Abstract
Aim
To compare the pharmacokinetics of mycophenolic acid when given with either ciclosporin or sirolimus, and investigate in vitro the potential effect of ciclosporin, sirolimus, tacrolimus and everolimus on mycophenolic acid metabolism.
Methods
In renal transplant patients given mycophenolate mofetil in combination with ciclosporin (n = 19) or sirolimus (n = 12), concentration-time profiles of mycophenolic acid, mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside were determined at one month post-transplant. The effect of immunosuppressive drugs on mycophenolic acid glucuronidation and glycosylation was investigated in vitro using human liver microsomes.
Results
The mean mycophenolic acid AUC0–9 h in the sirolimus group was 44.9 mg h−1 L−1 (95% CI: 34.7–55.1), vs. 30.5 mg h−1 L−1 (95% CI: 25.4–35.6) in the ciclosporin group, corresponding to 1.5-fold dose-normalized difference (95% CI: 1.1–1.9; P < 0.05). In addition, the metabolite/mycophenolic acid AUC0–9 h ratios were significantly higher in patients cotreated with ciclosporin than with sirolimus, giving values of 1.8-fold (95% CI: 1.3–2.3; P = 0.0009), 2.6-fold (95% CI: 2.0–3.3; P < 0.0001) and 4.3-fold (95% CI: 2.6–6.0; P = 0.0016) for mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside, respectively. In vitro, none of the immunosuppressive drugs tested inhibited mycophenolic acid metabolism.
Conclusion
Patients taking mycophenolate mofetil and sirolimus experience a higher exposure to mycophenolic acid and a lower exposure to mycophenolic acid metabolites than those being treated with mycophenolate mofetil and ciclosporin. This interaction is probably not caused by inhibition of mycophenolic acid glucuronidation or glycosylation, but is more likely to be due to the influence of ciclosporin on the excretion of mycophenolic acid metabolites into bile.
Keywords: mycophenolate mofetil, sirolimus, kidney transplantation, metabolism
Introduction
Mycophenolate mofetil is an immunosuppressive agent used in combination therapy for the prevention or treatment of acute rejection after solid organ transplantation. Mycophenolic acid, the active metabolite of mycophenolate mofetil, acts by inhibiting the de novo pathway of purine biosynthesis. In kidney transplant recipients, mycophenolate mofetil is currently administered at a fixed dose of 1 g twice daily, but several studies have demonstrated a marked relationship between mycophenolic acid exposure (AUC0–12 h or trough concentration) and the incidence of acute rejection [1–3]. Thus it is now recommended that plasma mycophenolic acid concentrations should be monitored with the aim of achieving adequate immunosuppression with limited adverse effects [4].
Mycophenolic acid is mainly converted to one active metabolite (mycophenolic-acid-acyl-glucuronide) and two inactive metabolites: mycophenolic-acid-phenyl-glucuronide and mycophenolic-acid-phenyl-glucoside [5]. The plasma concentrations of mycophenolic-acid-phenyl-glucuronide largely exceed those of mycophenolic acid and it competes with the latter for the cationic binding sites of plasma albumin. In addition, mycophenolic-acid-phenyl-glucuronide is partly excreted into the bile and contributes to the enterohepatic circulation of mycophenolic acid after deconjugation in the small intestine [6]. The minor metabolite mycophenolic-acid-acyl-glucuronide inhibits lymphocyte proliferation in vitro [7] and has a potentially pro-inflammatory effect through induction of cytokine release and cytokine mRNA expression in human mononuclear leucocytes. Such an action could contribute to the digestive and haematological adverse effects of mycophenolate mofetil [8]. A prospective study in adult renal allograft recipients showed that patients who developed anaemia had a higher mycophenolic-acid-acyl-glucuronide/mycophenolic acid trough plasma concentration ratio [9].
Several studies have reported a lower exposure to mycophenolic acid in patients receiving mycophenolate mofetil in combination with ciclosporin than in those receiving mycophenolate mofetil and tacrolimus [10, 11] or mycophenolate mofetil alone [12]. Most studies indirectly support the hypothesis that ciclosporin decreases mycophenolic acid enterohepatic cycling through inhibition of mycophenolic-acid-phenyl-glucuronide excretion into the bile. In contrast to tacrolimus, ciclosporin is an inhibitor of the multidrug resistance-associated protein 2 (MRP2) involved in the biliary excretion of mycophenolic-acid-phenyl-glucuronide [13]. However, there are no data regarding biliary excretion and plasma concentration of the active and presumably toxic metabolite mycophenolic-acid-acyl-glucuronide in the presence or absence of ciclosporin. Tacrolimus was reported to be an inhibitor of mycophenolic-acid-phenyl-glucuronidation [14] in vitro, but further studies have not confirmed this observation [15, 16].
Recently, sirolimus was introduced into kidney transplantation as an alternative to calcineurin inhibitors [17, 18], owing to its distinctive mechanism of action and absence of nephrotoxicity [19]. There are no data comparing the pharmacokinetics of mycophenolic acid and its metabolites during sirolimus or ciclosporin therapy, and data evaluating the effect of sirolimus on mycophenolic-acid-phenyl-glucuronidation, and the effects of immunosuppressive drugs on the formation of mycophenolic acid-acyl-glucuronide and mycophenolic acid-glycosides are also lacking.
The objectives of this study were to compare the concentration-time profiles of mycophenolic acid and mycophenolic acid metabolites between patients receiving mycophenolate mofetil and either ciclosporin or sirolimus, and to investigate the effects of ciclosporin, sirolimus, tacrolimus and everolimus on mycophenolic acid phase II metabolism in vitro.
Methods
Patients
Thirty-one kidney transplant recipients, participating in two multicentre clinical trials were studied. Both trials were approved by the regional ethics committee of Limousin and written informed consent was obtained from each patient. Nineteen received mycophenolate mofetil in combination with ciclosporin and 12 mycophenolate mofetil in combination with sirolimus. Mycophenolate mofetil was administered at an initial fixed dose of 1 g twice daily, which was decreased if necessary based on clinical criteria. Ciclosporin was administered twice daily and sirolimus once daily. The morning dose of sirolimus or ciclosporin was given at the same time as that of mycophenolate mofetil. Dosing of sirolimus and ciclosporin was based on whole-blood concentration, with the targets being trough values between 0.010 and 0.015 mg L−1 for sirolimus, and between 0.15 and 0.20 mg L−1 for ciclosporin. The patients had a standardized breakfast 30 min after the morning dose and lunch 4–5 h later. All patients received prednisolone orally.
Sampling protocol
Blood samples were collected from each patient at one month post-transplant in EDTA-tubes before (C0) and at 20, 30 (patients under sirolimus) or 40 (patients under ciclosporin), 60, 90, 120 min and 3, 4, 6 and 9 h after the dose of mycophenolate mofetil. Plasma was separated by centrifugation, acidified by addition of 10 µL mL−1 phosphoric acid to prevent mycophenolic-acid-acyl-glucuronide degradation [20], and stored at −20 °C until analysis.
Chemicals, reagents and human microsomes
Mycophenolic acid, UDP-glucuronic acid trisodium salt (UDPGA), UDP-glucose, niflumic acid and triton X-100 were purchased from Sigma-Aldrich (St-Louis, MO, USA) and mycophenolic-acid-phenyl-glucuronide from Analytical Services International Ltd (London, UK). Everolimus and ciclosporin were obtained from Novartis Pharma AG (Basel, Switzerland), sirolimus from Wyeth Ayerst (Pearl River, NY, USA), and tacrolimus from Fujisawa Pharmaceuticals (Osaka, Japan). All other chemicals and reagents were of analytical reagent grade.
Human liver microsomes pooled from 29 donors (16 males and 13 females) including 26 Caucasian, 1 Hispanic and 2 Asian people were purchased from BD GENTEST (Woburn, MA, USA). The mean age of donors was 55 ± 15. The preparation was formulated to represent the profile of enzyme activities of the population at large. Liver samples were obtained after donors had given their informed consent, in accordance with the Uniform Anatomical Gift Act.
Microsomal incubations
Mycophenolic acid was incubated with human liver microsomes in the presence or absence (control) of tacrolimus, sirolimus, everolimus (0.01, 0.1, 1 mg L−1) or ciclosporin (0.1, 1, 10 mg L−1). As recommended for in vitro metabolic inhibition studies [21], the mycophenolic acid concentration (0.1 mm) was chosen to be lower than the Michaelis constant (Km) for mycophenolic acid-glucuronidation previously determined in our laboratory [22]. Inhibition tests with the immunosuppressive drugs were performed at three concentrations, close to and above their respective therapeutic blood concentrations to try and detect even minor inhibitory effects. The effect of 100 µm niflumic acid was also investigated and served as a positive control of mycophenolic-acid-phenyl-glucuronidation inhibition [16, 23]. Incubations were performed following a method recently developed in our laboratory [22], with minor modifications. Briefly, incubation mixtures (200 µL) consisted of: 0.1 mg microsomal protein; 10 mm MgCl2; 0.1 m, pH 7.4 TRIS-HCl buffer; 2 mm UDPGA or UDP-glucose; 0.1 mm mycophenolic acid; and tacrolimus, sirolimus, everolimus or ciclosporin in acetonitrile/water (50/50, v/v). Control incubations were spiked with the same amount of acetonitrile (1.25% v/v). Microsomes were detergent-activated by preincubation with Triton X-100 during 30 min on ice, with a detergent-to-microsomal protein ratio of 0.4 (w/w). Mycophenolic acid, potential inhibitors and microsomes were preincubated at 37 °C for 3 min before starting the reaction by addition of the cosubstrate. After 30 min of incubation at 37 °C, the reaction was stopped by adding 20 µL perchloric acid (24%, v/v). After centrifugation, the supernatant was stored at −20 °C until analysis.
LC-MS/MS determination of mycophenolic acid and metabolites
In renal transplant patients, mycophenolic acid and mycophenolic-acid-phenyl-glucuronide were determined using a previously described and fully validated LC-MS/MS method [24], whose limit of quantification is 0.1 mg L−1 for mycophenolic acid and 1 mg L−1 for mycophenolic-acid-phenyl-glucuronide. The linearity was verified up to 30 mg L−1 for mycophenolic acid and 300 mg L−1 for mycophenolic-acid-phenyl-glucuronide (r = 0.999). The within-day CV% was less than 10% and the between-day CV% less than 15% over the linearity range. mycophenolic-acid-acyl-glucuronide and Mycophenolic-acid-phenyl-glucoside were determined using a modification of this procedure, as previously described [22]. Briefly, mycophenolic - acid - acyl -glucuronide and mycophenolic-acid-phenyl-glucoside concentrations were estimated as mycophenolic-acid-phenyl-glucuronide molar equivalents with respect to the mycophenolic-acid-phenyl-glucuronide calibration curve, due to the lack of pure standards for mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside. The limit of quantification was 0.010 mg mL−1 as mycophenolic - acid - phenyl -glucuronide equivalent. Linearity was verified up to 1.5 mg L−1 (r = 0.999). The between-day precision CV% and mean relative error were less than 10% over the linearity range. Determination of mycophenolic acid metabolites in microsomal incubations was performed following the same method except that calibrators were prepared in incubation buffer.
Data analysis
Areas under the concentration-time curves (AUC0–9 h) for mycophenolic acid and metabolites were estimated using the trapezoidal rule. Pharmacokinetic parameters obtained in the two groups were compared using the nonparametric Mann–Whitney U-test. The sex ratio, dose distribution and numbers of patients with mycophenolic acid AUC0–12 h below, within or above the target range in the two groups were compared using the chi-square test. P-values = 0.05 were considered statistically significant.
Results
Renal function, bodyweight and prednisolone dose were not significantly different between the two patient groups (Table 1). The initial 2 g d−1 dose of mycophenolate mofetil was maintained until day 30 post-transplantation except for three patients cotreated with sirolimus and four cotreated with ciclosporin, who experienced adverse effects and for whom the dose was decreased within 3 days after the visit at day 14. Therefore at month 1, all patients could be regarded as at steady-state with respect to mycophenolic acid. The distribution of the dose at 1 month post-transplantation did not differ significantly between the two groups (Table 2; P = 0.8088).
Table 1.
Patient demographic characteristics, renal function and prednisolone dose
| Sirolimus group (n = 12) | Ciclosporin group (n = 19) | P | |
|---|---|---|---|
| Age (years) | 58 (20–68) | 46 (20–66) | 0.1048 |
| Sex ratio (M:F) | 7 : 5 | 10 : 9 | 0.0970 |
| Weight (kg) | 59 (41–90) | 64 (43–87) | 0.5980 |
| Creatinine (µmol L−1) | 131 (65–339) | 124 (65–185) | 0.6265 |
| Prednisolone dose (mg day−1) | 25 (15–35) | 20 (12.5–45) | 0.1443 |
Table 2.
Distribution of the daily dose of mycophenolate mofetil at 30 days post-transplant in the two groups
| Number (%) of patients | ||
|---|---|---|
| Mycophenolate mofetil (g day−1) | Sirolimus group (n = 12) | Ciclosporin group (n = 19) |
| 2.0 | 9 (75) | 15 (79) |
| 1.5 | 2 (17) | 2 (11) |
| 1.0 | 0 (0) | 1 (5) |
| 0.5 | 1 (8) | 1 (5) |
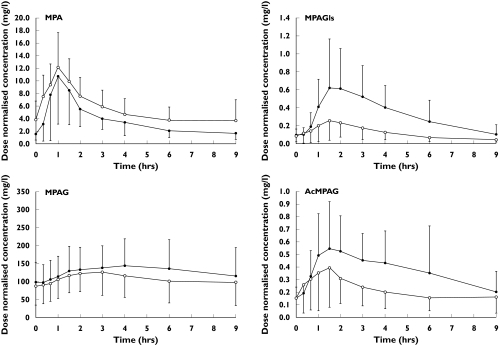
On average, mycophenolic acid concentrations at each sampling time were higher in patients receiving sirolimus than in those receiving ciclosporin (Figure 1), resulting in a mean AUC0–9 h of 44.9 mg h−1 L−1 (95% CI: 34.7–55.1) vs. 30.5 mg h−1 L−1 (95% CI: 25.4–35.6) (P = 0.0084). The corresponding mycophenolic acid AUC0–9 h ratio/dose was 1.5 fold higher in the former (95% CI: 1.1–1.9; P = 0.0066). Mycophenolic acid dose-normalized trough concentrations (C0/dose) were also significantly higher in the sirolimus group, whereas dose-normalized mycophenolic acid maximum concentrations (Cmax/dose) did not differ significantly between the treatments (Table 3).
Figure 1.
Mean (±SD) dose normalized plasma mycophenolic acid, mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-phenyl-glucoside and mycophenolic-acid-acyl-glucuronide concentration-time profiles in patients receiving mycophenolate mofetil in combination with ciclosporin (full circles) or with sirolimus (open circles)
Table 3.
Mean values(95% CI) and differences between means(95% CI) for the dose-normalized exposure indices of mycophenolic acid and metabolites at 30 days post-transplant
| Sirolimus group | Ciclosporin group | Differences | P | |
|---|---|---|---|---|
| Mycophenolic acid | ||||
| C0 (mg L−1) dose−1 | 3.8 (2.1; 5.5) | 1.5 (1.1; 1.9) | 2.3 (0.8; 3.8) | 0.0141 |
| Cmax (mg L−1) dose−1 | 12.8 (10.0; 15.7) | 11.7 (8.1; 15.2) | 1.3 (−3.9; 6.4) | 0.2710 |
| Tmax (h) | 0.9 (0.7; 1.2) | 1.6 (0.7; 2.5) | 0.7 (−0.5; 1.8) | 0.2237 |
| AUC0–9h (mg h−1 L−1) dose−1 | 51.1 (39.8; 62.3) | 34.0 (28.1; 39.9) | 17.1 (5.0; 29.2) | 0.0066 |
| Mycophenolic-acid-phenyl-glucuronide | ||||
| C0 (mg L−1) dose−1 | 88.2 (57.8; 118.5) | 101.7 (78.2; 125.2) | 13.5 (−26.3; 53.4) | 0.4531 |
| Cmax (mg L−1) dose−1 | 137.5 (102.6; 172.3) | 164.5 (130.6; 198.3) | 27.0 (−26.1; 80.1) | 0.2478 |
| Tmax (h) | 2.1 (1.5; 2.8) | 3.0 (2.3; 3.7) | 0.9 (−0.2; 2.0) | 0.0964 |
| AUC0-9h (mg h−1 L−1) dose−1 | 980.0 (689.5; 1270.5) | 1200.4 (933.8; 1466.9) | 220.4 (−204.4; 645.7) | 0.1679 |
| Mycophenolic-acid-acyl-glucuronide | ||||
| C0 (mg L−1) dose−1 | 0.2 (0.1; 0.2) | 0.2 (0.1; 0.2) | 0.0 (−0.1; 0.1) | 0.3010 |
| Cmax (mg L−1) dose−1 | 0.4 (0.3; 0.6) | 0.7 (0.5; 0.9) | 0.3 (−0.1; 0.6) | 0.0257 |
| Tmax (h) | 1.7 (1.2; 2.2) | 2.2 (1.6; 2.8) | 0.5 (−0.4; 1.4) | 0.2237 |
| AUC0–9h (mg h−1 L−1) dose−1 | 2.0 (1.2; 2.7) | 3.3 (2.5; 4.2) | 1.4 (0.1; 2.7) | 0.0167 |
| Mycophenolic-acid-phenyl-glucoside | ||||
| C0 (mg L−1) dose−1 | 0.1 (0.0; 0.1) | 0.1 (0.1; 0.1) | 0.0 (0.0; 0.1) | 0.8235 |
| Cmax (mg L−1) dose−1 | 0.3 (0.1; 0.4) | 0.7 (0.5; 1.0) | 0.5 (0.1; 0.8) | 0.0024 |
| Tmax (h) | 1.7 (1.5; 1.9) | 2.2 (1.7; 2.6) | 0.5 (−0.1; 1.1) | 0.0426 |
| AUC0–9h (mg h−1 L−1) dose−1 | 1.1 (0.; 1.5) | 3.0 (2.1; 3.8) | 1.9 (0.7; 3.0) | 0.0018 |
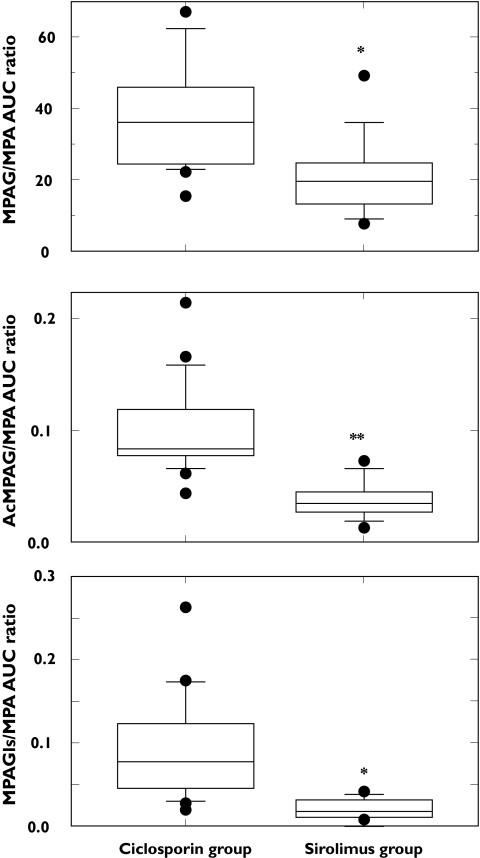
Patients with mycophenolate mofetil and ciclosporin experienced a higher exposure to mycophenolic acid metabolites (mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-phenyl-glucoside, mycophenolic-acid-acyl-glucuronide) than those treated with mycophenolate mofetil and sirolimus (Figure 1, Table 3). Consequently, the metabolite/mycophenolic acid AUC0–9 h ratios were significantly higher in patients cotreated with ciclosporin than with sirolimus, with a difference of 1.8-fold (95% CI: 1.3–2.3; P = 0.0009), 2.6-fold (95% CI: 2.0–3.3; P < 0.0001) and 4.3-fold (95% CI: 2.6–6.0; P = 0.0016) for mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside, respectively (Figure 2).
Figure 2.
The AUC0–9 h metabolite/AUC0–9 h mycophenolic acid ratios in patients receiving mycophenolate mofetil and sirolimus or ciclosporin. *P < 0.01; **P < 0.0001 ciclosporin vs. sirolimus
To evaluate mycophenolic acid exposure between doses in the two groups, mycophenolic acid AUC0–12 h was estimated assuming that the concentration at 12 h postdose was equal to C0. The distribution of mycophenolic acid AUC0–12 h with regard to the target range (30–60 mg h−1 L−1) was significantly different between the two groups (P = 0.0434). Thus, in the ciclosporin group (n = 19), seven patients (37%) were below and only one patient was above this target range. In the sirolimus group (n = 12), three patients (25%) were below and five patients (42%) above this target range, including two patients with values higher than 90 mg h−1 L−1.
Human liver microsomes produced mycophenolic-acid-phenyl-glucuronide and mycophenolic-acid-acyl-glucuronide when incubated with UDP-glucuronic acid and mycophenolic-acid-phenyl-glucoside when incubated with UDP-glucose. The mean mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside formation rates in the absence of potential inhibitors (n = 2 experiments) were 1220, 15 and 448 pmole mg−1 min−1, respectively. None of the immunosuppressants tested (sirolimus, ciclosporin, tacrolimus and everolimus) affected the rate of mycophenolic-acid-phenyl-glucuronide, mycophenolic-acid-acyl-glucuronide, or mycophenolic-acid-phenyl-glucoside production, even at high concentrations. In contrast, niflumic acid, a known inhibitor of mycophenolic acid phenyl glucuronation [16, 23], caused a 98% inhibition of the pathway and a 25% increase in mycophenolic-acid-acyl-glucuronide production.
Discussion
In the present study, patients receiving mycophenolate mofetil in combination with sirolimus were significantly more exposed to mycophenolic acid than those receiving the drug with ciclosporin. This finding emphasizes the need for mycophenolate mofetil concentrations to be monitored, and for guidelines when switching patients from ciclosporin to sirolimus. The same dose of mycophenolate mofetil would lead on average to a 50% increase in the AUC of mycophenolic acid in patients also receiving sirolimus compared with ciclosporin though with a large inter-individual variability. A similar increased exposure to mycophenolic acid has already been observed in patients receiving either tacrolimus and mycophenolate mofetil [10, 11] or mycophenolate mofetil alone [12], compared with those receiving mycophenolate mofetil and ciclosporin.
We have also demonstrated a greater exposure to mycophenolic acid phase II metabolites in patients cotreated with ciclosporin, which was significant for the AUC0–9 h and Cmax for mycophenolic-acid-acyl-glucuronide and mycophenolic-acid-phenyl-glucoside, but not mycophenolic-acid-phenyl-glucuronide. This comparison was performed at 1 month post-transplantation with the initial dose being maintained in all patients, except for four who were cotreated with ciclosporin and three with sirolimus and whose dose was decreased several days before blood sampling. Thus, all patients were presumed to be at steady-state with respect to mycophenolic acid and metabolites when studied.
Since inhibition of mycophenolic acid phase II metabolism by tacrolimus or sirolimus was a possible explanation for the above findings, the inhibitory potential of these drugs on mycophenolic acid phase II metabolism was investigated in human liver microsomes. Reports on the effect of tacrolimus on mycophenolic-acid-phenyl-glucuronidation, are contradictory. Zucker et al. [14] found that tacrolimus and ciclosporin were competitive inhibitors of mycophenolic-acid-phenyl-glucuronidation. The Ki values reported (0.03 and 2.1 µm for tacrolimus and ciclosporin, respectively) were much smaller than the Km for mycophenolic acid glucuronidation (between 0.18 and 0.35 mm) [22, 25, 26] which would indicate a greater affinity of the glucuronyl transferase for tacrolimus and ciclosporin than for mycophenolic acid. In contrast, Vietri et al. [15] and Balogh et al. [16] found that tacrolimus did not inhibit mycophenolic acid-glucuronidation. Similarly, no inhibition of mycophenolic acid glucuronidation or glycosylation by tacrolimus or ciclosporin was found in the present study, even at concentrations (1.24 µm and 8.31 µm, respectively) much higher than their respective therapeutic blood concentrations and their previously reported Ki values [14]. Like ciclosporin or tacrolimus, sirolimus and everolimus did not affect mycophenolic acid glucuronidation or glycosylation in vitro.
The increase in the mycophenolic acid metabolic ratios as well as the trend to a higher AUC for its metabolites are consistent with the hypothesis that ciclosporin interacts with the enterohepatic cycling of mycophenolic acid. Ciclosporin presumably decreases mycophenolic-acid-phenyl-glucuronide biliary excretion by inhibiting the multidrug resistance-associated protein 2 (MRP2), as suggested by data from mutant rats not expressing MRP2 [13]. Consequently, less mycophenolic-acid-phenyl-glucuronide is subject to deconjugation by the intestinal flora, resulting in a decrease in the re-circulation of mycophenolic acid. In addition, the lack of a significant difference in the Cmax of mycophenolic acid suggests that the initial absorption of mycophenolate mofetil remains unchanged. Given its high concentrations, mycophenolic-acid-phenyl-glucuronide is clearly the main metabolite contributing to the re-circulation of mycophenolic acid. We have also demonstrated that the active and presumably toxic metabolite mycophenolic-acid-acyl-glucuronide is subject to the same interaction with ciclosporin, which apparently decreases its biliary excretion. Increased exposure to mycophenolic-acid-phenyl-glucuronide (based on AUC/dose and Cmax/dose) was less marked than for mycophenolic-acid-acyl-glucuronide. Mycophenolic-acid-phenyl-glucuronide concentration time profiles, but not those for mycophenolic-acid-phenyl-glucuronide showed a flattened shape with a late Tmax suggesting that its formation is delayed. Several in vitro studies have demonstrated that the kidney produces a large amount of mycophenolic-acid-phenyl-glucuronide [22, 25, 26], and that renal elimination is the main disposition pathway for this metabolite although about 40% is excreted in the bile [6]. Thus, despite decreasing mycophenolic acid re-circulation, inhibition of biliary excretion of this metabolite seems to have a moderate effect on its overall elimination.
Acyl-glucuronides are reactive metabolites that undergo intramolecular acyl-migration to isomeric conjugates and react chemically with plasma proteins, tissue proteins or nucleic acids [27]. These properties could contribute to drug toxicity. In the case of mycophenolic acid, Wieland et al. demonstrated in vitro that mycophenolic-acid-acyl-glucuronide can induce release of cytokines [8], which could contribute to the intestinal adverse effects of the drug (i.e. abdominal pain, diarrhoea). In the intestine, mycophenolic-acid-acyl-glucuronide rearranges to iso-mycophenolic-acid-acyl-glucuronide, which is relatively stable to bacterial beta-glucuronidase, as demonstrated in vitro [5] and is likely to reach the colon. In the present study, ciclosporin cotreatment with mycophenolate mofetil led to an increase in systemic exposure to mycophenolic-acid-acyl-glucuronide, and possibly to a reduction of its excretion in bile and of its intestinal toxicity, though further studies are required. Ciclosporin has been used to decrease the digestive toxicity of the anticancer agent irinotecan by impairing the biliary excretion of its active metabolite SN-38 [28].
In conclusion, ciclosporin interacts with mycophenolic acid pharmacokinetics in vivo. This interaction could be due to the inhibition of the MRP2-dependent biliary excretion of mycophenolic acid metabolites. Inter-individual variation in the enterohepatic cycling of mycophenolic acid and its interaction with drugs should be investigated further, as should the role of this process in adverse effects caused by mycophenolate mofetil.
Acknowledgments
This study was funded by the Limousin Regional Council and Limoges University hospital.
References
- 1.van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hene RJ, Verpooten GA, Navarro MT, Hale MD, Nicholls AJ. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–6. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 2.Pillans PI, Rigby RJ, Kubler P, Willis C, Salm P, Tett SE, Taylor PJ. A retrospective analysis of mycophenolic acid and ciclosporin concentrations with acute rejection in renal transplant recipients. Clin Biochem. 2001;34:77–81. doi: 10.1016/s0009-9120(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 3.Kiberd BA, Lawen J, Fraser AD, Keough-Ryan T, Belitsky P. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant. 2004;4:1079–83. doi: 10.1111/j.1600-6143.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit. 2001;23:305–15. doi: 10.1097/00007691-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schutz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol. 1999;126:1075–82. doi: 10.1038/sj.bjp.0702399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34:429–55. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 7.Shipkova M, Wieland E, Schutz E, Wiese C, Niedmann PD, Oellerich M, Armstrong VW. The acyl glucuronide metabolite of mycophenolic acid inhibits the proliferation of human mononuclear leukocytes. Transplant Proc. 2001;33:1080–1. doi: 10.1016/s0041-1345(00)02424-6. [DOI] [PubMed] [Google Scholar]
- 8.Wieland E, Shipkova M, Schellhaas U, Schutz E, Niedmann PD, Armstrong VW, Oellerich M. Induction of cytokine release by the acyl glucuronide of mycophenolic acid: a link to side effects? Clin Biochem. 2000;33:107–13. doi: 10.1016/s0009-9120(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 9.Kuypers DR, Vanrenterghem Y, Squifflet JP, Mourad M, Abramowicz D, Oellerich M, Armstrong V, Shipkova M, Daems J. Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipients on low dose tacrolimus in combination with mycophenolate mofetil. Ther Drug Monit. 2003;25:609–22. doi: 10.1097/00007691-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Zucker K, Rosen A, Tsaroucha A, de Faria L, Roth D, Ciancio G, Esquenazi V, Burke G, Tzakis A, Miller J. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol. 1997;5:225–32. doi: 10.1016/s0966-3274(97)80042-1. [DOI] [PubMed] [Google Scholar]
- 11.Hubner GI, Eismann R, Sziegoleit W. Drug interaction between mycophenolate mofetil and tacrolimus detectable within therapeutic mycophenolic acid monitoring in renal transplant patients. Ther Drug Monit. 1999;21:536–9. doi: 10.1097/00007691-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Smak Gregoor P, van Gelder T, Hesse CJ, van der Mast BJ, van Besouw NM, Weimar W. Mycophenolic acid plasma concentrations in kidney allograft recipients with or without ciclosporin: a cross-sectional study. Nephrol Dial Transplant. 1999;14:706–8. doi: 10.1093/ndt/14.3.706. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Saitoh H, Tadano K, Takahashi Y, Hirano T. Ciclosporin A but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther. 2004;309:1029–35. doi: 10.1124/jpet.103.063073. [DOI] [PubMed] [Google Scholar]
- 14.Zucker K, Tsaroucha A, Olson L, Esquenazi V, Tzakis A, Miller J. Evidence that tacrolimus augments the bioavailability of mycophenolate mofetil through the inhibition of mycophenolic acid glucuronidation. Ther Drug Monit. 1999;21:35–43. doi: 10.1097/00007691-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Vietri M, Pietrabissa A, Mosca F, Pacifici GM. Mycophenolic acid glucuronidation and its inhibition by non-steroidal anti-inflammatory drugs in human liver and kidney. Eur J Clin Pharmacol. 2000;56:659–64. doi: 10.1007/s002280000227. [DOI] [PubMed] [Google Scholar]
- 16.Balogh A, Merkel U, Muller D. Can xipamide or tacrolimus inhibit the glucuronidation of mycophenolic acid in rat liver slices? Exp Toxicol Pathol. 2003;54:375–9. doi: 10.1078/0940-2993-00273. [DOI] [PubMed] [Google Scholar]
- 17.Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, Touraine JL, Claesson K, Campistol JM, Durand D, Wramner L, Brattstrom C, Charpentier B. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with ciclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67:1036–42. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, Savas K, Cook DJ, Novick AC. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus ciclosporine. Transplantation. 2002;74:1070–6. doi: 10.1097/00007890-200210270-00002. [DOI] [PubMed] [Google Scholar]
- 19.Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit. 2001;23:559–86. doi: 10.1097/00007691-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Shipkova M, Schutz E, Armstrong VW, Niedmann PD, Oellerich M, Wieland E. Determination of the acyl glucuronide metabolite of mycophenolic acid in human plasma by HPLC and Emit. Clin Chem. 2000;46:365–72. [PubMed] [Google Scholar]
- 21.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug–drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–32. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 22.Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the udp-glucuronosyltransferase isoforms involved in mycophenolic Acid phase II metabolism. Drug Metab Dispos. 2005;33:139–46. doi: 10.1124/dmd.104.001651. [DOI] [PubMed] [Google Scholar]
- 23.Vietri M, Pietrabissa A, Mosca F, Pacifici GM. Inhibition of mycophenolic acid glucuronidation by niflumic acid in human liver microsomes. Eur J Clin Pharmacol. 2002;58:93–7. doi: 10.1007/s00228-001-0407-4. [DOI] [PubMed] [Google Scholar]
- 24.Premaud A, Rousseau A, Le Meur Y, Lachatre G, Marquet P. Comparison of liquid chromatography-tandem mass spectrometry with a commercial enzyme-multiplied immunoassay for the determination of plasma MPA in renal transplant recipients and consequences for therapeutic drug monitoring. Ther Drug Monit. 2004;26:609–19. doi: 10.1097/00007691-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Bowalgaha K, Miners JO. The glucuronidation of mycophenolic acid by human liver, kidney and jejunum microsomes. Br J Clin Pharmacol. 2001;52:605–9. doi: 10.1046/j.0306-5251.2001.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipkova M, Strassburg CP, Braun F, Streit F, Grone HJ, Armstrong VW, Tukey RH, Oellerich M, Wieland E. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol. 2001;132:1027–34. doi: 10.1038/sj.bjp.0703898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shipkova M, Armstrong VW, Oellerich M, Wieland E. Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther Drug Monit. 2003;25:1–16. doi: 10.1097/00007691-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Innocenti F, Undevia SD, Ramirez J, Mani S, Schilsky RL, Vogelzang NJ, Prado M, Ratain MJ. A phase I trial of pharmacologic modulation of irinotecan with ciclosporine and phenobarbital. Clin Pharmacol Ther. 2004;76:490–502. doi: 10.1016/j.clpt.2004.07.016. [DOI] [PubMed] [Google Scholar]