a rise in creatinine kinase to a level > 75% above normal, in the absence of other confounding causes. Troponin I assays were not available in our hospital at the time of the study.
Cases were matched to both community and hospital controls. Community controls for each index case were selected within 3 months by visit to the general practice from which the case had come. A suitable community control was the first case from the same general practice as the index case identified by random record selection that was of the same sex and within 5 years of age of the index case. Two community controls were selected to allow for drop-out but all community controls who consented to interviewed were included in the analysis. Hospital controls were the first suitable non-MI patients admitted on the same day as the index case and chosen and matched to cases similarly to community controls. Patients admitted because of any form of arthritis or ulcer disease were excluded from being a control to avoid a spurious amplification of NSAID use in hospital controls, but those with coincidental arthritis or ulcer disease who were admitted for other reasons were included in order to avoid an opposite bias.
Data collection
Data were collected by interview (G.M.H. and S.E.) according to a structured questionnaire, as previously described [26]. Current NSAID exposure was defined as any exposure to any non-aspirin NSAID during the week prior to the first onset of symptoms (cases and hospital controls) or interview (community controls). Use of individual NSAIDs was noted. Previous exposure was defined as any exposure in the last year that finished more than 7 days prior to admission. Current and previous aspirin use were similarly defined. World Health Organization criteria [27] were used to calculate the defined daily dose of aspirin and non-aspirin NSAIDs. Body mass index (BMI) was calculated from height and weight measurements or self report and categorized as low, average or increased [28].
Analyses
Our primary analysis concerned the influence of non-aspirin NSAIDs on the risk of MI. In view of the argument that naproxen should be considered as more like aspirin than other NSAIDs [15–17, 29], a secondary analysis, which grouped naproxen with aspirin, was carried out.
Potential influences on the risk of MI were first analysed by univariate unconditional logistic regression analysis. Variables analysed in this way were current smoking, current use of antidiabetic medication (as a marker for diabetes), current use of antihypertensive medication (as a possible surrogate for hypertension), current and past use of non-aspirin NSAIDs, current and past use of aspirin and BMI category. Dose dependence was investigated using total current defined daily doses (DDDs) to categorize patients as taking lower or higher doses of NSAIDs (<1 DDD vs.≥ 1 DDD, respectively, and of aspirin (75 mg daily vs. higher). The influence of age, anti-anginal medication and composite cardiovascular risk profile was not investigated because these factors were constrained by the criteria for selection, the influence of the clinical event on parameters such as hypertension or the availability of data. Multivariate logistic regression analysis was used to calculate adjusted estimates of risk. All potential factors (whether significant or not on univariate analysis) were entered into the initial model and a backward elimination technique used to remove nonsignificant influences from the model in a step-wise way. The same factors remained significant in the multivariate analysis as had been identified in the univariate analysis. Because of studies suggesting an interaction between aspirin and NSAIDs, we re-ran the main analysis with a term for an interaction between aspirin and non-aspirin NSAIDs.
Based on equal numbers, 248 subjects would be required in each group to detect an increase in NSAID use from 15% to 25%. For staffing reasons, the study was terminated after enrolment of 205 cases but, because there were more community controls than cases, the power to detect an increase from 15% to 25% was 85%vs. all controls, 77%vs. community controls and 72%vs. hospital controls.
Results
Demographic data for cases and controls are shown in Table 1. Cases and controls were well matched for relevant demographic findings, although community controls were slightly older than the cases and the hospital controls. Only one case, one community control and two hospital controls used more than one non-aspirin NSAID. Concurrent aspirin use was reported by seven cases, three community controls and one hospital control. No case or control on naproxen used another non-aspirin NSAID. As shown in Table 2, our cases had typical symptoms and findings of MI. The anatomical distribution of the infarct reflected normal clinical patterns with anterior and inferior infarcts predominating. There were no significant differences in the site of infarction between those on and off NSAIDs (data not shown).The reasons for admission of hospital controls are shown in Table 3.
Table 1.
Demographic features of cases and controls
| Controls | ||||
|---|---|---|---|---|
| Cases | All | Community | Hospital | |
| n | 205 | 463 | 258 | 205 |
| Age, years, mean (SD) | 61.7 (11.0) | 63.7 (10.9) | 65.3 (10.4) | 61.7 (11.3) |
| Age range, years | 33–84 | 34–86 | 34–85 | 36–86 |
| Male, % | 74.3 | 70.4 | 68.2 | 73.2 |
| Current smoker, % | 41.7 | 20.1*** | 15.5*** | 26.0*** |
| Cigarettes/day | 19.3 (10.8) | 12.6 (16.9)** | 12.2 (8.9) | 16.5 (11.9) |
| Alcohol use, % | 64.1 | 71.9 | 79.8*** | 62.0 |
| Units/day in users | 13.8 (14.6) | 14.5 (14.5) | 11.5 (11.9) | 19.5 (16.9)** |
| Body mass index | 26.1 (4.2) | 25.5 (4.5) | 25.5 (3.5) | 25.4 (5.4) |
| Anti-diabetic Rx, %. | 5.8 | 3.0(*) | 1.6* | 4.9 |
| Anti-hypertensive Rx, % | 14.6 | 10.8 | 12.8 | 8.3* |
| Current aspirin, % | 13.3 | 14.6 | 17.2 | 11.4 |
| Mean DDDs (SD) | 0.24 (0.21) | 0.23 (0.20) | 0.24 (0.21) | 0.22 (0.18) |
| Range DDDs | 0.1–1.0 | 0.1–1.0 | 0.1–1.0 | 0.1–1.0 |
| Previous aspirin, past year, % | 8.8 | 10.6 | 12.0 | 8.8 |
| Current NSAIDs, % | 17.4 | 9.0** | 10.6* | 7.0*** |
| Current non-naproxen NSAIDs, % | 16.9 | 8.3*** | 9.4* | 7.0** |
| Ibuprofen, % | 10.2 | 4.5 | 3.4 | 5.4 |
| Diclofenac, % | 2.9 | 2.4 | 2.5 | 2.3 |
| Mean DDDs (SD) | 0.73 (0.78) | 0.60 (0.51) | 0.54 (0.42) | 0.69 (0.62) |
| DDDs range | 0.2–3.0 | 0.2–3.00 | 0.20–2.0 | 0.16–3.0 |
| Previous NSAIDs, past year, % | 14.4 | 12.3 | 14.1 | 10.0 |
| Previous non-naproxen NSAIDs, past year, % | 13.9 | 8.8 | 14.1 | 2.0*** |
| Previous aspirin or naproxen, past year, % | 6.8 | 6.9 | 9.7 | 3.4 |
| Haemoglobin, mean (SD) | 14.6 (1.7) | † | † | 14.0 (7.7) |
| Albumin, mean (SD) | 38.2 (4.1) | † | † | 33.9 (6.0) |
| Creatinine, mean (SD) | 93.9 (38.6) | † | † | 87.1 (34.7) |
P < 0.001;
P < 0.01;
P < 0.05;[*]P = 0.068.
Blood tests not taken from community controls. Based on χ2or Fisher’s exact test if < 5 values per cell. DDD, Defined daily dose; NSAID, nonsteroidal anti-inflammatory drug.
Table 2.
Characteristics of infarct in cases
| No of cases | % cases | ||
|---|---|---|---|
| Principal symptom | Chest pain | 183 | 88.8 |
| Collapse, shock syncope | 14 | 6.8 | |
| Abdominal pain | 7 | 3.4 | |
| Breathless | 2 | 1.0 | |
| Site of infarction | Anterior | 95 | 47.0 |
| Inferior | 95 | 47.0 | |
| Septal | 3 | 1.5 | |
| Posterior | 4 | 2.0 | |
| Non-Q-wave infarct | 2 | 1.0 | |
| Uncertain | 3 | 1.5 | |
| Evidence for infarction | ST elevation >1 mm | 138 | 72.3 |
| CK rise > 75% above normal | 198 | 96.6 |
Table 3.
Reasons for admission (hospital controls)
| ICD 10 chapter | Code range | Abbreviated descriptor | No of controls | Main*/common†diagnosis |
|---|---|---|---|---|
| I | A00–B99 | Infections and parasitic | 4 | – |
| II | C00–D48 | Neoplasms | 19 | Lung cancer 7 |
| III | D50–D89 | Blood and immune | 2 | – |
| IV | E00–E90 | Endocrine/nutrition/metabolic | 21 | Diabetes mellitus 15 |
| V | F00–F99 | Mental and behavioural | 1 | – |
| VI | G00–G99 | Nervous system | 2 | – |
| IX | I00–I99 | Circulatory | 18 | Pulmonary embolus 7 |
| X | J00–J99 | Respiratory | 57 | COPD 20 Bacterial pneumonia 13 Asthma 7 |
| X1 | K00–K63 | Gastrointestinal | 19 | Ulcerative colitis 7 |
| XI | K70–K87 | Hepatopancreaticobiliary | 23 | Alcoholic cirrhosis 4 |
| XII | L00–L99 | Skin and subcutaneous | 10 | Cellulitis 10 |
| XIII | M00–M99 | Musculoskeletal/connective tissue | 2 | – |
| XIV | N00–N99 | Genitourinary | 1 | – |
| XVIII | R00–R99 | Symptoms, signs, findings | 17 | Fever of unknown origin 3 |
| XIX | S00–T98 | Injury/poisoning | 6 | – |
| Total | 202 |
All main diagnoses within code range if≥2.
All diagnoses with≥5 instances.
ICD, International Classification of Diseases; COPD, chronic obstructive pulmonary disease.
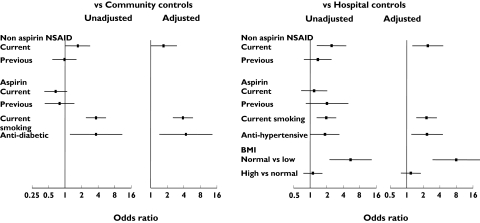
In univariate analyses, NSAID use was associated with an unadjusted odds ratio for MI of 1.77 (1.03, 3.03) vs. community controls and 2.61 (1.38, 4.95) vs. hospital controls (Table 4, Figure 1). Although cases took a higher average dose of their NSAID than controls, the gradient of risk was not significantly dose-related in either comparison. Odds ratios for smoking were 3.91 (2.52, 6.04) vs. community controls and 2.00 (1.32, 3.04) vs. hospital controls, whereas for use of antidiabetic medication the value was 3.92 (1.25, 12.33) vs. community controls (NS vs. hospital controls). Use of antidiabetic medication as surrogate for diabetes may exclude a number of patients with mild diabetes from consideration. Trends towards a lower risk with aspirin use (0.67, 0.41, 1.11, P = 0.122) for cases vs. community controls did not reach significance. The odds ratio for antihypertensive drug use was 1.89 (1.00, 3.34, P = 0.048) vs. hospital controls (NS vs. community controls). A higher BMI was associated with increased risk of MI (Table 4) in comparison with hospital controls, but this was due to these controls having a lower average BMI rather than cases having a higher BMI. Data for factors entered into the univariate analysis and those that remained significant in the multivariate analysis are shown in Table 4 and Figure 1.
Table 4.
Univariate estimates of risk
| Vs. community controls | Vs. hospital controls | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Current smoker | 3.91 | 2.52, 6.04 | <0.001 | 2.00 | 1.32, 3.04 | < 0.001 |
| Diabetic medication | 3.92 | 1.25, 12.33 | 0.02 | 1.21 | 0.51, 2.86 | 0.670 |
| Hypertensive medication | 1.16 | 0.68, 1.98 | 0.580 | 1.89 | 1.00, 3.34 | 0.048 |
| Body mass index | 0.057 | < 0.001 | ||||
| Body mass index 20–24.99 vs. < 20 | 1.17 | 0.39, 3.46 | 0.781 | 5.75 | 2.30, 14.41 | < 0.001 |
| Body mass index 20–24.99 vs. ≥ 25 | 1.23 | 0.84, 1.79 | 0.293 | 1.12 | 0.74, 1.69 | 0.603 |
| Current non-aspirin NSAID | 1.77 | 1.03, 3.03 | 0.039 | 2.61 | 1.38, 4.95 | 0.003 |
| Previous non-aspirin NSAID | 0.99 | 0.58, 1.67 | 0.96 | 1.00 | 0.49, 2.01 | 0.988 |
| Current aspirin | 0.67 | 0.41, 1.11 | 0.122 | 1.19 | 0.66, 2.13 | 0.566 |
| Previous aspirin | 0.80 | 0.42, 1.52 | 0.501 | 1.44 | 0.79, 2.63 | 0.229 |
| Current non-aspirin, non-naproxen NSAID | 1.95 | 1.11, 3.40 | 0.020 | 2.52 | 1.33, 4.79 | 0.005 |
| Previous non-aspirin non-naproxen NSAID | 0.95 | 0.56, 1.61 | 0.840 | 1.39 | 0.76, 2.53 | 0.288 |
NSAID, Nonsteroidal anti-inflammatory drug.
Figure 1.
Unadjusted and significant adjusted odds ratios
When aspirin and naproxen were grouped and distinguished from all other NSAIDs, similar patterns emerged, with an odds ratio for current non-aspirin, non-naproxen NSAIDs of 1.95 (1.11, 3.40, P = 0.020) vs. community controls and 2.52 (1.33, 4.79, P = 0.005) vs. hospital controls.
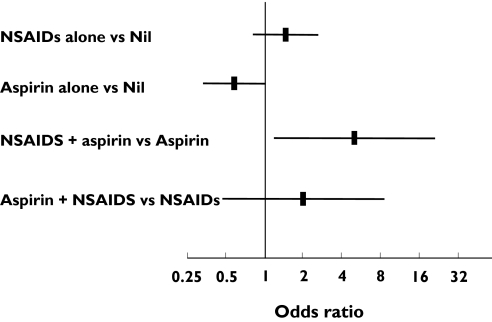
In an exploratory analysis of possible interactions between aspirin and NSAIDs, the overall effect of NSAIDs remained significant overall, for both community (P= 0.013) and hospital (P= 0.014) controls. In patients using aspirin, the odds ratios for MI with NSAID use were 5.00 (1.18, 21.28, P = 0.023, Figure 2) and 7.66 (0.97, 67.48, P = 0.067) for comparisons with community and hospital controls, respectively, suggesting a comparatively large effect in these patients. Comparable odds ratios in non-users of aspirin were 1.46 (0.81, 2.63, P = 0.20) and 2.25 (1.14, 4.43, P = 0.026), respectively.
Figure 2.
Exploratory analysis: nonsteroidal anti-inflammatory drug (NSAID) and aspirin interactions in comparisons vs. community controls
Discussion
Our data show that use of non-aspirin NSAIDs was associated with an increased risk of first MI when compared with either hospital or community controls. We included both community and hospital controls as results with each can be misleading alone. Because troponin measurements were not available, it is possible that our study included a higher proportion of ST-elevation MIs than would be the case now. The process of selection can result in community controls being healthier than average because the notes of patients who are unwell are more likely to be out of file. The reason for admission can be a bias with hospital controls if this is influenced by the factor under investigation. Consequently, we anticipated that hospital controls, even after exclusion of unsuitable patients, might give a more conservative estimate of risk than community controls, but this was not so.
The magnitude of the effect we have reported is somewhat higher than for other studies [12–21], although our confidence intervals overlap with them. Many previous studies have been conducted using electronic databases, which have many advantages, principally the ability to study large numbers of patients, but lack some of the diagnostic precision that characterizes conventional case–control methodology with direct patient interview [30, 31]. Bias in questioning intensity seems unlikely to account for our results, since the Research Assistants conducting the study were not aware of the precise hypothesis of the study. Finally, our decision to study first MI makes our study less prone to confounding than studies of all MI, although it is possible that our data are specific to this subgroup.
The increased risk with smoking and use of antidiabetic medication and the (statistically insignificant) trends to a reduction with aspirin are of a similar magnitude to recent estimates [32, 33], suggesting validity of the approach we have taken. An influence of antidiabetic medication or aspirin was of course not seen in the comparison with hospital controls because some were admitted because of complications of diabetes or conditions influenced by aspirin use. Our model included terms for BMI, but differences between cases and hospital controls principally arose not because of more obesity in the patients but because of more underweight individuals in the hospital controls. We used antihypertensive medication as a surrogate for blood pressure because reliable estimates of pre-event blood pressure cannot be obtained once a patient has had a MI. Our study may therefore miss some risks associated with untreated hypertension. We did not calculate infarct risk profiles or study age or antianginal medication because these factors formed part of our selection protocol or were influenced by it. Potential cases with cardiovascular indications for aspirin were not included from the study. Most aspirin use was for reasons such as pain relief.
An increased risk of MI with NSAIDs could arise through an immediate pharmacological effect such as inhibition of vascular prostacyclin or as a consequence of accelerated vascular disease secondary to hypertension. In addition, ibuprofen has been suggested to interfere with the antiplatelet effect of aspirin [25] and possibly to increase the risk of MI in aspirin users [22–24]. Because of this, we conducted an exploratory analysis to investigate whether there was an interaction between aspirin and NSAID use. Our data show a greater effect of NSAIDs in aspirin users, compatible with pharmacodynamic interference with aspirin as a partial contributor to our results. However, an effect in non-users is not excluded. Our data should be regarded as tentative since this was not a prespecified analysis and the study was not powered or designed to investigate such interactions.
If inhibition of vascular prostacyclin is the mechanism by which COX-2 inhibition enhances risk of MI, one would predict that ‘nonselective’ NSAIDs would be associated with a generally lower risk than selective COX-2 inhibitors and differ amongst themselves. In the interaction between platelets and endothelium, prostacyclin limits the response to thromboxane A2[34]. Theoretically, nonselective NSAIDs that have a substantial effect on thromboxane should limit the thrombotic tendency compared with selective COX-2 inhibitors and there is evidence favouring this for naproxen [15–17, 29]. Results and claims about other recent studies [10, 19, 20, 35] have provided contrary data. We anticipated this uncertainty in our analyses but our study had too few patients taking naproxen for detailed analysis. Most patients were taking ibuprofen or diclofenac, which have limited effects on platelet thromboxane synthesis.
Our data did not show dose dependence, in contrast to studies of selective COX-2 inhibitors [1, 2, 4, 10, 18–20]. This may represent a type 2 error but could be real if nonselective NSAIDs affect platelet COX-1 and endothelial COX-2 differently. Recently, an association between discontinuation of NSAIDs and MI [36] has been reported. Our study did not show this effect, even though NSAID use that finished ≥ 7 days prior to admission was categorized as previous use.
Our results add to growing evidence of possible hazard from nonselective NSAIDs. Use of NSAIDs is common and a twofold increase in risk of MI, if confirmed, would have substantial public health implications. In Oxfordshire, the incidence of MI for 50–79-year-olds has been estimated at 0.86% for men and 0.40% for women [37]. Assuming two-thirds of NSAID usage is in women, a doubling of risk with NSAID use would increase risk from 0.55% to 1.10% per annum. Assuming a 15% prevalence of NSAID use in this, our data would imply that this would be reduced by 13% to 0.48% if NSAIDs were not used and assuming the effect we have demonstrated persisted for second and subsequent infarcts. The number needed to treat to result in one MI per annum would be 116 for men and 250 for women.
Most NSAID use by our patients concerned ibuprofen and diclofenac. Our study was not powerful enough to establish whether there were differences between them. The overall safety of selective and nonselective cyclooxygenase inhibitors can be established only on a drug by drug basis. Funding of such research on nonselective NSAIDs will not be forthcoming from industry since most NSAIDs are out of patent and should be a priority for Health Services and Medical Research Councils.
Acknowledgments
The study was supported by an unrestricted grant from Boehringer Ingleheim Limited. The authors thank Mrs Yola Booth for preparing this manuscript.
References
- 1.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 2.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 3.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 4.Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, Gertz BJ. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation. 2001;104:2280–8. doi: 10.1161/hc4401.100078. [DOI] [PubMed] [Google Scholar]
- 5.Hawkey CJ, Langman MJ. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut. 2003;52:600–8. doi: 10.1136/gut.52.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–11. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 7.Topol EJ. Failing the public health—rofecoxib, Merck, and the FDA. N Engl J Med. 2004;351:1707–9. doi: 10.1056/NEJMp048286. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Vioxx: an unequal partnership between safety and efficacy. Lancet. 2004;364(9442):1287–8. doi: 10.1016/S0140-6736(04)17198-5. [DOI] [PubMed] [Google Scholar]
- 9.Dieppe PA, Ebrahim S, Martin RM, Juni P. Lessons from the withdrawal of rofecoxib. BMJ. 2004;329:867–8. doi: 10.1136/bmj.329.7471.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray W. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case–control study. Lancet. 2005;365:475–81. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 11.Daniels B, Seidenberg B. Cardiovascular safety profile of rofecoxib in controlled clinical trials. Arthritis Rheum. 1999;42(Suppl):S143. [Google Scholar]
- 12.Weir MR, Sperling RS, Reicin A, Gertz BJ. Selective COX-2 inhibition and cardiovascular effects: a review of the rofecoxib development program. Am Heart J. 2003;146:591–604. doi: 10.1016/S0002-8703(03)00398-3. [DOI] [PubMed] [Google Scholar]
- 13.Reicin A, Shapiro D, Sperling RS, Barr E, Yu Q. Comparison of cardiovascular thrombotic events in patients with osteoarthritis treated with rofecoxib versus nonselective nonsteroidal anti-inflammatory drugs (ibuprofen, diclofenac, and nabumetone) Am J Cardiol. 2002;89:204–9. doi: 10.1016/s0002-9149(01)02201-9. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Rodriguez LA, Varas C, Patrono C. Differential effects of aspirin and non-aspirin nonsteroidal antiinflammatory drugs in the primary prevention of myocardial infarction in postmenopausal women. Epidemiology. 2000;11:382–7. doi: 10.1097/00001648-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. 2002;162:1099–104. doi: 10.1001/archinte.162.10.1099. [DOI] [PubMed] [Google Scholar]
- 16.Rahme E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med. 2002;162:1111–5. doi: 10.1001/archinte.162.10.1111. [DOI] [PubMed] [Google Scholar]
- 17.Watson DJ, Rhodes T, Cai B, Guess HA. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med. 2002;162:1105–10. doi: 10.1001/archinte.162.10.1105. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet. 2002;359(9301):118–23. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- 19.Mamdani M, Rochon P, Juurlink DN, Anderson GM, Kopp A, Naglie G, Austin PC, Laupacis A. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med. 2003;163:481–6. doi: 10.1001/archinte.163.4.481. [DOI] [PubMed] [Google Scholar]
- 20.Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Avorn J. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–73. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, Gonzalez-Perez A. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109:3000–6. doi: 10.1161/01.CIR.0000132491.96623.04. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361(9357):573–4. doi: 10.1016/s0140-6736(03)12509-3. [DOI] [PubMed] [Google Scholar]
- 23.Kurth T, Glynn RJ, Walker AM, Chan KA, Buring JE, Hennekens CH, Gaziano JM. Inhibition of clinical benefits of aspirin on first myocardial infarction by non steroidal anti inflammatory drugs. Circulation. 2003;108:1191–5. doi: 10.1161/01.CIR.0000087593.07533.9B. [DOI] [PubMed] [Google Scholar]
- 24.Patel TN, Goldberg KC. Use of aspirin and ibuprofen compared with aspirin alone and the risk of myocardial infarction. Arch Intern Med. 2004;164:852–6. doi: 10.1001/archinte.164.8.852. [DOI] [PubMed] [Google Scholar]
- 25.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 26.Stack WA, Atherton JC, Hawkey GM, Logan RF, Hawkey CJ. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther. 2002;16:497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 27.ATC Index With DDDs. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2002. [Google Scholar]
- 28.17. Wiley, USA: 1992. The Merck Manual of Diagnosis and Therapy. [Google Scholar]
- 29.Capone ML, Tacconelli S, Sciulli MG, Grana M, Ricciotti E, Minuz P, Di Gregorio P, Merciaro G, Patrono C, Patrignani P. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468–71. doi: 10.1161/01.CIR.0000124715.27937.78. [DOI] [PubMed] [Google Scholar]
- 30.Walley T, Mantgani A. The UK general practice research database. Lancet. 1997;350(9084):1097–9. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 31.Peabody JW, Luck J, Jain S, Bertenthal D, Glassman P. Assessing the accuracy of administrative data in health information systems. Med Care. 2004;42:1066–72. doi: 10.1097/00005650-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;136:161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 33.Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovascular Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 34.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296(5567):539–41. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 35.FDA Statement on Naproxen. [29 November 2005]. http://www.fda.gov/bbs/topics/news/2004/NEW01148.htmlLast.
- 36.Fischer LM, Schlienger RG, Matter CM, Jick H, Meier CR. Discontinuation of non-steroidal anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med. 2004;164:2472–6. doi: 10.1001/archinte.164.22.2472. [DOI] [PubMed] [Google Scholar]
- 37.Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HAW on behalf of the Oxford Myocardial Infarction Incidence Study Group. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. Heart. 1998;80:40–4. doi: 10.1136/hrt.80.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]