Abstract
Aims
Pre-clinical data suggest that the racemic phyto-oestrogen 8-prenylnaringenin (8-PN) may have beneficial effects in postmenopausal women and may become an alternative to classical hormone replacement therapy (HRT) treatment regimes. The aim of this study was to investigate the pharmacokinetics, endocrine effects and tolerability of chemically synthesized 8-PN in postmenopausal women.
Methods
The study was performed using a randomized, double-blind, placebo-controlled, dose-escalation design with three groups of eight healthy postmenopausal women. In each group six subjects received 8-PN and two subjects placebo. 8-PN was given orally in doses of 50, 250 or 750 mg. Drug concentrations in serum, urine and faeces were measured up to 48 h and follicle-stimulating hormone/luteinizing hormone (LH) concentrations up to 24 h.
Results
All treatments were well tolerated and associated with a low incidence of (drug unrelated) adverse events. Serum concentrations of free 8-PN showed rapid drug absorption and secondary peaks suggestive of marked enterohepatic recirculation. Independent of the treatment group, approximately 30% of the dose was recovered in excreta as free compound or conjugates over the 48-h observation period. The first Cmax and AUC0–48 h showed dose linearity with ratios of 1 : 4.5 : 13.6 (Cmax) and 1 : 5.2 : 17.1 (AUC). The 750- mg dose decreased LH concentrations by 16.7% (95% confidence interval 0.5, 30.2).
Conclusion
Single oral doses of up to 750 mg 8-PN were well tolerated by postmenopausal women. The pharmacokinetic profile of 8-PN was characterized by rapid and probably complete enteral absorption, high metabolic stability, pronounced enterohepatic recirculation and tight dose linearity. The decrease in LH serum concentrations found after the highest dose demonstrates the ability of 8-PN to exert systemic endocrine effects in postmenopausal women.
Keywords: 8-PN, enterohepatic recirculation, HRT, LH, phyto-oestrogen
Introduction
Doubts have emerged about the benefits of long-term hormonal replacement therapy (HRT) since the publication of the Women’s Health Initiative trial showing a low benefit/risk ratio of the daily intake of conjugated equine oestrogens plus medroxyprogesterone acetate [1]. This landmark study has initiated much debate on HRT for menopausal women [2–4] and was followed by a report indicating an increased risk of breast cancer in current HRT users [5]. Whatever the outcome of the ongoing debate, there is a clear medical need for HRT, as the menopause is associated with a variety of troublesome symptoms caused by oestrogen deficiency and an increased risk of osteoporosis.
Secondary metabolites with oestrogenic activity (phyto-oestrogens) derived from plants have been suggested as an alternative to classical HRT. However, in spite of more than 100 published studies, an unequivocal answer with respect to clinical effectiveness of these compounds is lacking. Indeed, a recent meta-analysis demonstrated that the benefits of plant products on menopausal symptoms are negligible [6]. Nevertheless, high dietary intake of phyto-oestrogens from soy products has been shown to be associated with beneficial effects with respect to (peri-)menopausal symptoms [7]. Irrespective of reports on the positive effects of phyto-oestrogens, the oestrogenic effects of these compounds must be negligible, since they possess low potency and activate predominantly the oestrogen receptor β (ERβ) rather than the more important and abundant oestrogen receptor α (ERα). In addition, because phyto-oestrogens are used in nonpharmaceutical preparations and are given in combination with other natural compounds at unknown doses, these compounds escape the strict regulations relating to the efficacy of prescription drugs.
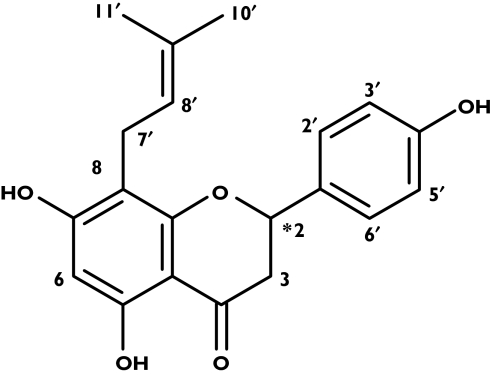
8 - Prenylnaringenin [8 - PN; 5, 7 - dihydroxy - 2 - (4-hydroxyphenyl)-8-(3-methylbut-2-enyl) chroman-4-one; Figure 1] has been identified as the most potent phyto-oestrogen [8]. The racemic compound (code name ZK 222302) was synthesized according to a published method [9] with modifications. A pharmaceutical preparation containing the micronized drug is now available.
Figure 1.
Chemical structure of racemic 8-prenylnaringenin (8-PN) – the star indicates the chiral centre
Studies with racemic 8-PN and its enantiomers 2S(−)8-PN and 2R(+)8-PN have revealed that 8-PN is the first ERα selective phyto-oestrogen with a two- to threefold higher affinity for the ERα receptor than the ERβ receptor measured by an in vitro competitive binding assay, and a 3.6-fold higher oestrogenic activity at the ERα than at the ERβ receptor in transactivational analysis [10]. The compound is a pure ER agonist. Qualitative differences between the racemate and the enantiomers were not found, with 2S(−)8-PN being slightly (two- to threefold) more potent than 2R(+)8-PN.
The pharmacological profile of 8-PN or its enantiomers in vivo was investigated using three techniques: the short-term uterus growth test in juvenile rats [10], the 4-week bone mineral density (BMD) model in adult ovariectomized rats [11] and the ERE-luc transgenic mouse model [11]. Although no qualitative differences were observed with respect to estradiolin in the short term test in juvenile rats, in the other models clear tissue specificity of 8-PN was found. In the BMD model, equipotent bone-protective doses of 8-PN and estradiol showed large differences with respect to their oestrogenic effects on the uterus and the endometrium. Whereas estradiol maximally stimulated uterus growth and epithelial height, the effect of 8-PN was marginal and not different from that in ovariectomized control animals. A pronounced bone tissue-specific oestrogenic effect was also seen in the transgenic ERE-luc mouse model.
In summary, 8-PN can be termed a natural selective oestrogen receptor modulator (SERM) with 10-fold higher selectivity for bone tissue compared with 17β-estradiol. The relative potency of 8-PN is almost equal to that of estrone and 70 times weaker than that of 17β-estradiol [10].
The pharmacokinetics of 8-PN in rats and dogs is characterized by a low oral bioavailability caused by extensive presystemic elimination after complete enteral absorption. The drug is almost completely excreted via the bile in unchanged form and in urine in the form of conjugates. Intestinal and hepatic phase 1 metabolism plays a minor role in the inactivation of 8-PN (data on file; Schering AG). This is in complete contrast to other natural or synthetic oestrogens, whose decreased oral bioavailability is due to intestinal conjugation and hepatic metabolic inactivation [12]. The comparatively high metabolic stability of 8-PN and its pronounced presystemic elimination via the bile resulted in enterohepatic recirculation in both rats and dogs.
The aim of the present study was to investigate in humans the pharmacokinetics, tolerability and endocrine effects of single oral doses of 8-PN. Based upon the pharmacokinetic profile of the compound in rats and dogs, excreta were collected for two days in addition to routine blood samplings. To control for one gastrointestinal passage, the drug was administered together with carmine red (also known as carminic acid) [13]. The endocrine effects on the hypothalamo-pituitary gonadotrophic axis were assessed by investigating the influence on follicle-stimulating hormone (FSH) and luteinising hormone (LH).
Subjects and methods
The Ethics Committee of Leiden University Medical Centre approved the study protocol and subjects gave written informed consent. The study was conducted according to the principles of the Declaration of Helsinki and in accordance with the Guideline for Good Clinical Practice. The study was carried out using a randomized, double blind, placebo-controlled, dose-escalation design with three groups of eight healthy women. In each group six subjects received 8-PN and two subjects received placebo.
Subjects
Subjects were included after an eligibility screening that consisted of medical history, physical examination, 12-lead ECG and routine blood and urine analysis. The women, who had a body mass index (BMI) between 19 and 29 kg m−2, were between 46 and 65 years of age and their last menstruation had occurred more than 1 year ago. Subjects were excluded if they had a history of thromboembolic disease or any condition associated with increased susceptibility to thromboembolism, or if they used medication or smoked >10 cigarettes per day.
Study procedures
The treatments consisted of single oral dose of 50 mg, 250 mg or 750 mg 8-PN or placeboafter an overnight fast. The drug was formulated in a hard gelatine capsule (size: zero) with micronized 8-PN (µ20) mixed with µ-lactose (1 : 1 w/w). Each unit contained 50 mg of active drug, and placebo capsules were filled with 100 mg lactose. Preparations were all from a single batch prepared and released by Klosterfrau GmbH (Berlin, Germany). Together with treatments a capsule containing 250 mg of carmine red (Spruyt Hillen BV, Utrecht, the Netherlands) was administered after being prepared for human use by the LUMC hospital pharmacy.
Blood sampling, urine and faeces collection and measurement of vital signs took place regularly over a 48-h observation period. Subjects were required to refrain from alcohol for at least 10 h before dosing and did not eat until 4 h after dosing. Thereafter, a light lunch was provided and dinner was served approximately 9–10 h after dosing. The following morning, breakfast was provided after the blood sampling was completed. At the 48-h observation point a poststudy screening was performed in fasted state.
Venous blood samples were taken by venepuncture or from an indwelling intravenous cannula. Blood samples for drug assay (plain tubes) were taken predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24 and 48 h after dosing. Blood was drawn for follicle-stimulating hormone (FSH) and luteinizing hormone (LH) analysis (plain tubes) before and 1, 2, 3, 4, 6, 8, 12, 16 and 24 h after drug administration. Blood samples were left at room temperature for 30–60 min to allow clotting and then centrifuged for 10 min at 2000 g at 4 °C. The separated serum was stored at −20 °C until analysis. Samples for haematology, biochemistry and electrolyte analysis were analysed at the central laboratories of LUMC.
Fractionated urine collection was performed over the following time intervals: predose (assay blank), 0–8 h, 8–16 h and 16–24 h. The volume passed over each time interval was determined by weighing. From each urine collection, aliquots were taken and stored at −20 °C until analysis. The subjects were required to collect all faeces passed during the observation period or until carmine red was detectable in a stool sample. After weighing, the faeces were stored at −20 °C until analysis.
Drug assays
Radioimmunoassay of PN
Serum and urine samples were analysed for drug concentrations using a recently described radioimmunoassay (RIA) making use of anti-C4′-carboxy-methoxy-rac-8-PN and a tritiated tracer [14]. Serum and urine samples (or dilutions thereof) (≤0.4 ml) were diluted with physiological saline to give 0.5 ml and then extracted with 2.5 ml t-butyl methyl ether for 50 min. After phase separation (low-speed centrifugation) and freezing the aqueous phase, the organic layer was separated and evaporated to dryness by a stream of nitrogen. Extract residues were reconstituted in 0.8 ml assay buffer prior to RIA analysis.
Serum and urine samples were also analysed for conjugated drug content after cleavage of conjugates using β-glucuronidase/arylsulphatase as described previously [15]. Enzyme-treated samples were extracted as described before and drug content determined by RIA.
The volume used in the RIA was 1.0 ml and consisted of 0.1 ml of standard (75–10 000 pg ml−1) plus 0.7 ml of buffer or 0.8 ml extracts of unknowns, 0.1 ml tracer (10 000 dpm per test tube) and 0.1 ml of antiserum solution (final dilution in test tube 1 : 100 000). The mixture was incubated at 4 °C overnight. Separation of free and bound antigen was achieved by addition of 0.2 ml of 1.25% dextran-coated charcoal suspension at 0 °C for 15 min, subsequent low-speed centrifugation (2300 g) and decantation of supernatant. Radioactivity was counted in a liquid scintillation counter after addition of 4 ml of Atomlight to the supernatant. TECAN easyWIN fitting Version 6.1 was used to fit the standard curve and to read the concentration of the unknowns.
The assay had a sensitivity limit of 0.3 ng ml−1 biological matrix (urine and serum) when 0.1 ml of the matrix was extracted. When necessary (as in case of some serum samples obtained from the 50-mg subject group) sensitivity was lowered to 0.1 ng ml−1 by extraction of 0.3 ml of serum sample. As complete extraction of PN occurs the standards did not need to be co-extracted [14]. There was no detectable cross-reactivity with endogenous sex steroids.
The intra-assay coefficient of variation (CV, N = 7) was 8.8% and 7.0% for concentrations of 0.3 and 1.0 ng ml−1 urine and 12.2% and 10.6% for 0.3 and 1 ng ml−1 serum, respectively. Respective figures for the interassay CV (N = 8) were 26.8% and 17.6% (urine) and 25.7% and 13.9% (serum). To determine the accuracy, five blank urine and serum samples were spiked with 1.0 and 3.0 ng 8-PN per ml and analysed. In serum (urine) 1.43 ± 0.14 ng ml−1 (1.36 ± 0.14 ng ml−1) and 3.52 ± 0.47 ng ml−1 (3.29 ± 0.24 ng ml−1) were recovered.
High-performance liquid chromatography assay of PN
The high-performance liquid chromatography (HPLC) system developed and validated for examining the release and the stability testing of the gelatine capsules was also used for the analysis of 8-PN in faeces. The system consisted of a Purospher 100, RP-C18e column (5 µm, 250 × 4 mm) operated with a mobile phase of 0.05 m phosphoric acid (adjusted to pH 2.5 with ammonium acetate) and acetonitrile (4 : 6 v/v). The flow rate was 1 ml min−1 at 30 °C and detection was at 210 nm 8-PN eluted after 4.9 min. Faeces were lyophilized, weighed, homogenized in the dry state by automatic grinding, and weighed amounts (35–650 mg) were extracted with 10 ml of methanol. The alcoholic layer was separated, diluted with methanol and kept at −20 °C until analysis. The lower limit of quantification (LLOQ) was set to 0.1 µg ml−1 of diluted extracts.
Data analysis
All serum samples were analysed for free 8-PN, whereas the determination of conjugates was carried out in individual and weighed serum pool samples. Concentrations of conjugated 8-PN in the latter were multiplied by 24 h to give the area under the curve (AUC) 0–24 h [16]. Individual serum concentration–time curves were evaluated model-independently. The AUC was calculated by the trapezoidal rule and allowing the estimation of the mean residence time (MRT). The weights of the dry faeces and the urine volumes were used to calculate total excretion. Data were evaluated by summary measures and are given as means ± SD. Dose linearity for the first occurring Cmax and the AUC0–48 h was analysed by dividing these parameters by dose and subjecting the data to analysis of variance.
The 24-h serum concentration time profiles of FSH and LH were analysed by mixed-model analyses of variance (using SAS PROC MIXED) with subject as a random effect and treatment, time and treatment by time as fixed effects, with baseline as covariate. An unstructured covariance matrix was used and baseline was defined as the predose value. For graphical purposes, an analysis was performed with change from baseline values using the latter as covariate. This leads to statistically identical results compared with the alternate analysis, but allows graphs of change from baseline to be generated. Data were analysed after log-transformation and results were back-transformed and either presented as percentage change from baseline with 95% confidence interval (CI) (graphs) or as percentage difference from placebo with 95% CI (treatment contrasts). Calculation of time and treatment by time effects was for graphical presentation purposes only. Contrasts within the overall treatment effect were estimated, resulting in mean response estimates over 24 h. A P-value <0.05 was considered significant. The analyses were performed using SAS for Windows version 9.1.2 (SAS Institute, Cary, NC, USA).
Results
The study population consisted of 24 healthy postmenopausal women (23 caucasian subjects and one subject of mixed ethnic origin). The age range of the population was 46–65 years and their mean (SD) BMI was 24.9 (1.9) kg m−2. All subjects had had their last menstrual period more than 1 year before screening for the study.
Only few mild and transient adverse events (AEs) occurred. Nine AEs were observed after administration of the 50-mg dose. The most frequently observed AE in this cohort was headache occurring twice on active drug and once on placebo. In addition, there was one case of epistaxis, one case of restlessness due to a known allergy for a food additive, redness and itching at the site of the adhesive tape and an intercurrent upper respiratory tract infection (most likely viral). In the second cohort 13 AEs were noted. The most frequently observed ones were headache (three episodes after placebo and four episodes after active drug) and gastrointestinal complaints (twice on placebo and once on active drug). There was also one haematoma at the cannulation site (after active drug) and one subject on placebo experienced erythema and itching in the neck and scalp region. After administration of the highest dose four AEs occurred. Two subjects experienced an intercurrent upper respiratory tract infection (one on placebo and one on active drug) and in one of these subjects an episode of headache occurred. In another subject a vasovagal reaction occurred after cannula manipulation. Hence, there is no obvious relationship between the AEs and the administration of PN at any of the doses. In addition, there were no clinically significant effects of the drug on heart rate, blood pressure, ECG parameters or on the routine blood and urine laboratory parameters.
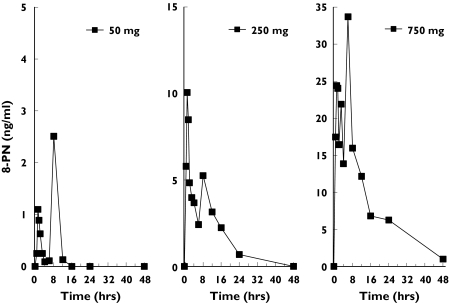
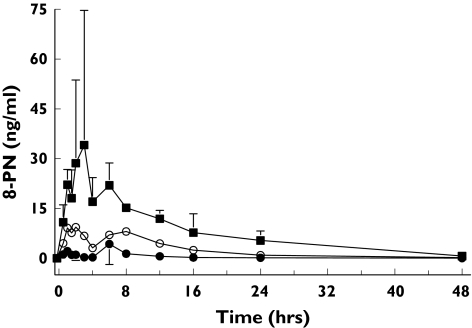
The high sensitivity of the assay enabled the determination of all free 8-PN concentrations in all dose groups. In samples from placebo-treated subjects no 8-PN was found. A representative individual curve from each dose group is given in Figure 2 and the mean serum concentration profiles for all doses are shown in Figure 3. The individual profiles show that 8-PN was rapidly absorbed leading to maximum serum drug concentrations 1.0–1.5 h after administration. Thereafter, drug serum concentrations decreased sharply, followed by a further increase in concentrations leading to a second peak occurring at 7–10 h. As a result, it was impossible to estimate adequately the terminal half-life of drug disposition. The means (SD) of the pharmacokinetic parameters in each dose group are summarized in Table 1.
Figure 2.
Representative individual serum concentration–time profiles of free 8-prenylnaringenin (8-PN) after oral administration. Note the different ordinate scale for each curve
Figure 3.
Mean (SD; N = 6) serum concentrations of free 8-prenylnaringenin (8-PN) following single oral doses of 50 mg (•), 250 (○), or 750 mg (▪) to healthy postmenopausal women
Table 1.
Mean (± SD) pharmacokinetic parameters after a single oral administration of 8-prenylnaringenin (8-PN) to healthy postmenopausal women (n = 6 per group)
| Dose | |||
|---|---|---|---|
| 50 mg | 250 mg | 750 mg | |
| Tmax 1 (h) | 1.2 ± 0.4 | 1.0 ± 0.5 | 1.5 ± 0.8 |
| Cmax 1 (ng ml−1) | 2.74 ± 1.3 | 12,2 ± 11.1 | 37.3 ± 37.8 |
| Cmax 1 per dose (ng ml−1 mg−1) | 0.055 ± 0.026 | 0.049 ± 0.045 | 0.050 ± 0.050 |
| Tmax 2 (h) | 7.7 ± 2.3 | 7.5 ± 2.9 | 10.2 ± 8.1 |
| Cmax 2 (ng ml−1) | 4.89 ± 5.7 | 12.1 ± 6.1 | 22.6 ± 9.6 |
| AUC0–48 h (ng/mlxh) | 22.5 ± 22.2 | 115.9 ± 49.3 | 383.5 ± 111 |
| AUC0–48 h per dose (ng ml−1 h−1 mg−1) | 0.45 ± 0.44 | 0.46 ± 0.20 | 0.51 ± 0.15 |
| Mean residence time (h) | 8.7 ± 3.1 | 10.1 ± 2.3 | 12.2 ± 1.9 |
The first occurring Cmax and AUC0–48 h increased linearly with dose giving ratios of 1 : 4.5 : 13.6 (Cmax) and 1 : 5.2 : 17.1 (AUC) at a dose ratio of 1 : 5 : 15. Statistical testing of the dose-normalized Cmax (P = 0.97) and AUC (P = 0.93) confirmed the absence of dose-dependency in these parameters. With increasing dose, a higher proportion of total AUC was shifted to later times, which is reflected by increases in the MRT at higher doses. Data for the recovery of 8-PN in urine and faeces are shown in Table 2. A direct comparison of AUC0–24 h for free and conjugated 8-PN from the pooled samples is also presented. Conjugated serum drug concentrations were higher than those of free drug, although the ratio of these concentrations clearly decreased with increasing dose (the ratios of AUCconj to AUCfree = 42 ± 20, 21 ± 3.8, 7.0 ± 1.9 for the 50, 250 and 750-mg dose groups, respectively). Consequently, compared with dose ratios (1 : 5 : 15) those for the AUCs of conjugated 8-PN increased less than proportionally to the dose (1 : 4.4 : 6.1).
Table 2.
Area under the curves (AUC) for free and conjugated 8-prenylnaringenin (8-PN) and mass balance data for 8-PN after a single oral dose to healthy postmenopausal women (n = 6 per group); data are given as means (± SD)
| Dose | |||
|---|---|---|---|
| 50 mg | 250 mg | 750 mg | |
| AUC0–24 h free drug (ng ml−1 h−1) | 13.3 ± 9.2 | 97.4 ± 43.7 | 394 ± 76 |
| AUC0–24 h conjugated (µg ml−1 h−1) | 0.44 ± 0.09 | 1.94 ± 0.71 | 2.65 ± 0.48 |
| Urinary excretion free 8-PN (% dose in 48 h) | 0.23 ± 0.11 | 0.21 ± 0.09 | 0.15 ± 0.07 |
| Urinary excretion conjugated 8-PN (% dose in 48 h) | 8.1 ± 3.9 | 7.2 ± 2.1 | 4.6 ± 1.5 |
| Faeces free (% dose in 48 h) | 22.8 ± 13.6 | 23.7 ± 11.0 | 21.8 ± 12.2 |
| Balance (% dose in 48 h) | 31.0 ± 11.9 | 30.9 ± 11.2 | 26.4 ± 13.2 |
The urinary excretion of free 8-PN was only 0.2% of each dose. Drug conjugates showed a higher renal excretion averaging to 8.1 ± 3.9% (50-mg dose), 7.2 ± 2.1% (250-mg dose) and 4.6 ± 1.5% (750-mg dose) of the dose. The lower urinary excretion in the highest dose group might indicate that the collection period of 2 days was not sufficient.
To control for at least one gastrointestinal transit, subjects received carmine red. The dry weights of the samples varied substantially, ranging between 34 and 317 g (50-mg group), 23–145 g (250-mg group) and 30–215 g (750-mg group). No drug-related substance was detected in any of the faeces collected by subjects on placebo. On average, 21–24% of dose was found in the faeces. Although individual portions of faecal samples were pooled prior to analysis, there were six subjects for whom the samples were separately pooled up to 24 h and the remaining time, mostly up to 40 or 48 h. The samples from these six subjects were analysed separately. Between 20 and 62% of the respective doses were found in the later collected samples. The data suggest that a substantial part of the dose is excreted over an extended time period after administration of PN. In total, about 30% of the dose was recovered in urine and faeces, either as unchanged drug or as conjugated metabolites.
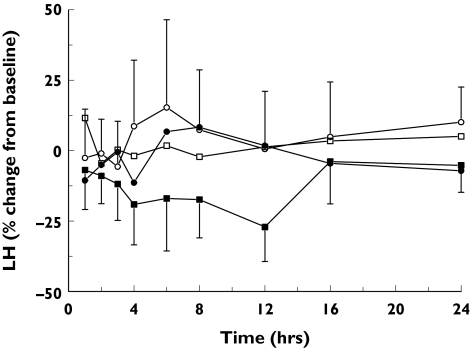
The effects of 8-PN on FSH and LH are summarized in Table 3. Whereas FSH concentrations were not significantly influenced by the drug at any of the doses, there was a significant decrease in serum LH concentrations after the 750-mg dose (Figure 4). The mean percentage decrease in LH concentration from placebo over 24 h was 16.7% (95% CI 0.5, 30.2).
Table 3.
Mean effect of different doses of 8-prenylnaringenin (8-PN) on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) serum concentrations; values are given as least square means and percentage change from placebo with the 95% confidence interval
| Dose | FSH (U ml−1) | LH (U ml−1) |
|---|---|---|
| Placebo | 63.0 | 30.6 |
| 50 mg | 64.6, 2.5% (−3.1, 8.3) | 28.6, −6.4% (−21.5, 11.5) |
| 250 mg | 66.5, 5.5% (−0.7, 12.1) | 29.9, −2.3% (−18.0, 16.2) |
| 750 mg | 60.8, −3.5% (−9.0, 2.4) | 25.5, −16.7% (−30.2, −0.5) |
Figure 4.
The time course of the percentage change from baseline in serum luteinizing hormone (LH) concentrations after single oral doses of 50 mg (•), 250 mg (□) or 750 mg (▪) 8-prenylnaringenin (8-PN) or placebo (□); 95% CI error bars are given for the placebo and the 750-mg groups
Discussion
Phyto-oestrogens comprise a set of secondary plant metabolites belonging to the chemical classes of coumestans, isoflavones, flavanones and lignans, which all activate the human oestrogen receptor. These molecules share at least one phenolic group as their structural hallmark. Epidemiological data suggest that phyto-oestrogens ingested with common foodstuffs help to prevent breast cancer and atherosclerosis and to alleviate perimenopausal symptoms. However, potential negative effects have also been suggested [17] and beneficial ones questioned [6]. Pharmaceutical development of phyto-oestrogens has been greatly limited by their low potency leading to the need of high doses and by the fact that marketed crude plant extracts mainly contain bulky amounts of hormonally inactive substances. However, the assumption that a specific and potent phyto-oestrogen, which is both pharmacologically and pharmaceutically well characterized, may have therapeutic use and is supported by a significant body of data.
The prenylated flavanone 8-prenylnaringenin (8-PN) was recently isolated [18] and also identified as the oestrogenic component in hops and beer [8, 19–21] and may have therapeutic value. 8-PN has been shown to be the phyto-oestrogen with the greatest affinity for the oestrogen receptor and its in vitro and in vivo pharmacology has been extensively investigated [10, 18, 22–24]. In addition, pharmaceutical development of racemic 8-PN has become a realistic option using scaled up chemical synthesis.
We report the first study of the pharmacokinetics and pharmacodynamics of pharmaceutically pure 8-PN in healthy postmenopausal women. Single oral doses of 50–750 mg were well tolerated and were associated with a low incidence of nondrug-related AEs. There were no effects on blood pressure, pulse rate or ECG parameters. It was also shown that, compared with placebo, none of the doses of PN had a significant influence on FSH concentrations. In contrast, the 750-mg dose decreased LH concentrations significantly. This is strong evidence that 8-PN is able to cross the blood–brain barrier and interact with the hypothalamo–pituitary axis, which is regarded as a prerequisite for the successful treatment of menopausal symptoms. Although the significant 17% decrease in LH serum concentrations and the nonsignificant 4% decrease in FSH concentrations are of similar magnitude to those observed after the administration of 2 mg 17β-estradiol to postmenopausal women [25, 26], a direct comparison is difficult, because in the previous studies there was no correction for placebo effects (diurnal changes) on gonadotrophins.
The pharmacokinetic profile of 8-PN in humans confirmed important findings from animal studies. The tight dose linearity of Cmax 1, AUC0–48 h of free drug and renally excreted free and conjugated drug, is compatible with either complete enteral drug absorption or absorption of a constant but unknown fraction of the dose. The former mechanism would be in line with data from animal experiments showing that doses up to 250 mg kg−1 are completely absorbed (data on file, Schering AG). The time to reach Cmax was short (1–1.5 h), reflecting rapid absorption from the proximal part of the intestinal tract. Secondary peaks of unchanged drug clearly indicate the occurrence of enterohepatic recirculation. A second maximum (7–10 h after administration) was present at all doses and was especially prominent in the 50-mg group. Most probably the first meal, taken 4 h after drug intake, induced gall bladder emptying and started the enterohepatic recirculation process. At higher doses primary absorption seemed to occur at the same time as secondary absorption after enterohepatic recirculation, leading to broader secondary peaks. The proportion of the dose that underwent presystemic elimination and wa subsequently re-absorbed is difficult to estimate from the present study design. As the secondary peaks in the 50-mg dose group accounted for 54 ± 20% of total AUC, it is conceivable that about half of the dose underwent presystemic elimination.
Secondary peaks hindered the calculation of the disposition half-life of PM. However, the rapid decrease in serum concentration after the peaks suggests a short elimination phase with a half-life of 1–3 h. Drug elimination seems to be a combination of hepatic conjugation, hepatic elimination and renal excretion of conjugates. Conjugation of 8-PN to its β-glucuronide or sulphate is likely to be the predominant metabolic pathway. Whether unchanged drug found in faeces was in the conjugated form when excreted in the bile and was subsequently de-conjugated in the gastrointestinal tract, is unknown. The nonlinear increase in AUCconj in the 750-mg dose group might indicate that conjugation was less effective at the highest dose. This might also explain the lower proportion of the dose found in urine at the highest dose. However, the extent of conjugation of the total dose is not known. Faecal and renal excretion was estimated by analysis of the parent compound. On the basis of the animal studies it was expected that 60–70% of dose would be recovered in free or conjugated form. The mass balance over 48 h in the present study accounted for approximately one-third of the dose at all the doses. However, owing to incomplete collection of excreta by subjects (see range of dry faecal weights), the mean mass balance data are probably an underestimation.
Notwithstanding the uncertainty of the amount of drug recovered, it is clear that a substantial part (at least 30%) of orally administered 8-PN escapes phase 1 metabolism in vivo. Compared with estradiol, ethinyl estradiol and conjugated equine oestrogens, which all undergo extensive phase 1 metabolism and for which only negligible amounts are excreted as free or conjugated compounds [12], 8-PN can be regarded as a comparatively stable oestrogen in vivo. This is in agreement with the recently published data on the metabolism of PN in human liver microsomes [27].
The pharmacokinetic profile of 8-PN can be used to estimate the total dose of 8-PN to which consumers of hop-spiced beers might be exposed. As shown recently, the systemically available dose is the sum of the amount of 8-PN in beer plus the amount of 8-PN arising from the in vivo demethylation of isoxanthohumol [14]. Comparing the amount of 8-PN and/or its conjugates in urine or the serum concentrations found in beer consumers with the present data should lead to a fairly good estimation of total exposure to oestrogen.
In summary, single oral doses of 8-PN up to 750 mg were well tolerated by postmenopausal women. No drug-related adverse events were recorded. At the highest dose LH serum concentrations were significantly decreased, clearly demonstrating the intended biological activity necessary for the successful treatment of menopausal symptoms. The pharmacokinetic profile of 8-PN was characterized by rapid enteral absorption, a pronounced presystemic elimination with subsequent enterohepatic recirculation, dose linear pharmacokinetics and comparatively little phase 1 metabolism. Conjugation to β-glucuronides or sulphates seems to constitute the main metabolic pathway. The results warrant further investigations of this compound and its biological effects after multiple doses.
Acknowledgments
This study was financially supported by Schering AG, Berlin, Germany and the data will be used for further development of the compound by 8-PN GmbH (Berlin) owned by M.H., W-D.S. and Catenion Group GmbH (Berlin).
References
- 1.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Lemay A. The relevance of the Women’s Health Initiative results on combined hormone replacement therapy in clinical practice. J Obstet Gynaecol Can. 2002;24:711–5. doi: 10.1016/s1701-2163(16)30326-7. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Stuenkel CA. Hormone replacement therapy (HRT)—risks and benefits. Int J Epidemiol. 2001;30:423–6. doi: 10.1093/ije/30.3.423. [DOI] [PubMed] [Google Scholar]
- 5.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 6.Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824–36. doi: 10.1097/01.AOG.0000140688.71638.d3. [DOI] [PubMed] [Google Scholar]
- 7.Nagata C, Takatsuka N, Kawakami N, Shimizu H. Soy product intake and hot flashes in Japanese women: results from a community-based prospective study. Am J Epidemiol. 2001;153:790–3. doi: 10.1093/aje/153.8.790. [DOI] [PubMed] [Google Scholar]
- 8.Milligan SR, Kalita JC, Van Pocock VDKV, Stevens JF, Deinzer ML, Rong H, De Keukeleire D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab. 2000;85:4912–5. doi: 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- 9.Gester S, Metz P, Zierau O, Vollmer G. An efficient synthesis of the potent phytoestrogens 8-prenylnaringenin and 6-(1,1-dimethylallyl) naringenin by europium (III)-catalyzed Claisen rearrangement. Tetrahedron. 2001;57:1015–8. [Google Scholar]
- 10.Schaefer O, Hümpel M, Fritzemeier KH, Bohlmann R, Schleuning WD. 8-Prenyl naringenin is a potent ERalpha selective phytoestrogen present in hops and beer. J Steroid Biochem Mol Biol. 2003;84:359–60. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 11.Hümpel M, Isaksson P, Schaefer O, Kaufmann U, Ciana P, Maggi A, Schleuning WD. Tissue specificity of 8-prenylnaringenin: protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J Steroid Biochem Mol Biol. 2005;97:299–305. doi: 10.1016/j.jsbmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell MB. Pharmacokinetic and pharmacologic variation between different estrogen products. J Clin Pharmacol. 1995;35:18S–24S. doi: 10.1002/j.1552-4604.1995.tb04143.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JB. Use of non-absorbable markers in studies of human nutrient absorption. Hum Nutr Appl Nutr. 1986;40:399–411. [PubMed] [Google Scholar]
- 14.Schaefer O, Bohlmann R, Schleuning WD, Schulze-Forster K, Hümpel M. Development of a radioimmunoassay for the quantitative determination of 8-prenylnaringenin in biological matrices. J Agric Food Chem. 2005;53:2881–9. doi: 10.1021/jf047897u. [DOI] [PubMed] [Google Scholar]
- 15.Hümpel M, Illi V, Milius W, Wendt H, Kurowski M. The pharmacokinetics and biotransformation of the new benzodiazepine lormetazepam in humans. I. Absorption, distribution, elimination and metabolism of lormetazepam-5-14C. Eur J Drug Metab Pharmacokinet. 1979;4:237–43. doi: 10.1007/BF03189433. [DOI] [PubMed] [Google Scholar]
- 16.Louton T, Kuhnz W, Dibbelt L, Knuppen R. Weighted serum pools in comparison to the trapezoidal rule for estimating AUCs for ethinyl estradiol. The relationship of the variance of the determination to the interindividual variance. Eur J Clin Pharmacol. 1994;46:77–81. doi: 10.1007/BF00195920. [DOI] [PubMed] [Google Scholar]
- 17.Russell L, Hicks GS, Low AK, Shepherd JM, Brown CA. Phytoestrogens: a viable option? Am J Med Sci. 2002;324:185–8. doi: 10.1097/00000441-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kitaoka M, Kadokawa H, Sugano M, Ichikawa K, Taki M, Takaishi S, Iijima Y, Tsutsumi S, Boriboon M, Akiyama T. Prenylflavonoids: a new class of non-steroidal phytoestrogen (Part 1). Isolation of 8-isopentenylnaringenin and an initial study on its structure–activity relationship. Planta Med. 1998;64:511–5. doi: 10.1055/s-2006-957504. [DOI] [PubMed] [Google Scholar]
- 19.Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, De Keukeleire D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–42. [PubMed] [Google Scholar]
- 20.Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, De Keukeleire D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab. 1999;84:2249–52. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- 21.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–30. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Rong H, Boterberg T, Maubach J, Stove C, Depypere H, Van Slambrouck S, Serreyn R, De Keukeleire D, Mareel M, Bracke M. 8-Prenylnaringenin, the phytoestrogen in hops and beer, upregulates the function of the E-cadherin/catenin complex in human mammary carcinoma cells. Eur J Cell Biol. 2001;80:580–5. doi: 10.1078/0171-9335-00190. [DOI] [PubMed] [Google Scholar]
- 23.Shirataki Y, Motohashi N, Tani S, Sakagami H, Satoh K, Nakashima H, Mahapatra SK, Ganguly K, Dastidar SG, Chakrabarty AN. In vitro biological activity of prenylflavanones. Anticancer Res. 2001;21:275–80. [PubMed] [Google Scholar]
- 24.Pepper MS, Hazel SJ, Hümpel M, Schleuning WD. 8-prenylnaringenin, a novel phytoestrogen, inhibits angiogenesis in vitro and in vivo. J Cell Physiol. 2004;199:98–107. doi: 10.1002/jcp.10460. [DOI] [PubMed] [Google Scholar]
- 25.Yen SS, Martin PL, Burnier AM, Czekala NM, Greaney MO, Jr, Callantine MR. Circulating estradiol, estrone and gonadotropin levels following the administration of orally active 17beta-estradiol in postmenopausal women. J Clin Endocrinol Metab. 1975;40:518–21. doi: 10.1210/jcem-40-3-518. [DOI] [PubMed] [Google Scholar]
- 26.Englund DE, Johansson ED. Plasma levels of oestrone, oestradiol and gonadotrophins in postmenopausal women after oral and vaginal administration of conjugated equine oestrogens (Premarin) Br J Obstet Gynaecol. 1978;85:957–64. doi: 10.1111/j.1471-0528.1978.tb15860.x. [DOI] [PubMed] [Google Scholar]
- 27.Nikolic D, Li Y, Chadwick LR, Grubjesic S, Schwab P, Metz P, van Breemen RB. Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes. Drug Metab Dispos. 2004;32:272–9. doi: 10.1124/dmd.32.2.272. [DOI] [PubMed] [Google Scholar]