Abstract
Aims
Case reports suggest an interaction between rofecoxib and the CYP1A2 substrate tizanidine. Our objectives were to explore the extent and mechanism of this possible interaction and to determine the CYP1A2 inhibitory potency of rofecoxib.
Methods
In a randomized, double-blind, two-phase cross-over study, nine healthy subjects took 25 mg rofecoxib or placebo daily for 4 days and, on day 4, each ingested 4 mg tizanidine. Plasma concentrations and the urinary excretion of tizanidine, its metabolites (M) and rofecoxib, and pharmacodynamic variables were measured up to 24 h. On day 3, a caffeine test was performed to estimate CYP1A2 activity.
Results
Rofecoxib increased the area under the plasma concentration–time curve (AUC0–∞) of tizanidine by 13.6-fold [95% confidence interval (CI) 8.0, 15.6; P < 0.001), peak plasma concentration (Cmax) by 6.1-fold (4.8, 7.3; P < 0.001) and elimination half-life (t1/2) from 1.6 to 3.0 h (P< 0.001). Consequently, rofecoxib markedly increased the blood pressure-lowering and sedative effects of tizanidine (P < 0.05). Rofecoxib increased several fold the tizanidine/M-3 and tizanidine/M-4 ratios in plasma and urine and the tizanidine/M-5, tizanidine/M-9 and tizanidine/M-10 ratios in urine (P < 0.05). In addition, it increased the plasma caffeine/paraxanthine ratio by 2.4-fold (95% CI 1.4, 3.4; P = 0.008) and this ratio correlated with the tizanidine/metabolite ratios. Finally, the AUC0–25 of rofecoxib correlated with the placebo phase caffeine/paraxanthine ratio (r = 0.80, P = 0.01).
Conclusions
Rofecoxib is a potent inhibitor of CYP1A2 and it greatly increases the plasma concentrations and adverse effects of tizanidine. The findings suggest that rofecoxib itself is also metabolized by CYP1A2, raising concerns about interactions between rofecoxib and other CYP1A2 substrate and inhibitor drugs.
Keywords: caffeine, CYP1A2, interaction, rofecoxib, tizanidine
Introduction
Recent case reports have suggested an interaction between rofecoxib and tizanidine [1, 2]. The adverse reactions observed involved both the central nervous and cardiovascular systems. However, the likelihood and mechanism of this possible interaction have not been documented in controlled studies.
Rofecoxib is a COX-2 selective nonsteroidal anti-inflammatory drug (NSAID). Prior to its possibly temporary withdrawal, due to an increased risk of myocardial infarction and stroke [3], rofecoxib was one of the most widely used coxibs. Its daily dose ranged from 12.5 to 50 mg and the drug could be taken once daily due to its long elimination half-life of about 20 h. Rofecoxib is extensively metabolized in the liver, probably by enzymes other than cytochromes P450 [4, 5]. According to a recent study, rofecoxib may be a modest inhibitor of CYP1A2 in humans [6].
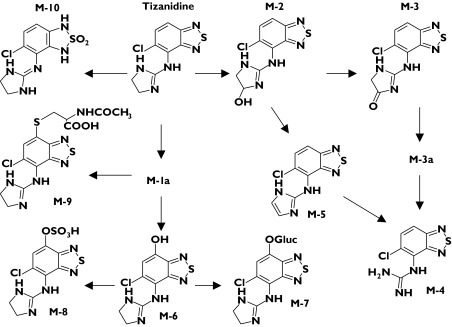
Tizanidine is a centrally acting muscle relaxant, which has sedative and blood pressure-lowering properties. It undergoes extensive first-pass metabolism, its oral bio-availability being about 10–30% [7, 8], and it is metabolized down several pathways (Figure 1) [9]. CYP1A2 catalyses the elimination of tizanidine both in vitro and in vivo [10, 11]. Tizanidine can be more sensitive than theophylline and caffeine as a probe substrate for CYP1A2 in detecting small or transient effects on its activity, particularly during first-pass metabolism [8, 11].
Figure 1.
Pathways of tizanidine metabolism [9]
Our aims were to study the effect of rofecoxib on CYP1A2 activity in humans, using tizanidine and caffeine as probe substrates, and to investigate the mechanism and consequences of a possible rofecoxib–tizanidine interaction.
Methods
Subjects
Nine healthy male nonsmokers (age range 20–25 years; weight range 64–87 kg) completed the study (Table 1). One subject withdrew from the study for personal reasons before the first tizanidine dose. Male subjects were chosen to avoid possible effects of the menstrual cycle on tizanidine pharmacokinetics. Prior to enrolment, subjects were ascertained to be healthy by a physical examination, a 12-lead electrocardiogram and routine laboratory tests. Subjects with a systolic blood pressure < 110 mmHg were excluded for safety reasons. All subjects provided written informed consent before the physical examination.
Table 1.
Characteristics of the subjects, the pharmacokinetic variables for rofecoxib, and the results of the caffeine test
| Rofecoxib | Caffeine/paraxanthine ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| Subject number | Age, years | Weight, kg | Cmax, ng ml−1 | AUC0–25, ng h ml−1 | AUC0–∞, ng h ml−1 | t1/2, h | During placebo (control) | During rofecoxib |
| 2 | 21 | 84 | 367 | 5453 | 8846 | 9.1 | 1.66 | 3.74 |
| 3 | 22 | 80 | 243 | 3271 | 4332 | 10.9 | 0.89 | 1.58 |
| 4 | 21 | 83 | 196 | 2905 | 4347 | 14.4 | 0.91 | 2.37 |
| 5 | 22 | 80 | 320 | 4043 | 6700 | 18.2 | 1.26 | 3.58 |
| 6 | 21 | 73 | 302 | 3906 | 5374 | 12.3 | 1.45 | 2.78 |
| 7 | 23 | 71 | 324 | 4396 | 7929 | 20.9 | 1.75 | 7.66 |
| 8 | 25 | 87 | 331 | 5885 | 18818 | 40.0 | 3.26 | 7.79 |
| 9 | 20 | 64 | 407 | 4891 | 9648 | 24.6 | 1.32 | 1.81 |
| 10 | 22 | 86 | 241 | 3949 | 12490 | 44.2 | 1.66 | 2.38 |
| Mean | 22 | 79 | 303 | 4300 | 8721 | 21.6 | 1.57 | 3.74* |
| SD | 1 | 6 | 66 | 971 | 4628 | 12.7 | 0.70 | 2.37 |
AUC0–25, Area under plasma concentration–time curve from time 0–25 h; AUC0–∞, area under plasma concentration–time curve from time 0 to infinity; Cmax, maximum plasma concentration; t1/2, half-life.
P = 0.008, vs. control.
Study design
The study protocol was approved by the Ethics Committee for Studies in Healthy Subjects of the Hospital District of Helsinki and Uusimaa, and the Finnish National Agency for Medicines, Helsinki, Finland. It was carried out according to a randomized, double-blind, two-phase cross-over design with a 2-week wash-out period. The subjects took 25 mg rofecoxib (one 25-mg Vioxx tablet; Merck & Co, Whitehouse Station, NJ, USA) or placebo once daily at 08.00 h for 4 days. On day 4, they ingested a single oral dose of 4 mg tizanidine (one 4-mg Sirdalud tablet; Novartis Pharma, Wehr, Germany) with 150 ml water at 09.00 h. A standard meal was served 3 and 7 h after tizanidine ingestion. Subjects were not allowed to drink grapefruit juice for 1 week before each study day. Alcohol and drinks containing caffeine were not permitted on the study days.
The subjects were under close medical supervision during the days of tizanidine administration. Fluids for intravenous infusion were available for immediate use, but were not needed.
Sampling
On the days of administration of tizanidine, a forearm vein of each subject was cannulated with a plastic cannula and kept patent with an obturator. Timed blood samples were drawn before the administration of tizanidine and at 20, 40, 60 and 90 min and 2, 3, 4, 5, 7, 9, 12 and 24 h later. Blood samples (10 ml each) were drawn to ethylenediaminetetraacetic acid-containing tubes. Plasma was separated within 30 min and stored at −40 °C until analysis. Urine was collected cumulatively in two fractions, 0–12 h and 12–24 h. The samples were stored at −40 °C for later analysis.
Assessment of CYP1A2 activity
A caffeine test was performed on the third day of pretreatment during both phases [12–14]. The subjects ingested 100 mg caffeine (one 100-mg Cofi-Tabs tablet; Vitabalans, Hämeenlinna, Finland) at 09.00 h, after having abstained from caffeine intake for at least 12 h. A blood sample for the analysis of plasma caffeine and paraxanthine (1,7-dimethylxanthine) concentrations was taken from each subject 6 h after caffeine intake.
Determination of drug concentrations in plasma and urine
Plasma and urine concentrations of unchanged tizanidine and its metabolites (M) were quantified using an API 2000 liquid chromatography-tandem mass spectrometry system (MDS Sciex, Toronto, Ontario, Canada). Chromatography was performed on an XTerra RP C18 column (3.9 × 100 mm; Waters Corp.; Milford, MA, USA) using gradient elution. The mobile phase consisted of 10 mmol l−1 ammonium acetate (pH 9.5, adjusted with 25% ammonia solution) and acetonitrile. The mass spectrometer was operated in the atmospheric pressure ionization mode with positive ion detection. The ion transitions monitored were mass-to-charge ratio (m/z) 254 to m/z 44 for tizanidine, m/z 268 to m/z 211 for M-3, m/z 228 to m/z 211 for M-4, m/z 252 to m/z 216 for M-5, m/z 415 to m/z 286 for M-9, m/z 288 to m/z 188 for M-10 and m/z 230 to m/z 44 for the internal standard, clonidine. These transitions represent the product ion of the [M + H]+ ion. The limit of quantification for tizanidine was 0.05 ng ml−1 and the day-to-day coefficient of variation (CV) was 5.6% at 0.096 ng ml−1, 3.1% at 0.96 ng ml−1 and 4.6% at 9.6 ng ml−1 (n = 7). A signal-to-noise ratio of 10 : 1 was used as the limit of detection for tizanidine metabolites and their concentrations are given in arbitrary units relative to the ratio of the peak height of the metabolite to the peak height of the internal standard. The detector response for each metabolite was confirmed to be linear over the relevant concentration range by means of sample dilution. The CVs were between 3 and 11% for the different tizanidine metabolites at relevant concentrations (n = 8). Rofecoxib did not interfere with the analysis of tizanidine and its metabolites.
The plasma concentrations of rofecoxib were determined by high-performance liquid chromatography (HPLC) with etoricoxib as the internal standard [15]. The limit of quantification was 5 ng ml−1 and the day-to-day CVs were 0.9% at 20 ng ml−1, 4.1% at 100 ng ml−1 and 0.6% at 400 ng ml−1 (n = 6). Tizanidine did not interfere with the determination of rofecoxib in plasma.
Plasma caffeine and paraxanthine concentrations were determined by HPLC with ultraviolet detection, with [beta]-hydroxy-ethyltheophylline as the internal standard [16, 17]. The limit of quantification was 50 ng ml−1 for both compounds. The within-day CV for caffeine and paraxanthine was <6% at relevant concentrations (n = 7).
The sources of the reference compounds were: tizanidine hydrochloride (Farmak, Olomouc, Czech Republic), rofecoxib (Merck & Co., and Sequoia Research Products Ltd, Pangbourne, UK), clonidine hydrochloride (Boehringer Ingelheim GmbH, Ingelheim, Germany), caffeine (Sigma-Aldrich Corp., St Louis, MO, USA), paraxanthine (Sigma-Aldrich Corp.) and etoricoxib (Merck & Co.).
Pharmacokinetics
The pharmacokinetics of tizanidine and its metabolites were evaluated by the peak concentration in plasma (Cmax), time to Cmax (tmax), area under the plasma concentration–time curve (AUC) from 0 to 24 h (AUC0–24) and to infinity (AUC0–∞), and elimination half-life (t1/2). The terminal log-linear part of the concentration–time curve was identified visually for each subject. The elimination rate constant (ke) was determined with the use of linear regression analysis of the log-linear part of the plasma concentration–time curve. The t1/2 was calculated by the following equation: t1/2 = ln2/ke. The AUC values were calculated by use of the linear trapezoidal rule for the rising phase of the plasma drug concentration–time curve and the log-linear trapezoidal rule for the descending phase, with extrapolation to infinity, when appropriate, by division of the last measured concentration by ke. The amount of tizanidine and its metabolites excreted into urine (Ae) was determined. The pharmacokinetics of rofecoxib were characterized by Cmax, tmax, t1/2, the AUC from 0 to 25 h after the last dose of rofecoxib (AUC0–25) and the AUC from 0 to infinity (AUC0–∞). Of note, the extrapolated fraction of the AUC0–∞ of the tizanidine metabolite M-3 during the rofecoxib phase and that for rofecoxib exceeded 20%. The pharmacokinetic calculations were performed using the program MK-model, version 5.0 (Biosoft, Cambridge, UK).
Pharmacodynamics
The pharmacodynamic variables were assessed before administration of tizanidine and immediately after each blood sample was taken, up to 24 h after drug intake. The systolic and diastolic blood pressure and heart rate were measured twice from the forearm with the subject in a sitting position and the mean value was used in the calculations. The blood pressures and heart rates were measured with an automatic oscillometric blood pressure monitor (HEM-711; Omron Healthcare GmbH, Hamburg, Germany). Before the beginning of the study, the subjects were trained to perform three psychomotor tests. In the Digit Symbol Substitution Test (DSST), the number of digits correctly substituted in 2 min was recorded [18]. Subjective drowsiness and drug effect were measured using a 100 mm long horizontal visual analogue scale (VAS) [19]. For each pharmacodynamic variable, the maximum response was recorded and the area under the effect vs. time curve from 0 to 12 h (AUC0–12) was calculated using the trapezoidal rule. A 12-lead electrocardiogram (ECG) was recorded prior to and at 2 h after tizanidine intake.
Statistical analysis
All results are given as mean ± SD. The pharmacokinetic and pharmacodynamic variables after the two pretreatments were compared by repeated-measures anova with treatment sequence as a factor or, in the case of tmax, using the Wilcoxon signed-rank test. For all variables except tmax, 95% confidence intervals were calculated on the mean differences between the placebo and rofecoxib phases. The Pearson correlation coefficient and linear regression analysis were used to investigate possible relationships between the pharmacokinetics of tizanidine and rofecoxib and the caffeine/paraxanthine ratio. All data were analysed using the statistical program Systat for Windows, version 6.0.1 (SPSS Inc., Chicago, IL, USA). The differences were considered statistically significant at P < 0.05.
Results
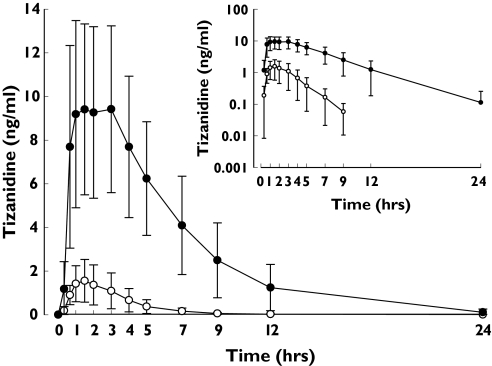
Rofecoxib raised the Cmax of tizanidine 6.1-fold (range 4.3–14.7-fold; P < 0.001) and its AUC0–∞ 13.6-fold (range 7.4–22.5-fold; P < 0.001) compared with the placebo phase (Figure 2, Table 2). The t1/2 of tizanidine was increased from 1.6 ± 0.2 h to 3.0 ± 0.6 h (P < 0.001) by rofecoxib.
Figure 2.
Plasma concentrations (mean ± SD) of tizanidine after a single oral dose of 4 mg tizanidine during the placebo and rofecoxib (25 mg day−1) phases in nine healthy subjects. ○, placebo phase; •, rofecoxib phase. Inset depicts the same data on a semilogarithmic scale
Table 2.
Pharmacokinetic variables for tizanidine (4 mg) during the placebo and rofecoxib (25 mg day−1) phases in nine healthy subjects
| Variable | Placebo phase (control) | Rofecoxib phase | 95% CI | P-value |
|---|---|---|---|---|
| Cmax (ng ml−1) | 1.7 ± 0.9 | 10.5 ± 4.0 | <0.001 | |
| Relative to control (range) | 6.1 (4.3–14.7) | 4.9, 7.4 | ||
| tmax (min) | 60 (40, 120) | 90 (40, 180) | 0.74 | |
| t1/2 (h) | 1.6 ± 0.2 | 3.0 ± 0.6 | <0.001 | |
| Relative to control (range) | 1.90 (1.36–2.70) | 1.56, 2.13 | ||
| AUC0–∞ (ng h ml−1) | 5.7 ± 3.6 | 66.7 ± 33.7 | <0.001 | |
| Relative to control (range) | 13.6 (7.4–22.5) | 7.9, 15.5 |
Data are mean ± SD or mean with 95% CI; tmax data are given as median with range. CI, Confidence interval; Cmax, maximum plasma concentration; tmax, time to reach Cmax; t1/2, half-life; AUC0–∞, area under plasma concentration–time curve from time 0 to infinity.
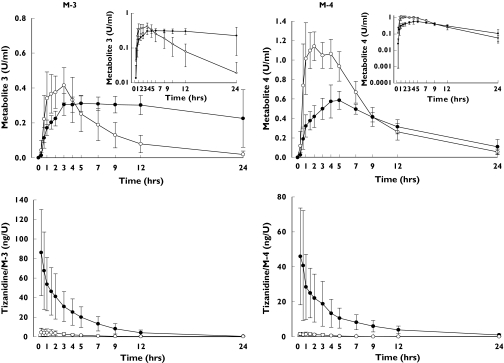
Rofecoxib caused considerable prolongation of the tmax of the tizanidine metabolite M-3 (from 90 min to 300 min; P = 0.003) and of its further metabolite M-4 (from 60 min to 300 min; P = 0.003) (Figure 3, Table 3). In addition, the t1/2 values of M-3 and M-4 were increased by 4.4-fold and 1.6-fold, respectively. Rofecoxib increased the AUC0–∞ of M-3 (P = 0.01) and decreased the Cmax of M-4 (P < 0.001). The tizanidine/M-3 and tizanidine/M-4 ratios in plasma were increased over 10-fold by rofecoxib (P < 0.001; Figure 3).
Figure 3.
Plasma concentrations (mean ± SD) of tizanidine metabolites M-3 and M-4 (top) and the plasma tizanidine to metabolite concentration ratios (bottom) after a single dose of 4 mg tizanidine during the placebo (○) and rofecoxib (25 mg day−1) (•) phases in nine healthy subjects. The insets depict the same data on a semilogarithmic scale
Table 3.
Pharmacokinetic variables for tizanidine metabolites 3, 4 and 5 during the placebo and rofecoxib (25 mg day−1) phases in nine healthy subjects
| Variable | Placebo phase (control) | Rofecoxib phase | 95% CI | P-value |
|---|---|---|---|---|
| Metabolite 3 | ||||
| Cmax (U ml−1) | 0.45 ± 0.11 | 0.38 ± 0.81 | 0.14 | |
| Relative to control (range) | 0.84 (0.50–1.50) | 0.53, 1.11 | ||
| tmax (min) | 90 (60, 180) | 300 (180, 1400) | 0.003 | |
| t1/2 (h)* | 4.3 ± 1.5 | 17.5 ± 8.1 | 0.002 | |
| Relative to control (range) | 4.40 (2.90–6.80) | 2.56, 5.65 | ||
| AUC0–24 (U h ml−1) | 3.1 ± 1.1 | 6.4 ± 1.4 | <0.001 | |
| Relative to control (range) | 2.06 (1.40–3.90) | 1.77, 2.32 | ||
| AUC0–∞ (U h ml−1)* | 3.0 ± 1.3 | 12.3 ± 9.1 | 0.011 | |
| Relative to control (range) | 4.10 (2.70–8.60) | 1.73, 6.47 | ||
| Metabolite 4 | ||||
| Cmax (U ml−1) | 1.3 ± 0.1 | 0.64 ± 0.13 | <0.001 | |
| Relative to control (range) | 0.51 (0.38–0.70) | 0.42, 0.64 | ||
| tmax (min) | 60 (40, 240) | 300 (240, 420) | 0.003 | |
| t1/2 (h) | 4.7 ± 0.7 | 8.0 ± 4.0 | 0.016 | |
| Relative to control (range) | 1.60 (1.20–2.80) | 1.15, 2.23 | ||
| AUC0–24 (U h ml−1) | 10.0 ± 1.3 | 7.4 ± 1.1 | <0.001 | |
| Relative to control (range) | 0.75 (0.59–0.86) | 0.65, 0.85 | ||
| AUC0–∞ (U h ml−1) | 10.4 ± 1.6 | 9.1 ± 3.2 | 0.16 | |
| Relative to control (range) | 0.87 (0.69–1.40) | 0.71, 1.05 | ||
| Metabolite 5 | ||||
| Cmax (U ml−1) | 0.10 ± 0 06 | 0.17 ± 0.07 | 0.09 | |
| Relative to control (range) | 1.70 (0.22–5.60) | 0.9, 2.5 | ||
| tmax (min) | 60 (40, 240) | 180 (60, 420) | 0.046 | |
Data are mean ± SD or mean with 95% CI; tmax data are given as median with range. CI, Confidence interval; Cmax, maximum plasma concentration; tmax, time to reach Cmax; t1/2, half-life; AUC0−24, area under plasma concentration–time curve from time 0–24 h; AUC0–∞, area under plasma concentration–time curve from time 0 to infinity.
Data from eight subjects only are shown, because the t1/2 and AUC0–∞ could not be determined for subject 8 (the individual with the lowest CYP1A2 activity), because M-3 concentration was rising up to the last time point (24 h).
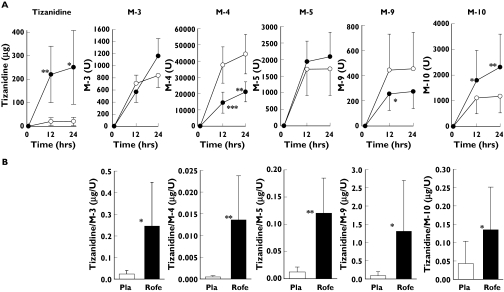
Rofecoxib increased the urinary excretion of tizanidine by 12-fold (P = 0.04) and that of M-10 by twofold (P < 0.001). However, the excretion of M-4 was decreased by 48% (P = 0.002; Figure 4A). Rofecoxib increased by several fold the tizanidine to metabolite (M-3, M-4, M-5, M-8, M-10) ratios in urine (Figure 4B). The renal clearance of tizanidine was not significantly altered by rofecoxib (data not shown).
Figure 4.
Cumulative excretion of tizanidine and its metabolites (mean ± SD) into urine (A) and the tizanidine to metabolite 24 h excretion ratios in urine (B) after a single dose of 4 mg tizanidine during the placebo (○ and white bars) and rofecoxib 25 mg day−1 (• and black bars) phases. *P < 0.05; **P < 0.01; ***P < 0.001 vs. placebo
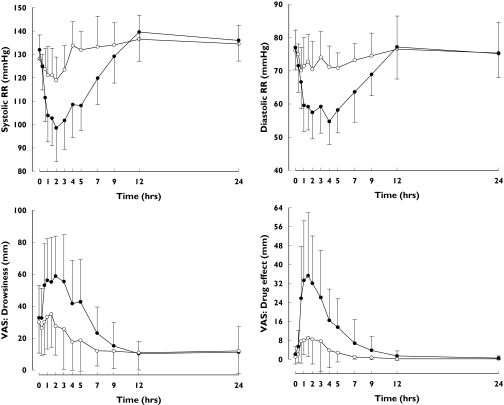
Tizanidine had a much greater pharmacodynamic effect during the rofecoxib phase than during the placebo phase (Table 4, Figure 5). During the rofecoxib phase, the mean minimum systolic blood pressure was 97 ± 12 mmHg, which is 20 mmHg lower than during the placebo phase (P = 0.003). Similarly, diastolic blood pressure was decreased by tizanidine more during the rofecoxib phase than during the placebo phase (P = 0.002). In addition, the effects of tizanidine on subjective drowsiness and overall drug effect were enhanced by rofecoxib (P < 0.05, Table 4).
Table 4.
Pharmacodynamic effects of tizanidine (4 mg) during the placebo and rofecoxib (25 mg day−1) phases in nine healthy subjects
| Variable | Placebo phase (control) | Rofecoxib phase | Difference between phases, 95% CI | P-value |
|---|---|---|---|---|
| Systolic blood pressure | ||||
| Baseline (mmHg) | 128 ± 10 | 132 ± 4 | 3.9 (−2.6, 10.3) | 0.21 |
| Minimum (mmHg) | 117 ± 10 | 97 ± 12 | −20 (−32, −9) | 0.004 |
| AUC0–12 (mmHg h) | 1570 ± 100 | 1410 ± 120 | −153 (−211, −94) | <0.001 |
| Diastolic blood pressure | ||||
| Baseline (mmHg) | 76 ± 6 | 77 ± 7 | 0.9 (−3.9, 5.8) | 0.78 |
| Minimum (mmHg) | 65 ± 8 | 53 ± 7 | −12 (−18, −5) | 0.003 |
| AUC0–12 (mmHg h) | 880 ± 72 | 770 ± 77 | −107 (−142, −72) | <0.001 |
| Heart rate | ||||
| Baseline (beats min−1) | 59 ± 7 | 56 ± 7 | −3 (−7, 1) | 0.08 |
| Minimum (beats min−1) | 50 ± 5 | 49 ± 8 | −1 (−5, 3) | 0.50 |
| AUC0–12 (beats min−1 h) | 696 ± 86 | 682 ± 89 | −14 (−50, 22) | 0.312 |
| VAS: drowsiness | ||||
| Baseline (mm) | 30 ± 19 | 33 ± 20 | 3 (−10, 16) | 0.95 |
| Maximum (mm) | 41 ± 19 | 71 ± 22 | 30 (13, 47) | 0.007 |
| AUC0–12 (mm h) | 215 ± 138 | 385 ± 185 | 169 (82, 255) | 0.005 |
| VAS: drug effect | ||||
| Baseline (mm) | 1 ± 3 | 2 ± 3 | 1 (−2, 4) | 0.47 |
| Maximum (mm) | 14 ± 13 | 41 ± 22 | 27 (12, 43) | 0.006 |
| AUC0–12 (mm h) | 36 ± 39 | 152 ± 120 | 116 (35196) | 0.016 |
| DSST | ||||
| Baseline (symbols per 2 min) | 104 ± 10 | 105 ± 13 | 0.2 (−5.9, 6.3) | 0.60 |
| Minimum (symbols per 2 min) | 96 ± 11 | 86 ± 18 | −11 (−22, 1) | 0.07 |
| AUC(0,12) (symbols per 2 min h) | 1255 ± 156 | 1199 ± 174 | −56 (−107, 4) | 0.06 |
The maximum effects (maximum or minimum value after tizanidine) and AUC0–12 are presented for systolic and diastolic blood pressure, heart rate and for the psychomotor tests. Data are mean ± SD or mean with 95% CI. AUC0–12, area under effect–time curve from time 0–12 h; VAS, visual analogue scale; DSST, digit symbol substitution test.
Figure 5.
Mean ± SD systolic and diastolic blood pressures (RR) and recordings of subjective drowsiness and drug effect (VAS, visual analogue scale) after a single dose of 4 mg tizanidine during the placebo (○) and rofecoxib 25 mg day−1 (•) phases in nine healthy subjects
There was no significant difference in the QT-interval or heart rate, recorded using a 12-lead electrocardiogram in the supine position, between the rofecoxib and placebo phases at baseline (data not shown). Two hours after tizanidine ingestion, heart rate was lower during the rofecoxib phase (42 ± 6 beats min−1) than during the placebo phase (47 ± 5 beats min−1) (P = 0.02). There was no significant difference between the phases in the heart rate corrected QT-interval (QTc) (P = 0.15).
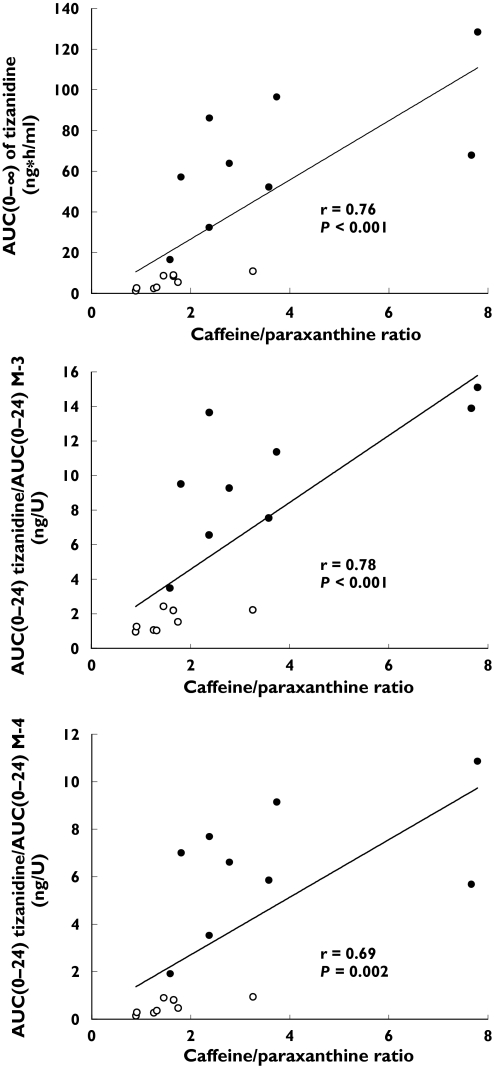
Rofecoxib increased the caffeine/paraxanthine ratio by 2.4-fold (from 1.6 ± 0.7 to 3.7 ± 2.4; P = 0.008) (Table 1). The AUC of tizanidine, the tizanidine/M-3 AUC ratio and the tizanidine/M-4 AUC ratio correlated well with the caffeine/paraxanthine ratio (r = 0.78, P = 0.014; r = 0.78, P < 0.001; and r = 0.69, P = 0.002, respectively) (Figure 6). During the placebo phase, the tizanidine/metabolite (M-4, M-5 and M-10) Ae ratios correlated significantly with the caffeine/paraxanthine ratio (Table 5). Moreover, the changes in tizanidine/metabolite (M-3, M-4, M-5 and M-9) Ae ratios caused by rofecoxib correlated with the changes in the caffeine/paraxanthine ratio caused by this drug (Table 5).
Figure 6.
Relationships between the AUC0–∞ tizanidine, and the tizanidine/metabolite (M-3 or M-4) AUC0–24 ratios and the caffeine/paraxanthine ratio. ○, Placebo phase; •, rofecoxib phase. Correlations were tested using linear regression analysis
Table 5.
Relationships between the tizanidine/metabolite urine Ae ratios and the caffeine/paraxanthine plasma concentration ratio during the placebo phase, and between their changes caused by rofecoxib
| Caffeine/paraxanthine ratio | ||||
|---|---|---|---|---|
| Values during placebo | Changes caused by rofecoxib | |||
| Variable | r | P-value | r | P-value |
| Urine Ae ratio | ||||
| Tizanidine/M-3 | 0.65 | 0.06 | 0.90 | < 0.001 |
| Tizanidine/M-4 | 0.79 | 0.011 | 0.73 | 0.025 |
| Tizanidine/M-5 | 0.76 | 0.018 | 0.92 | < 0.001 |
| Tizanidine/M-9 | 0.58 | 0.10 | 0.86 | 0.003 |
| Tizanidine/M-10 | 0.79 | 0.011 | 0.56 | 0.12 |
r, Pearson’s correlation coefficient; Ae, amount excreted in urine within 24 h.
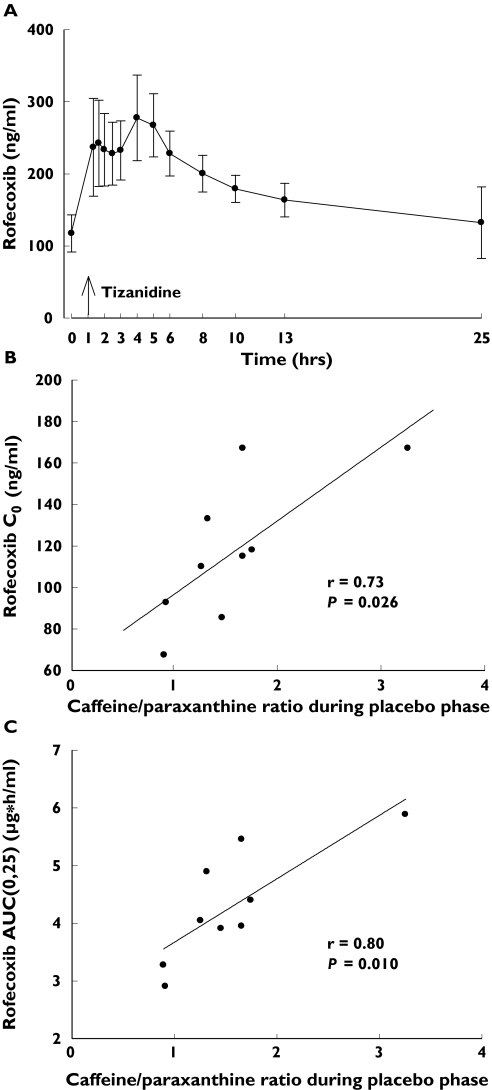
The concentration of rofecoxib in plasma was approximately 200 ng ml−1 at the time of tizanidine ingestion (Figure 7A). There was a fourfold interindividual variation in the AUC0–∞ and t1/2 of rofecoxib and a twofold variation in its Cmax (Table 1). The trough concentration (predose, C0) of rofecoxib on day 4 (r = 0.73, P = 0.026; Figure 7B), the AUC0–25 of rofecoxib (r = 0.80, P = 0.01; Figure 7C) and the AUC0–∞ of rofecoxib (r = 0.92, P = 0.0005) correlated significantly with the placebo phase caffeine/paraxanthine ratio. The AUC0–25 and AUC0–∞ of rofecoxib also correlated with the placebo phase AUC0–∞ of tizanidine (r = 0.62, P = 0.077, and r = 0.70, P = 0.037).
Figure 7.
Plasma concentrations of rofecoxib (mean ± SD) on day 4 of treatment with 25 mg rofecoxib once daily in nine healthy subjects (A). Time 0 refers to the administration of rofecoxib, which was given 1 h before the administration of tizanidine. Relationship between the plasma caffeine/paraxanthine ratio during the placebo phase and the C0 of rofecoxib (B) and between the plasma caffeine/paraxanthine ratio during the placebo phase and the AUC0–25 of rofecoxib (C) are also shown. Correlations were tested using linear regression analysis
The subjects complained more about dry mouth and tiredness after tizanidine ingestion during the rofecoxib phase than during the placebo phase. During the first 12 h, the urine volume was 764 ± 393 ml in the rofecoxib phase and 1226 ± 360 ml in the placebo phase (P = 0.004), but there was no significant difference between the phases in the urine volume excreted between 12 and 24 h.
Discussion
Our study using tizanidine and caffeine as probe substrates of CYP1A2 indicates that rofecoxib, at a normal daily dose of 25 mg, is a potent inhibitor of CYP1A2 activity. Rofecoxib showed a particularly strong pharmacokinetic interaction with tizanidine, which has potentially hazardous consequences. The AUC0–∞ of tizanidine was increased in each of the nine subjects by an average of nearly 14-fold, the greatest increase being more than 22-fold. Thus, it is most probable that the cardiovascular and central nervous system adverse reactions (e.g. hypotension, bradycardia, somnolence), observed in several patients after the concomitant intake of rofecoxib and tizanidine [1, 2], were caused by this pharmacokinetic interaction. Until now, the mechanism of this suspected interaction was unknown. Furthermore, our results indicate that inhibition of several CYP1A2 mediated routes of tizanidine metabolism by rofecoxib (and/or its metabolites) explains this pharmacokinetic interaction.
CYP1A2 makes a large contribution to the elimination of tizanidine [8, 10, 11], the major metabolites of which are M-3, M-4 and M-10 [9]. In the present study, M-3 and M-4 were detected in plasma, and M-3, M-4, M-5, M-9 and M-10 in urine. Owing to lack of reference compounds, the metabolite concentrations could be determined only in arbitrary units. However, this does not compromise the estimation of the correlation between CYP1A2 activity and the different metabolite ratios of tizanidine, or of the effect of rofecoxib on the formation of these metabolites.
In addition to increasing greatly the plasma concentrations of tizanidine, rofecoxib increased by several fold the tizanidine/metabolite ratios and by 2.4-fold the caffeine/paraxanthine ratio. The latter, an established indicator of CYP1A2 activity [12–14], correlated with the tizanidine/metabolite ratios in plasma and urine (Figure 6 and Table 5), suggesting that CYP1A2 is involved in the formation of all tizanidine metabolites. Moreover, the increases in the plasma concentration and urinary excretion of tizanidine and the tizanidine/metabolite (M-3, M-4, M-5 and M-9) ratios caused by rofecoxib correlated well with the increase in the caffeine/paraxanthine ratio. These findings indicate that rofecoxib is a potent inhibitor of CYP1A2 in vivo, and that the rofecoxib–tizanidine interaction is mediated by inhibition of the CYP1A2-mediated metabolism of tizanidine.
Although rofecoxib inhibited the formation of M-3 from tizanidine, the further metabolism of M-3 (a precursor of M-4) was also inhibited by rofecoxib. Consequently, the total AUC and t1/2 of M-3 were increased markedly, whereas the AUC of M-4 decreased slightly. M-4 can also be formed from M-5, the half-life of which seems to be relatively short. Therefore, the half-life of M-4 was not formation rate-limited by the long half-life of M-3. On the other hand, rofecoxib increased the excretion of M-10 into urine during the first 12 h after tizanidine intake, and the increase in the tizanidine/M-10 ratio by rofecoxib treatment did not correlate significantly with the increase in the caffeine/paraxanthine ratio. These findings suggest that, in addition to CYP1A2, other enzymes may be involved in the formation of M-10.
Recently, the effect of two established inhibitors of CYP1A2, fluvoxamine (100 mg given once daily for 4 days) and ciprofloxacin (500 mg twice daily for 3 days), on the pharmacokinetics and pharmacodynamics of tizanidine and on the caffeine test has been studied [8, 11]. The increase in tizanidine AUC0–∞ by rofecoxib (14-fold) in the present study was slightly greater than that seen by ciprofloxacin (10-fold) [8] but smaller than by fluvoxamine (33-fold) [11]. The Cmax of tizanidine was increased by rofecoxib (sixfold) to the same extent or less than by ciprofloxacin (sevenfold) and fluvoxamine (12-fold). On the other hand, the half-life of tizanidine was nearly doubled by rofecoxib (from 1.6 to 3.0 h), only slightly prolonged by ciprofloxacin (from 1.5 to 1.8 h) and nearly tripled by fluvoxamine (from 1.5 to 4.3 h). In these three studies, a single 4-mg dose of tizanidine was given 1 h after the other drug in order to allow the latter to dissolve and undergo significant absorption. However, the elimination half-lives of rofecoxib (22 h), ciprofloxacin (3–4 h) and fluvoxamine (7–60 h) differ considerably [20, 21]. Therefore, ciprofloxacin seems to have inhibited mainly the presystemic metabolism (reflected as an increased Cmax) of tizanidine, with a minimal effect during the elimination phase [8], whereas rofecoxib affected both presystemic and systemic metabolism (t1/2, AUC) of tizanidine. Fluvoxamine, which is a highly potent inhibitor of CYP1A2 [22] and has a long half-life, had the greatest effect on both the presystemic and systemic elimination of tizanidine [11].
In general, the cardiovascular and central nervous system effects of tizanidine correlate well with its plasma concentration [11]. Accordingly, rofecoxib caused substantial potentiation and prolongation of both the hypotensive and sedative effects of tizanidine. The hypotensive effect of tizanidine was increased by rofecoxib, although treatment with the latter itself, in line with previous findings [23, 24], also tended to increase blood pressure slightly. The maximum systolic hypotensive response to tizanidine during rofecoxib (−35 mmHg) was much greater than during placebo (−11 mmHg). In the present study, rofecoxib also slightly increased the negative chronotropic effect of tizanidine, as shown by the occurrence of bradycardia (42 beats min−1), evident in the supine position at 2 h postdose during the rofecoxib phase.
Cases of symptomatic hypotension, bradycardia and somnolence have been observed (one with a fatal outcome) during coadministration of rofecoxib and tizanidine [1, 2], demonstrating the potential hazards of this interaction. Similar exaggerated pharmacodynamic effects of tizanidine were seen in our healthy subjects after ingestion of tizanidine during the rofecoxib phase. In one case report, QT-prolongation was also observed during the combination [2]. However, we found no evidence of QTc-prolongation after tizanidine intake during either the rofecoxib or placebo phases. Urine volume during the first 12 h after tizanidine ingestion was markedly lower during the rofecoxib phase than during the placebo phase, even though the hypotensive subjects in particular were prompted to drink water. Thus, concomitant administration of rofecoxib and tizanidine seems to affect renal function adversely, and may increase the risk of fluid accumulation and oedema. This renal effect was probably mainly due to the hypotension caused by the high plasma concentrations of tizanidine. However, rofecoxib may also decrease renal function and cause fluid retention, which may have contributed to the decreased urine excretion [23, 24].
Rofecoxib, in daily doses of 12.5, 25 and 50 mg, increased the AUC of R(+)-warfarin by 1.27-, 1.38- and 1.40-fold [25] and the AUC of theophylline by 1.4-, 1.5- and 1.6-fold, respectively [6]. The product information on rofecoxib states that ‘these data suggest that rofecoxib may produce a modest inhibition of cytochrome P450 (CYP) 1A2’ [4]. However, the findings of the present study with caffeine and tizanidine indicate that rofecoxib is a potent inhibitor of CYP1A2. It is reasonable to assume that a larger daily dose of rofecoxib could have an even greater effect on tizanidine pharmacokinetics. Other CYP1A2 substrates include several important drugs, such as clozapine, olanzapine, riluzole, tacrine, zolmitriptan, lidocaine, ropivacaine and melatonin [26–35]. It is likely that rofecoxib, in a dose-dependent manner, will also increase their plasma concentrations and enhance their pharmacological actions. The effect of rofecoxib may depend on the pharmacokinetic properties of the substrate drug, e.g. the extent of first-pass metabolism. However, no studies seem to have been carried out to determine whether treatment with rofecoxib has lead to adverse events in patients taking CYP1A2 substrate drugs.
Rofecoxib itself is extensively metabolized via complex oxidative, reductive and reverse reductive pathways and it was suggested to have a relatively low propensity to interact with drugs that are metabolized by CYP enzymes [4, 36]. According to the product information, ‘cytochrome P450 plays a minor role in metabolism of rofecoxib’[4, 36]. However, CYP1A2 has been found to catalyse the oxidative metabolism of rofecoxib in vitro [36]. A fourfold interindividual variability in the AUC of rofecoxib was observed in our study, which was carried out in a homogeneous group of young healthy subjects. The AUC of rofecoxib correlated well with the caffeine/paraxanthine ratio (Figure 7), which suggests that CYP1A2 has a significant role in the elimination of the drug. A role for CYP1A2 in rofecoxib metabolism is further supported by the previous observations that the pharmacokinetics of the drug are nonlinear, being best described by auto-inhibition of its own CYP1A2-mediated metabolism [4, 37]. Accordingly, interindividual variability in CYP1A2 activity may contribute to the risk of concentration-dependent adverse effects of rofecoxib, such as oedema, sodium retention and, theoretically, even an increased risk of myocardial infarction [3, 4, 23, 24]. Thus, coadministration of other CYP1A2 inhibitors, such as oral contraceptives, ciprofloxacin or fluvoxamine [8, 11], might lead to an accumulation of rofecoxib during daily administration and an increased risk of adverse events.
In conclusion, rofecoxib is a potent inhibitor of CYP1A2 and it greatly increases the plasma concentrations and adverse effects of tizanidine, a CYP1A2 substrate undergoing substantial presystemic metabolism. Therefore, coadministration of rofecoxib and tizanidine is best avoided. The findings also suggest that CYP1A2 plays a significant role in the elimination of rofecoxib and raise concerns about the potential risks of interactions between the drug and other CYP1A2 substrates and inhibitors.
Conflict of interest
None declared.
Acknowledgments
This study was supported by grants from the Helsinki University Central Hospital Research Fund, the National Technology Agency and the Sigrid Jusélius Foundation, Finland.
References
- 1.Washington DC: FDA 2002; November 2002. [11 August 2005]. Summary of Safety-Related Drug Labeling Changes Approved by FDA Center for Drug Evaluation and Research (CDER) Available from URL: http://www.fda.gov/medwatch/SAFETY/2002/nov02.htm#zanafl. [Google Scholar]
- 2.Kick A, Bertoli R, Moschovitis G, Caduff Janosa P, Cerny A. [Extreme sinus bradycardia (30/min) with acute right heart failure under tizanidine (Sirdalud). Possible pharmacological interaction with rofecoxib (Vioxx)] Med Klin (Munich) 2005;100:213–6. doi: 10.1007/s00063-005-1024-2. [DOI] [PubMed] [Google Scholar]
- 3.Topol EJ. Failing the public health—rofecoxib, Merck, and the FDA. N Engl J Med. 2004;351:1707–9. doi: 10.1056/NEJMp048286. [DOI] [PubMed] [Google Scholar]
- 4.VIOXX (Rofecoxib Tablets and Oral Suspension) Whitehouse Station, NJ: Merck, Inc.; 2002. [11 August 2005]. Available from URL: http://www.fda.gov/medwatch/SAFETY/2003/03May_PI/vioxx_pi.pdf. [Google Scholar]
- 5.Halpin RA, Porras AG, Geer LA, Davis MR, Cui D, Doss GA, Woolf E, Musson D, Matthews C, Mazenko R, Schwartz JI, Lasseter KC, Vyas KP, Baillie TA. The disposition and metabolism of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in human subjects. Drug Metab Dispos. 2002;30:684–93. doi: 10.1124/dmd.30.6.684. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann K, White D, Jauregui L, Schwartz JI, Agrawal NG, Mazenko R, Larson PJ, Porras AG. An evaluation of the dose-dependent inhibition of CYP1A2 by rofecoxib using theophylline as a CYP1A2 probe. J Clin Pharmacol. 2003;43:1082–90. doi: 10.1177/0091270003257454. [DOI] [PubMed] [Google Scholar]
- 7.Wagstaff AJ, Bryson HM. Tizanidine. A review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs. 1997;53:435–52. doi: 10.2165/00003495-199753030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598–606. doi: 10.1016/j.clpt.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Koch P, Hirst DR, von Wartburg BR. Biological fate of sirdalud in animals and man. Xenobiotica. 1989;19:1255–65. doi: 10.3109/00498258909043177. [DOI] [PubMed] [Google Scholar]
- 10.Granfors MT, Backman JT, Laitila J, Neuvonen PJ. Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol. 2004;57:349–53. doi: 10.1046/j.1365-2125.2003.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granfors MT, Backman JT, Neuvonen M, Ahonen J, Neuvonen PJ. Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin Pharmacol Ther. 2004;75:331–41. doi: 10.1016/j.clpt.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kivistö KT, Wang JS, Backman JT, Nyman L, Taavitsainen P, Anttila M, Neuvonen PJ. Selegiline pharmacokinetics are unaffected by the CYP3A4 inhibitor itraconazole. Eur J Clin Pharmacol. 2001;57:37–42. doi: 10.1007/s002280100278. [DOI] [PubMed] [Google Scholar]
- 13.Fuhr U, Rost KL, Engelhardt R, Sachs M, Liermann D, Belloc C, Beaune P, Janezic S, Grant D, Meyer UA, Staib AH. Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics. 1996;6:159–76. doi: 10.1097/00008571-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Spigset O, Hägg S, Söderström E, Dahlqvist R. The paraxanthine:caffeine ratio in serum or in saliva as a measure of CYP1A2 activity: when should the sample be obtained? Pharmacogenetics. 1999;9:409–12. [PubMed] [Google Scholar]
- 15.Chavez-Eng CM, Constanzer ML, Matuszewski BK. Determination of rofecoxib (MK-0966), a cyclooxygenase-2 inhibitor, in human plasma by high-performance liquid chromatography with tandem mass spectrometric detection. J Chromatogr B Biomed Sci Appl. 2000;748:31–9. doi: 10.1016/s0378-4347(99)00565-4. [DOI] [PubMed] [Google Scholar]
- 16.Holland DT, Godfredsen KA, Page T, Connor JD. Simple high-performance liquid chromatography method for the simultaneous determination of serum caffeine and paraxanthine following rapid sample preparation. J Chromatogr B Biomed Sci Appl. 1998;707:105–10. doi: 10.1016/s0378-4347(97)00590-2. [DOI] [PubMed] [Google Scholar]
- 17.Pickard CE, Stewart AD, Hartley R, Lucock MD. A rapid HPLC method for monitoring plasma levels of caffeine and theophylline using solid phase extraction columns. Ann Clin Biochem. 1986;23:440–6. doi: 10.1177/000456328602300410. [DOI] [PubMed] [Google Scholar]
- 18.Stone B. Pencil and paper tests – sensitivity to psychotropic drugs. Br J Clin Pharmacol. 1984;18:15–20. doi: 10.1111/j.1365-2125.1984.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bond A, Lader M. The use of visual analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–8. [Google Scholar]
- 20.Vance-Bryan K, Guay DR, Rotschafer JC. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet. 1990;19:434–61. doi: 10.2165/00003088-199019060-00003. [DOI] [PubMed] [Google Scholar]
- 21.Dollery C. Therapeutic Drugs. 2. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 22.Brøsen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol. 1993;45:1211–4. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- 23.Whelton A, Fort JG, Puma JA, Normandin D, Bello AE, Verburg KM. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther. 2001;8:85–95. doi: 10.1097/00045391-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Huledal G, Jonzon B, Malmenäs M, Hedman A, Andersson LI, Odlind B, Brater DC. Renal effects of the cyclooxygenase-inhibiting nitric oxide donator AZD3582 compared with rofecoxib and naproxen during normal and low sodium intake. Clin Pharmacol Ther. 2005;77:437–50. doi: 10.1016/j.clpt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz JI, Bugianesi KJ, Ebel DL, De Smet M, Haesen R, Larson PJ, Ko A, Verbesselt R, Hunt TL, Lins R, Lens S, Porras AG, Dieck J, Keymeulen B, Gertz BJ. The effect of rofecoxib on the pharmacodynamics and pharmcokinetics of warfarin. Clin Pharmacol Ther. 2000;68:626–36. doi: 10.1067/mcp.2000.112244. [DOI] [PubMed] [Google Scholar]
- 26.Wang JS, Backman JT, Taavitsainen P, Neuvonen PJ, Kivistö KT. Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000;28:959–65. [PubMed] [Google Scholar]
- 27.Facciola G, Hidestrand M, von Bahr C, Tybring G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur J Clin Pharmacol. 2001;56:881–8. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 28.Sanderink GJ, Bournique B, Stevens J, Petry M, Martinet M. Involvement of human CYP1A isoenzymes in the metabolism and drug interactions of riluzole in vitro. J Pharmacol Exp Ther. 1997;282:1465–72. [PubMed] [Google Scholar]
- 29.Madden S, Spaldin V, Park BK. Clinical pharmacokinetics of tacrine. Clin Pharmacokinet. 1995;28:449–57. doi: 10.2165/00003088-199528060-00003. [DOI] [PubMed] [Google Scholar]
- 30.Wild MJ, McKillop D, Butters CJ. Determination of the human cytochrome P450 isoforms involved in the metabolism of zolmitriptan. Xenobiotica. 1999;29:847–57. doi: 10.1080/004982599238290. [DOI] [PubMed] [Google Scholar]
- 31.Arlander E, Ekström G, Alm C, Carrillo JA, Bielenstein M, Böttiger Y, Bertilsson L, Gustafsson LL. Metabolism of ropivacaine in humans is mediated by CYP1A2 and to a minor extent by CYP3A4: an interaction study with fluvoxamine and ketoconazole as in vivo inhibitors. Clin Pharmacol Ther. 1998;64:484–91. doi: 10.1016/S0009-9236(98)90131-X. [DOI] [PubMed] [Google Scholar]
- 32.Ekström G, Gunnarsson UB. Ropivacaine, a new amide-type local anesthetic agent, is metabolized by cytochromes P450 1A and 3A in human liver microsomes. Drug Metab Dispos. 1996;24:955–61. [PubMed] [Google Scholar]
- 33.Ring BJ, Catlow J, Lindsay TJ, Gillespie T, Roskos LK, Cerimele BJ, Swanson SP, Hamman MA, Wrighton SA. Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J Pharmacol Exp Ther. 1996;276:658–66. [PubMed] [Google Scholar]
- 34.Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–34. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- 35.Bertilsson L, Carrillo JA, Dahl ML, Llerena A, Alm C, Bondesson U, Lindstrom L, Rodriguez de la Rubia I, Ramos S, Benitez J. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994;38:471–3. doi: 10.1111/j.1365-2125.1994.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slaughter D, Takenaga N, Lu P, Assang C, Walsh DJ, Arison BH, Cui D, Halpin RA, Geer LA, Vyas KP, Baillie TA. Metabolism of rofecoxib in vitro using human liver subcellular fractions. Drug Metab Dispos. 2003;31:1398–408. doi: 10.1124/dmd.31.11.1398. [DOI] [PubMed] [Google Scholar]
- 37.Depre M, Ehrich E, Van Hecken A, De Lepeleire I, Dallob A, Wong P, Porras A, Gertz BJ, De Schepper PJ. Pharmacokinetics, COX-2 specificity, and tolerability of supratherapeutic doses of rofecoxib in humans. Eur J Clin Pharmacol. 2000;56:167–74. doi: 10.1007/s002280050736. [DOI] [PubMed] [Google Scholar]