Abstract
Aims
Thiazolidinediones (TZDs) not only enhance cellular glucose transport but are reported to have potent anti-inflammatory effects. These effects may play an important role in the insulin sensitizing mechanism, and possibly precede the effects on parameters of glucoregulation. We sought to investigate whether these anti-inflammatory effects could yield early responding biomarkers for TZD action in Type 2 diabetes mellitus (T2DM) patients and healthy volunteers (HV) to expedite early clinical development of novel compounds.
Methods
We investigated the timing of treatment effects on several proinflammatory cytokines and markers of inflammation in comparison with effects on typical measures of glucoregulation in T2DM patients and HV receiving rosiglitazone 4 mg or placebo twice daily for 6 weeks.
Results
We found a significant reduction in interleukin (IL)-6 [−39.4%, confidence interval (CI) −60.0, −8.2] and white blood cell count (−18.4%, CI −30.2, −4.5) after 4 weeks of treatment in the T2DM group. These anti-inflammatory effects did not precede the effects on typical parameters of glucoregulation in the T2DM group and there was no significant anti-inflammatory response in the HV group.
Conclusion
We could not identify biomarkers that precede the effects of rosiglitazone on parameters of glucoregulation in T2DM or that have a significant response in HV. However, the IL-6 response observed in this study indicates a potential role for this cytokine as complementary biomarker in clinical ‘proof of concept’ studies with novel TZDs.
Keywords: biomakers, IL-6, inflammation, rosiglitazone, thiazolidinediones, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a heterogeneous disorder characterized by impaired insulin secretion on a background of insulin resistance and is associated with a marked increase in cardiovascular disease (CVD) risk [1, 2].
A growing body of evidence suggests that chronic subclinical inflammation may play an important role in the pathogenesis of insulin resistance, T2DM and CVD [3–13]. This is illustrated by recent studies which showed that several markers of inflammation, in particular plasma levels of high-sensitivity C-reactive protein (HS-CRP) and interleukin (IL)-6, are independent predictors of T2DM and CVD risk [6, 8, 10]. Moreover, elevated plasma concentrations of proinflammatory cytokines secreted by adipose tissue (‘adipokines’) – tumour necrosis factor (TNF)-α, IL-1β and IL-6 – appear to be associated with insulin resistance and T2DM [14–16].
Thiazolidinediones (TZDs), such as rosiglitazone (RSG), are a class of oral antidiabetic drugs that primarily act as insulin sensitizers, ameliorating insulin resistance with associated improvements in glycaemic control [17, 18]. These drugs are agonists of the peroxisome proliferator activated receptor gamma (PPARγ), which is a nuclear transcription factor controlling the expression of its target genes in various tissues [19, 20]. Importantly, results from in vitro [21, 22], preclinical [23] and recent clininal [24–28] studies suggest that TZDs not only exert their actions by enhancing cellular glucose transport but also possess distinct anti-inflammatory properties. These properties may play an important role in the insulin sensitizing mechanisms and hold the promise of reduced CVD risk. In addition, Xiang et al. recently showed the potentially important role of troglitazone, and possibly of other TZDs, in the prevention of T2DM in a population at risk. Furthermore, they demonstrated that amelioration of insulin resistance can preserve pancreatic β-cell function and stabilize glycaemia at the time T2DM develops [29]. These properties may potentially turn the TZDs into the first class of disease-modifying drugs for the treatment of T2DM. However, the limited glucose-lowering efficacy [about 20% maximum decrease in fasting plasma glucose (FPG) at the highest approved dose] and side-effect profile (mainly weight gain and fluid retention) limit the use of currently available TZDs. Therefore, novel TZD compounds with enhanced glucose-lowering efficacy and potentially less side-effects were created.
To facilitate a more efficient early clinical development programme (‘proof of concept’) for these compounds, there is a high demand for a large array of early responding in vivo biomarkers, that are easily assessable in small groups of subjects and are more closely related to the insulin sensitizing mechanism. The traditional FPG concentration is a fairly nonspecific biomarker, and generally takes about 6–12 weeks to reach its lowest concentration for selected TZDs [30, 31]. In contrast, recent evidence suggests that the potent anti-inflammatory effects of TZDs may play an important role in the insulin sensitizing mechanism [27], and might therefore precede the effects on the FPG. An additional advantage may be that treatment effects, as previously reported for adiponectin [32], may also be observed in healthy volunteers. Hence, studying novel TZD compounds in relatively small groups of healthy volunteers using a broad array of early responding, mechanistically more closely related biomarkers, could circumvent the need for longer and more complicated studies in T2DM patients.
The primary objective of this study was to investigate the timing of treatment effects on several proinflammatory cytokines (IL-6, IL-1β, TNF-α) and markers of inflammation [HS-CRP and white blood cell count (WBC)] in comparison with effects on typical measures of glycaemic control in a small group of T2DM patients receiving RSG 4 mg twice daily for 6 weeks. As secondary objective we sought to investigate whether any of the effect parameters would respond in healthy volunteers (HV).
In addition, we investigated the effects of RSG on parameters of lipid metabolism, haemodynamics and haemodilution in both study groups, as well as the differences in effect parameters between the T2DM and HV groups at baseline.
Methods
Patients
Eight male and eight female T2DM patients uncontrolled by diet alone, aged between 40 and 75 years, with a body mass index (BMI) >25 kg m−2, increased FPG concentrations (>7.0 mm), and C-peptide >0.17 nmol l−1 were to be included. Patients were excluded if they had a significant medical history or current symptoms of clinically relevant conditions, or had used any nonsteroidal anti-inflammatory drug, thiazolidinedione or insulin preparation within 2 weeks of the expected study start.
In addition, eight male and eight female healthy subjects (as determined by medical history, physical examination and routine laboratory tests), aged between 18 and 45 years, were to be included.
Study design
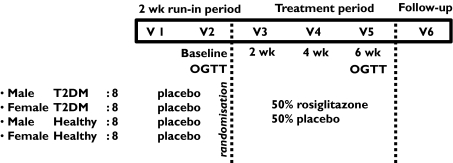
This was a randomized, double-blind, placebo-controlled, multiple oral dose study. The subjects were studied in a 8-week period consisting of six visits (Figure 1). Within 1 week after medical screening all subjects started with a 2-week single-blind placebo run-in period. At the end of the run-in period (baseline), subjects were randomly assigned to a 6-week treatment with capsules containing 4 mg RSG or matching placebo twice daily. Treatment allocation took place according to randomly permuted blocks and was stratified by gender and subject type (T2DM patient or HV).
Figure 1.
Study design. This figure gives an overview of the study populations, different treatment periods (placebo run-in and active treatment period) and double-blind, placebo-controlled, randomized design of the study. Blood samples for biomarker assessments were collected on visits 2 through 5. An oral glucose tolerance test (OGTT) was performed at visit 2 (baseline) and visit 5 (end of the active treatment period)
Blood samples for RSG concentrations and pharmacodynamic parameters were collected on all visits. At baseline three blood samples were collected; predose, 3.5 and 10 h postdose for RSG pharmacokinetic assessments. Throughout the study, blood and urine samples were collected for standard clinical (safety) laboratory measurements (including urine human chorionic gonadotropin test for female subjects). Moreover, frequent measurements of vital signs (heart rate and blood pressure) were performed. Finally, an oral glucose tolerance test (OGTT) was performed at baseline and at the end of the active treatment period.
The protocol for this study was approved by the Medical Ethical Committee of the Leiden University Medical Centre and performed according to the principles of International Conference on Harmonisation–Good Clinical Practice, the Helsinki Declaration and Dutch law, and all subjects gave their written informed consent. This study was part of a larger study in which comprehensive transcriptomic and metabolomic analyses were performed. The results of these analyses will be published separately.
Blood sampling
On each visit, an intravenous cannula was inserted in a forearm vein while the subject was in a supine position. Blood samples were collected after approximately 30 min of supine rest.
Cholesterol and triglycerides
Blood samples of 8.5 ml were collected in SST® Gel and Clot Activator tubes for measurements of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) (Friedewald formula). TC and TG concentrations were analysed on a fully automated Hitachi 747 apparatus. HDL-c was measured using a Hitachi 911 apparatus. LDL-c was estimated using the Friedewald equation [LDL-c (mmol l−1) = TC − (HDL-c − (TG/2.2)].
Glycosylated haemoglobin (HbA1c)
Blood samples were collected in plastic EDTA and plasma HbA1c concentrations were measured by high-performance liquid chromatography (Bio-Rad Laboratories, Richmond, CA, USA). The reference range was 4.5–6.5%.
Insulin, C-peptide and fructosamine
Blood samples were collected in 8.5-ml SST® Gel and Clot Activator tubes and were centrifuged within 45 min at 4 °C (10 min at 2000 g) and stored at −40 °C. Plasma insulin concentrations were measured by radioimmunoassay (RIA; Medgenix Diagnostics, Fleurus, Belgium) with a detection limit of 3 mU l−1 and an interassay coefficient of variance of 3.8–8.0%. Plasma C-peptide concentrations were measured with a RIA (Biolab, Brussels, Belgium). Plasma fructosamine concentrations were measured on a Hitachi gh (Hitachi, Tokyo, Japan) with a turbidimetric assay.
Free fatty acids (FFA)
Blood samples were collected in EDTA tubes, stored on ice-water, centrifuged within 30 min at 4 °C (10 min at 2000 g) and stored at −70 °C. FFAs were measured with an optimized enzymatic colorimetric assay (Roche Diagnostics, GmbH, Mannheim, Germany).
TNF-a, IL-6, IL-1β and HS-CRP
Blood samples were collected in EDTA tubes, centrifuged at 4 °C within 30 min (10 min at 2000 g) and stored at −80 °C. Plasma IL-1β, IL-6 and TNF-α were measured using high sensitivity ELISAs of R&D Systems (Abingdon, UK). Plasma HS-CRP concentrations were measured using a custom-made validated highly sensitive sandwich ELISA with coating and detecting polyclonal antibodies against human CRP (Dako, Glostrup, Denmark).
RSG bioanalysis
Blood samples (5 ml) were collected in sodium heparin tubes, immediately stored on ice-water, centrifuged at 4 °C within 30 min (10 min at 2000 g) and stored at −20 °C. The human plasma samples were analysed for RSG by a validated high-performance single quadrupole-liquid chromatographic mass spectrometric (LC-MS) procedure (MDS Pharma Services, St-Laurent (Montreal), Canada).
Glucose tolerance test
Blood samples of 2.5 ml were collected in SST® Double Gel and Clot Activator tubes, centrifuged at 4 °C within 45 min (10 min at 2000 g) and serum was stored at −40 °C. Glucose concentrations were measured in an automated assay.
Compliance monitoring
Study medication was delivered to the subjects in vials with automated recording of the time of vial opening [Aardex® electronic drug exposure monitor (eDEM™)]. Registered opening times and capsule counting were used for monitoring of subject compliance, and as input for modelling antidiabetic drug effects. In addition, RSG trough concentrations were measured to support compliance monitoring.
Statistical analysis
Included data
All subjects that dropped out before visit 4 (three subjects: one male and two female T2DM patients) were replaced with newly recruited subjects receiving the same treatment. Data of subjects dropping out after visit 2 (baseline) were included in the statistical analysis even when a subject was replaced. Consequently, data of subjects dropping out during the run-in period (one female T2DM patient) were not used in the analysis. Therefore, the statistical analysis at baseline was restricted to nine male and nine female T2DM patients and eight male and eight female HV (Tables 1a, 1b and 2). Furthermore, for the T2DM patient group, the exact number of subjects in each treatment group during the study (and thus the number of dropouts) is indicated in Table 4.
Table 1a.
Demographics and baseline characteristics. The demographics of both study populations as well as the baseline characteristics for the glycaemic control parameters and lipid profile as measured after the 2-week placebo run-in period. The data between parentheses represent the SD
| T2DM patients | Healthy volunteers | |||
|---|---|---|---|---|
| Parameter | Male | Female | Male | Female |
| Gender, N | 9 | 9 | 8 | 8 |
| Age (years) | 56.8 (10.53) | 54.0 (8.56) | 22.1 (4.79) | 24.4 (7.25) |
| Body mass index (kg m−2) | 28.8 (2.39) | 32.7 (4.61) | 24.0 (4.07) | 24.6 (5.07) |
| Waist/hip ratio | 1.01 (0.047) | 0.92 (0.056) | 0.85 (0.061) | 0.77 (0.072) |
| Disease duration (years) | 5 (2.1) | 2 (1.6) | NA | NA |
| Prior treatment: No medication, N | 0 | 0 | 8 | 8 |
| OAD monotherapy, N | 7 | 5 | 0 | 0 |
| OAD combination, N | 2 | 4 | 0 | 0 |
| Statin, N | 3 | 3 | 0 | 0 |
| Antihypertensive, N | 3 | 2 | 0 | 0 |
| Glucose (mmol l−1) | 11.8 (3.09) | 11.2 (4.96) | 4.7 (0.31) | 4.6 (0.63) |
| C-peptide (nmol l−1) | 1.1 (0.25) | 1.0 (0.37) | 0.6 (0.33) | 0.8 (0.38) |
| Insulin (mU l−1) | 11.7 (3.00) | 12.6 (6.67) | 10.4 (8.03) | 9.8 (4.89) |
| Fructosamine (mmol l−1) | 294 (50.5) | 278 (67.8) | 196 (11.5) | 192 (10.4) |
| HbA1c percentage (%) | 7.3 (0.84) | 6.9 (1.74) | 4.6 (0.32) | 4.7 (0.27) |
| AUE glucose (nmol l−1) after OGTT | 17.2 (3.643) | 15.7 (5.155) | 7.37 (1.116) | 7.21 (0.977) |
| AUE Insulin (mU l−1) after OGTT | 23.5 (9.831) | 33.7 (23.01) | 68.7 (56.33) | 58.5 (13.93) |
| Peak glucose (nmol l−1) after OGTT | 22.8 (4.222) | 21.4 (6.679) | 8.96 (2.168) | 8.71 (1.019) |
| Peak insulin (mU l−1) after OGTT | 38.1 (15.42) | 66.3 (44.24) | 106 (93.11) | 91.0 (24.62) |
| Cholesterol (mmol l−1) | 5.4 (0.62) | 5.4 (0.86) | 3.9 (0.54) | 4.1 (0.67) |
| Triglycerides (mmol l−1) | 3.4 (2.19) | 1.8 (0.72) | 1.3 (0.44) | 1.0 (0.28) |
| HDL-cholesterol (mmol l−1) | 1.05 (0.179) | 1.20 (0.135) | 1.18 (0.324) | 1.51 (0.417) |
| LDL-cholesterol | 2.8 (1.23) | 3.3 (0.87) | 2.1 (0.58) | 2.1 (0.89) |
| Free fatty acids (mmol l−1) | 0.31 (0.099) | 0.41 (0.083) | 0.27 (0.073) | 0.43 (0.128) |
OAD, oral antidiabetic drug; AUE, area under the effect curve; OGTT, oral glucose tolerance test.
NA, not applicable.
Table 1b.
Baseline characteristics (continued). The baseline characteristics of proinflammatory cytokines and inflammation markers as well as haemodynamic parameters as measured after the 2-week placebo run-in period. The data between parentheses represent the SD
| T2DM patients | Healthy volunteers | |||
|---|---|---|---|---|
| Parameter | Male | Female | Male | Female |
| White blood cell count (109 l−1) | 5.3 (1.06) | 5.5 (1.32) | 5.6 (1.01) | 4.8 (0.58) |
| High-sensitivity C-reactive protein (mg l−1) | 1.5 (0.80) | 3.2 (2.63) | 1.1 (2.23) | 1.7 (2.34) |
| IL-1β level (pg ml−1) | 0.19 (0.184) | 0.08 (0.047) | 0.08 (0.089) | 0.08 (0.075) |
| IL-6 level (pg ml−1) | 2.5 (1.58) | 2.7 (1.15) | 1.9 (1.87) | 1.1 (0.50) |
| Tumour necrosis factor-α (pg ml−1) | 1.7 (0.65) | 3.5 (3.94) | 3.4 (2.58) | 2.3 (1.66) |
| Systolic blood pressure (mmHg) | 129 (11.4) | 126 (24.0) | 119 (9.8) | 115 (5.9) |
| Diastolic blood pressure (mmHg) | 78 (5.5) | 75 (8.5) | 69 (8.1) | 70 (8.5) |
| Heart rate (bpm) | 66 (10.7) | 72 (10.8) | 69 (12.9) | 69 (12.0) |
Table 2.
Baseline comparisons between T2DM and HV groups. The results of the baseline comparisons (anova; ln transformed data) between the T2DM and HV groups for glycaemic control, lipid profile, inflammation and haemodynamic parameters
| Back transformed least square means | ||||
|---|---|---|---|---|
| Parameter | Diabetic (n = 18) | Healthy (n = 16) | Estimate of difference diabetic–healthy in % (95% CI) | P-value |
| Glucose (mmol l−1) | 10.871 | 4.596 | 136.5 (97.0, 184.0) | <0.0001 |
| C-peptide (nmol l−1) | 1.014 | 0.635 | 59.6 (23.5, 106.2) | 0.0008 |
| Insulin (mU l−1) | 11.367 | 8.936 | 27.2 (− 3.8, 68.3) | 0.0891 |
| Fructosamine (mmol l−1) | 280.58 | 193.84 | 44.7 (29.7, 61.6) | <0.0001 |
| HbA1c percentage (%) | 6.977 | 4.652 | 50.0 (35.3, 66.3) | <0.0001 |
| AUE insulin (mU l−1) after OGTT | 22.646 | 45.165 | −49.9 (− 64.9, −28.3) | 0.0004 |
| Peak insulin (mU l−1) after OGTT | 42.293 | 86.917 | −51.3 (− 68.0, −26.1) | 0.0014 |
| AUE glucose (nmol l−1) after OGTT | 15.566 | 7.064 | 120.4 (88.8, 157.2) | <0.0001 |
| Peak glucose (nmol l−1) after OGTT | 21.377 | 8.696 | 145.8 (107.2, 191.7) | <0.0001 |
| Cholesterol (mmol l−1) | 5.361 | 3.971 | 35.0 (22.1, 49.3) | <0.0001 |
| Triglycerides (mmol l−1) | 2.197 | 1.095 | 100.7 (42.4, 182.9) | 0.0002 |
| HDL-cholesterol (mmol l−1) | 1.110 | 1.306 | −15.0 (− 28.2, 0.5) | 0.0565 |
| LDL-cholesterol | 2.841 | 1.973 | 44.0 (6.4, 95.0) | 0.0200 |
| Free fatty acids (mmol l−1) | 0.345 | 0.327 | 5.3 (− 17.9, 35.0) | 0.6748 |
| White blood cell count (109 l−1) | 5.245 | 5.158 | 1.7 (− 11.8, 17.3) | 0.8115 |
| High-sensitivity C-reactive protein (mg l−1) | 1.625 | 0.572 | 184.2 (28.2, 529.8) | 0.0117 |
| IL-1β level (pg ml−1) | 0.088 | 0.055 | 59.7 (− 14.4, 197.9) | 0.1358 |
| IL-6 level (pg ml−1) | 2.354 | 1.203 | 95.7 (32.8, 188.4) | 0.0013 |
| Tumour necrosis factor-α (pg ml−1) | 1.897 | 2.202 | −13.9 (− 47.5, 41.3) | 0.5436 |
| Heart rate (bpm) | 68.833 | 68.750 | 0.1 (− 7.9, 8.1) | 0.9832 |
| Systolic blood pressure (mmHg) | 127.39 | 117.00 | 10.4 (0.3, 20.5) | 0.0446 |
| Diastolic blood pressure (mmHg | 76.889 | 69.500 | 7.4 (2.1, 12.7) | 0.0077 |
AUE, area under the curve; OGTT, oral glucose tolerance test.
Table 4.
Timing of rosiglitazone (RSG) treatment effects. The estimates of the average percentage change in effect parameters in the T2DM group per visit, for RSG vs. placebo with corresponding 95% confidence intervals and P-values. This analysis was restricted to parameters which were (nearly) significant for the overall RSG treatment
| Estimate of percentage change; 95% CI and P-value | ||||
|---|---|---|---|---|
| Parameter | Overall | 2 weeks placebo: n = 9 RSG: n = 8 | 4 weeks placebo: n = 6 RSG: n = 7 | 6 weeks placebo: n = 5 RSG: n = 6 |
| FPG | 16.2% (− 26.2, −4.8) | − 12.0% (− 22.9, 0.5) | − 18.8% (− 30.4, −5.2) | − 17.5% (− 27.5, −6.1) |
| P = 0.008 | P = 0.058 | P = 0.010 | P = 0.010 | |
| Fructosamine | 12.6% (− 20.7, −3.7) | − 6.3% (− 13.4, 1.4) | − 14.4% (− 23.3, −4.5) | − 16.8% (− 26.5, −5.9) |
| P = 0.009 | P = 0.101 | P = 0.007 | P = 0.005 | |
| Insulin | 27.7% (− 42.6, −8.8) | − 26.9% (− 45.3, −2.3) | − 33.5% (− 50.5, −10.5) | − 22.1% (− 44.4, 9.0) |
| P = 0.008 | P = 0.035 | P = 0.010 | P = 0.137 | |
| C-peptide | 19.5% (− 31.8, −4.9) | − 15.2% (− 31.0, −4.1) | − 24.3% (− 38.4, −7.0) | −18.6% (−37.0, 5.0) |
| P = 0.013 | P = 0.111 | P = 0.010 | P = 0.109 | |
| IL-6 | 31.8% (− 48.9, −9.1) | − 17.7% (− 44.7, 22.3) | − 39.4% (− 60.0, −8.2) | − 36.4% (− 54.9, −10.3) |
| P = 0.011 | P = 0.321 | P = 0.020 | P = 0.012 | |
| HS-CRP | 44.0% (− 69.2, 1.9) | − 31.6% (− 72.6, 71.1) | −45.4% (−84.4, 69.6) | − 52.9% (− 78.3, 2.2) |
| P = 0.058 | P = 0.404 | P = 0.282 | P = 0.056 | |
| WBC | 13.0% (− 23.7, −0.8) | 0% (− 31.0, −4.1) | − 18.4% (− 30.2, −4.5) | − 19.4% (− 33.7, −2.1) |
| P = 0.038 | P = 0.996 | P = 0.013 | P = 0.031 | |
Since the analysis procedures use maximum likelihood-based techniques, the results will still be valid even in the presence of missing data, because response for missing measurements is estimated based upon the information contained in the data prior to the discontinuation [33]. The model chosen, with an unstructured covariance matrix, gives as unbiased a prediction as possible for the missing values.
Pharmacokinetics
Data at visits 4, 5 and 6 below the detection limit were set to the detection limit (5 ng ml−1). RSG data were ln transformed. Ln transformed RSG data at visit 3, 3.5 h and 10 h postdose, were analysed using anova with group as factor.
Ln transformed RSG data at visits 4, 5 and 6 were analysed with a mixed effect model with group, visit and group × visit as fixed factors and subject as random factor.
Pharmacodynamics
The pharmacodynamic measurements were analysed using a repeated measures mixed effect model, with group, treatment, gender, visit, visit × treatment, group × treatment, visit × group × treatment as factors and baseline measurement as covariate, using an unstructured covariance matrix. The results were presented as P-values, estimate of difference and 95% confidence intervals (CIs) for the estimated difference. In case of ln transformation, the estimate of difference and the CIs were presented as percentages. Least square means were also calculated for change from baseline and graphically represented as percentage change from baseline in case of ln transformation.
To assess the timing of treatment effects, contrasts for RSG and placebo treatment (corrected for baseline) were calculated for visits 4, 5 and 6. These calculations were performed for parameters showing an overall significant (P < 0.05) or nearly significant (P ≈ 0.05) treatment effect, and for which multiple measurements (>2) were available.
Groups (T2DM vs. HV) were compared at baseline using anova with treatment as factor, on the ln transformed data. Results were presented as (back transformed) least square means, P-values, estimates of difference in percentage and 95% CIs in percentage for the estimated difference.
Since these analyses are of an exploratory nature, no formal correction for multiple comparisons was implemented.
Compliance
The percentage of the incorrect number of capsules taken per study and the percentage of days that an incorrect number of capsules per day was taken, were compared between groups. Because the data were not normally distributed, the Wilcoxon two-sample test was used. All calculations were performed using SAS for Windows V8.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Subjects
Seven T2DM patients were withdrawn: one subject developed a clinically significant elevated triglyceride concentration (12.9 mmol l−1; RSG treatment group), five subjects had repetitive measurements of glucose exceeding 15 mmol l−1 (four subjects in the placebo and one subject in the RSG treatment group) and one subject was hospitalized (severe bronchitis) during the placebo run-in period. Three (placebo treated) patients were replaced because (in the opinion of the investigators) insufficient evaluable data were obtained up to the point of withdrawal (e.g. withdrawal prior to visit 4; after 2 weeks of active treatment). For all other subjects no clinically significant changes in routine laboratory parameters and vital signs were observed and none of the subjects developed clinical symptoms of oedema.
Compliance
Mean overall compliance was similar in both patient (84.4% of prescribed dose and 85.5% days correct dose regimen) and healthy subject groups (79.1% of prescribed dose and 77.8% days correct dose regimen). There was no significant difference (P = 0.12) in the mean number of capsules taken during the study period between the T2DM and HV groups.
RSG exposure
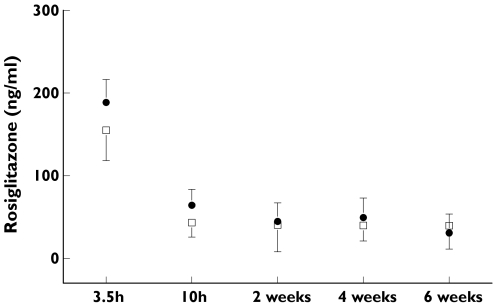
The results of the single dose pharmacokinetic analysis showed slightly higher mean RSG concentrations in the T2DM vs. HV group at 3.5 and 10 h postdosing [23.6%, CI −0.3, 53.4 (nonsignificant) and 53.7%, CI 6.6, 121.7]. There were no significant differences in RSG trough concentrations between the two groups at subsequent visits (Figure 2).
Figure 2.
Time profile of mean rosiglitazone (RSG) plasma concentrations. This figure shows the mean RSG concentrations (ng ml−1) at 3.5 h and 10 h (after first dose) and the mean trough levels at subsequent visits. T2DM group: • (+SD); HV group: □ (+SD). The virtually identical trough levels indicate equal RSG exposure in both study groups
Baseline characteristics
The demographics and baseline characteristics of both study populations are presented in Tables 1a and 1b.
Baseline group comparisons
Glycaemic control parameters
As shown in Table 2, mean baseline glucose, C-peptide, fructosamine, HbA1c, cholesterol, LDL-c and triglyceride concentrations were significantly higher in the T2DM vs. HV group. In addition, baseline HDL-c concentrations were lower and insulin concentrations higher in the T2DM vs. HV group. However, the latter differences were not statistically significant. Furthermore, baseline area under the effect curve (AUE) insulin and peak insulin concentrations after OGTT were significantly lower and baseline AUE glucose and peak glucose concentrations after OGTT were significantly higher in the T2DM vs. HV group.
Markers of inflammation
Mean baseline HS-CRP and IL-6 concentrations were significantly higher in the T2DM vs. HV group. Differences in mean IL-1β and TNF-α concentrations were not statistically significant (Table 2).
Haemodynamic parameters
Systolic and diastolic blood pressure were significantly higher in the T2DM vs. HV group. Heart rate did not differ between the two groups (Table 2).
Overall treatment effects
Glycaemic control parameters
In the T2DM RSG treatment group there was a significant decrease in FPG, C-peptide, insulin and fructosamine compared with placebo (Table 3), whereas these parameters showed no significant decrease in the HV group. In the HV RSG treatment group there was a sigificant decrease in mean peak insulin [−25.5% (−43.5 to −1.9), P = 0.0375] and peak glucose concentrations [−16.3% (−29.7 to −0.4)] after an oral glucose load (OGL) but no significant change in mean AUE insulin and AUE glucose after OGL compared with placebo (data not shown). No significant changes in glucose tolerance were noted in the T2DM RSG treatment group vs. placebo. Furthermore, in both treatment groups there were no significant changes in TC, HDL-c, LDL-c, TG, FFA and HbA1c compared with placebo (data not shown).
Table 3.
Rosiglitazone (RSG) treatment effects in T2DM group. The back transformed least square means for RSG and placebo treatments as well as the average (overall) % change in selected effect parameters for RSG vs. placebo in the T2DM group with corresponding 95% confidence intervals and P-values. All subjects with data points up to (and including) visit 4 or beyond were included in the statistical analyses
| RSG vs. placebo | ||||
|---|---|---|---|---|
| Back transformed least square means | ||||
| Parameter | RSG | Placebo | Estimate of difference RSG–placebo in % (95% CI) | P-value |
| Parameters of glucoregulation | ||||
| Glucose (mmol l−1) | 6.04 | 7.20 | −16.2 (− 26.1, −4.8) | 0.0082 |
| C-peptide (nmol l−1) | 0.63 | 0.78 | −19.5 (− 31.8, −4.9) | 0.0125 |
| Insulin (mU l−1) | 7.59 | 10.49 | −27.7 (− 42.6, −8.8) | 0.0080 |
| Fructosamine (mmol l−1) | 216.03 | 247.23 | −12.6 (− 20.7, −3.7) | 0.0085 |
| HbA1c percentage (%) | 5.92 | 5.90 | 0.4 (− 7.7, 9.2) | 0.9328 |
| Inflammation markers | ||||
| White blood cell count (109 l−1) | 4.50 | 5.17 | −13.0 (− 23.7, −0.8) | 0.0381 |
| High-sensitivity C-reactive protein (mg l−1) | 0.59 | 1.06 | −44.0 (− 69.2, 1.9) | 0.0572 |
| IL-1β level (pg ml−1) | 0.07 | 0.05 | 30.3 (− 22.9, 120.3) | 0.3083 |
| IL-6 level (pg ml−1) | 1.29 | 1.89 | −31.8 (− 48.9, −9.1) | 0.0109 |
| Tumour necrosis factor-α (pg ml−1) | 1.79 | 1.77 | 1.1 (− 16.8, 23.0) | 0.9068 |
Markers of inflammation
In the T2DM treatment group there was a significant decrease in mean IL-6 concentration and WBC vs. placebo. In addition, this group showed a decrease in mean HS-CRP concentration compared with placebo, but this was not statistically significant (Table 3). Furthermore, in the T2DM RSG treatment group there were no significant changes in IL-1β or TNF-α concentrations compared with placebo (Table 3). In the HV RSG treatment group there were no significant changes in plasma concentration for any of the inflammation markers and proinflammatory cytokines vs. placebo (data not shown).
Haemodynamic/haemodilution parameters
In both treatment groups there were no significant changes in blood pressure (systolic or diastolic), heart rate and haematocrit (as measure of haemodilution) compared with placebo (data not shown).
Timing of treatment effects
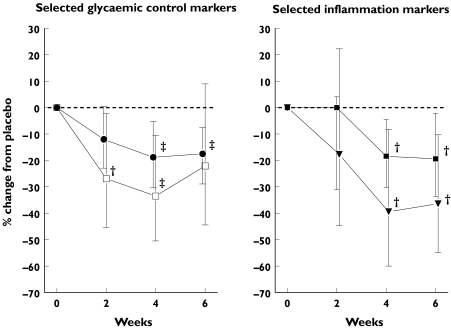
Since there were no significant overall treatment effects in the HV group for parameters with multiple (>2) measurements, this analysis was limited to the T2DM group. Results, including average relative change from baseline, 95% CIs and P-values, are presented in Table 4 and summarised for selected parameters in Figure 3.
Figure 3.
Timing of treatment effects relative to placebo for selected markers (T2DM group only). The left figure shows the percentage change in time of plasma insulin and fasting plasma glucose (FPG) concentrations for rosiglitazone (RSG) vs. placebo treatment in the T2DM group. The right figure shows the plasma IL-6 concentrations and white blood cell count (WBC) for RSG vs. placebo treatment in the T2DM group. •, Fasting plasma glucose (FPG); □, insulin; ▾, IL-6; ▪, WBC. The bars indicate 95% confidence intervals. †P ≤ 0.05; ‡P ≤ 0.01
Briefly, significant effects on glycaemic control parameters appeared to precede or parallel the effects on inflammation markers. This is illustrated by a significant decrease in mean fasting insulin, and a near significant decrease in FPG concentration after 2 weeks of RSG vs. placebo. At this point, no significant effect on IL-6 or WBC was present. After 4 weeks of active treatment, all glycaemic control parameters considered in this analysis (FPG, fructosamine, insulin and C-peptide) had significantly decreased compared with placebo treatment. This decrease was also observed for IL-6 and WBC. After 6 weeks of RSG treatment mean FPG, fructosamine and IL-6 concentrations as well as the WBC showed a significant decrease, coinciding with a near significant decrease in C-reactive protein concentration compared with placebo. The decrease in mean fasting C-peptide and insulin concentration in the RSG treated group was not significant compared with placebo at 6 weeks of treatment.
Discussion
The main finding of this study was that none of the proinflammatory cytokines and inflammation markers investigated in this study demonstrated an earlier response to treatment with RSG than typical parameters of glucoregulation in the T2DM group (Table 4). Also, we did not find significant treatment effects on any of the pharmacodynamic parameters in the HV group, apart from a slight reduction in peak glucose and peak insulin following an OGL.
The main question of the study was if (inflammatory) markers can be identified that precede or predict glycaemic response in T2DM patients that may also respond in HV. If found, these markers would be very helpful to expedite early clinical development of novel TZD. We have been unable to identify such parameters.
Although the duration of our study was short in comparison with the typical treatment periods in therapeutic T2DM trials, we chose this duration for practical purposes. It was our intention to find measures that would respond rapidly to treatment with TZD. Furthermore, in early drug development many variables like dose and dosing frequency need to be evaluated, and this cannot be done easily with relatively long treatment periods.
A potential interpretation issue of this study could lie within the drop-out rate of subjects in the T2DM placebo group. Unfortunately, drop-outs are inevitable in this type of study design, in which regular oral hypoglycaemic medication of T2DM patients was withdrawn. We chose to withdraw regular hypoglycaemic therapy since concerns were raised with regard to the glucose-lowering potential of RSG as add-on therapy. However, since this potential (drop-out rate) issue was anticipated, an appropriate statistical method was chosen (repeated measures mixed effect model) to provide as unbiased a prediction as possible for the missing values encountered [33] and thus allow valid conclusions.
The elevated IL-6 and HS-CRP baseline concentrations in the T2DM group are consistent with previous observations [34] and corroborate the notion that T2DM reflects a state of chronic inflammation [35]. However, as the groups were not matched for BMI and age, these findings cannot solely be attributed to the T2DM state and may reflect age-related effects.
The significant decrease in WBC coinciding with a borderline significant decrease in HS-CRP concentration in the present study is similar to observations made in previous studies [24–28], and illustrates the anti-inflammatory properties of RSG in vivo. Furthermore, in vitro studies with cultured hepatocytes have shown strong decreases in IL-6 expression and secretion after treatment with RSG [36]. Nonetheless, a significant decrease in IL-6 concentrations with TZD treatment in vivo has not been described before. Two previous studies investigating TZD effects in T2DM patients did not report significant effects on plasma IL-6 concentrations [26, 37]. Haffner et al. reported no significant effects after 26 weeks of treatment with RSG 4 or 8 mg day−1 [26]. Since adipose tissue is a significant source of IL-6 expression, the authors attribute their negative findings to a decrease in weight (and therefore probably loss of adipose tissue mass) in the placebo group coinciding with an increase in weight in the RSG-treated group. Subsequently, Tonelli et al. reported no significant effect on the plasma IL-6 concentration after 3 weeks of treatment with pioglitazone [37]. In this study we observed a significant decrease after 4 weeks of treatment with RSG which was still present after 6 weeks. Therefore, it is possible Tonelli et al. failed to show a significant effect because a longer treatment period is required.
Interestingly, the two study groups of HV and T2DM did not differ significantly in IL-1β and TNF-α plasma concentrations. IL-1β was mainly selected as candidate biomarker based on preclinical data, suggesting that plasma IL-1β is implicated in IL-6-induced HS-CRP production [38], and elevated plasma concentrations might therefore be present in T2DM. However, elevated IL-1β concentrations could not be confirmed in this clinical study. This may be explained by more recent data suggesting that IL-1β is mainly an autocrine/paracrine stimulator of IL-6 release from both human peripheral blood cells (PBC) and adipocytes, rather than an endocrine signal from adipose tissue [39]. As such, our data are in keeping with observations from a large recent study on inflammatory cytokines in T2DM patients in which no differences from nondiabetic controls could be demonstrated [16]. Furthermore, the normal TNF-α plasma concentrations in the T2DM group are consistent with some [14, 40, 41], but not with other previous studies [16, 42–44]. In addition, other studies have shown that its perilous effect on insulin signalling is mostly a consequence of paracrine/autocrine action [14, 40, 41, 45]. Therefore, our observations do not support the notion that elevated plasma TNF-α concentrations play a major role in the pathophysiology of T2DM. Nonetheless, there may still be important local tissue effects of this cytokine. Alternatively, since T2DM is a multifactorial and highly heterogeneous disorder, there is a possibility that plasma concentrations of particular cytokines are elevated in distinct subpopulations of T2DM patients, but not in others.
Some of the traditional glycaemic control markers responded earlier than expected from the results of previous studies with TZDs [30, 31]. This illustrates that (although no formal calculation was performed) the present study was properly powered to detect changes in parameters of glucoregulation. A significant effect on insulin and borderline significant effect on FPG was already present after 2 weeks of RSG treatment in the T2DM group (Table 4). In addition, the maximum (and significant) decrease in both insulin (34%) and FPG (19%) levels was reached after 4 weeks of treatment. In contrast, the first occurrence of a significant anti-inflammatory effect, illustrated by a decrease in IL-6 concentration and WBC, was observed after 4 weeks of RSG treatment. Our findings appear to differ from the previously reported timing of anti-inflammatory and metabolic effects of RSG in vivo. Mohanty et al. reported a significant decrease in CRP concentration from baseline after only 1 week of treatment with RSG 4 mg, whereas effects on glucose and insulin were not significant after 6 weeks using anova on ranks [27]. These contrasting findings may relate to differences in study design, including the nonrandomized design and lack of the use of placebo in the study by Mohanty et al.
Finally, the metabolic effects of RSG were independent of changes in fasting FFA and triglyceride concentrations. This observation is in keeping with results from a recent study by Tan et al. showing that the late postprandial FFA and triglyceride concentrations are decreased after RSG treatment, but fasting concentrations remain unaffected [46].
In conclusion, we could not identify novel plasma markers that respond earlier to treatment with rosiglitazone than typical parameters of glucoregulation or that have a significant response in HV. Nonetheless, the significant IL-6 response observed in this study suggests a place for this cytokine as nontraditional complementary biomarker in clinical ‘proof of concept’ studies with novel TZDs.
Acknowledgments
This study was supported by corporate funding from Johnson & Johnson Pharmaceutical Research & Development, Beerse, Belgium, to the Centre for Human Drug Research, Leiden, the Netherlands. One of the authors (E.v.H.) is employed by Johnson & Johnson Pharmaceutical Research & Development. We thank Xuejun Liu, Qingqin Li and Eric Helmer for their valuable comments during the review of the manuscript.
References
- 1.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–9. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 4.Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults–the ARIC study. Atherosclerosis Risk in Communities. Obes Res. 2000;8:279–86. doi: 10.1038/oby.2000.33. [DOI] [PubMed] [Google Scholar]
- 5.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom A, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 6.Festa A, D'Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 7.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–21. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23:650–5. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81–91. doi: 10.7326/0003-4819-133-2-200007180-00007. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl. 1):S68–S71. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- 14.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 15.Besedovsky HO, Del Rey A. Metabolic and endocrine actions of interleukin-1. Effects on insulin-resistant animals. Ann NY Acad Sci. 1990;594:214–21. doi: 10.1111/j.1749-6632.1990.tb40481.x. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 17.Berger J, Wagner JA. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol Ther. 2002;4:163–74. doi: 10.1089/15209150260007381. [DOI] [PubMed] [Google Scholar]
- 18.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 19.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–41. [PubMed] [Google Scholar]
- 20.Knouff C, Auwerx J. peroxisome proliferator-activated receptor-{gamma} calls for activation in moderation. Lessons from genetics and pharmacology. Endocrinol Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 22.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 23.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–9. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebeling P, Teppo AM, Koistinen HA, Viikari J, Ronnemaa T, Nissen M, Bergkulla S, Salmela P, Saltevo J, Koivisto VA. Troglitazone reduces hyperglycaemia and selectively acute-phase serum proteins in patients with Type II diabetes. Diabetologia. 1999;42:1433–8. doi: 10.1007/s001250051315. [DOI] [PubMed] [Google Scholar]
- 25.Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P. Suppression of nuclear factor-kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab. 2001;86:1306–12. doi: 10.1210/jcem.86.3.7309. [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–84. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 28.Chu NV, Kong AP, Kim DD, Armstrong D, Baxi S, Deutsch R, Caulfield M, Mudaliar SR, Reitz R, Henry RR, Reaven PD. Differential effects of metformin and troglitazone on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care. 2002;25:542–9. doi: 10.2337/diacare.25.3.542. [DOI] [PubMed] [Google Scholar]
- 29.Xiang AH, Peters RK, Kjos SL, Goico J, Ochoa C, Marroquin A, Tan S, Hodis HN, Azen SP, Buchanan TA. Pharmacological treatment of insulin resistance at two different stages in the evolution of type 2 diabetes: impact on glucose tolerance and beta-cell function. J Clin Endocrinol Metab. 2004;89:2846–51. doi: 10.1210/jc.2003-032044. [DOI] [PubMed] [Google Scholar]
- 30.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–93. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 31.Patel J, Anderson RJ, Rappaport EB. Rosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: a twelve-week, randomized, placebo-controlled study. Diabetes Obes Metab. 1999;1:165–72. doi: 10.1046/j.1463-1326.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 32.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 33.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 34.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372–9. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–9. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 38.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 39.Flower L, Gray R, Pinkney J, Mohamed-Ali V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21:32–7. doi: 10.1016/s1043-4666(02)00495-7. [DOI] [PubMed] [Google Scholar]
- 40.Bluher M, Kratzsch J, Paschke R. Plasma levels of tumor necrosis factor-alpha, angiotensin II, growth hormone, and IGF-I are not elevated in insulin-resistant obese individuals with impaired glucose tolerance. Diabetes Care. 2001;24:328–34. doi: 10.2337/diacare.24.2.328. [DOI] [PubMed] [Google Scholar]
- 41.Febbraio MA, Steensberg A, Starkie RL, McConell GK, Kingwell BA. Skeletal muscle interleukin-6 and tumor necrosis factor-alpha release in healthy subjects and patients with type 2 diabetes at rest and during exercise. Metabolism. 2003;52:939–44. doi: 10.1016/s0026-0495(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer A, Janott J, Mohlig M, Ristow M, Rochlitz H, Busch K, Schatz H, Schifferdecker E. Circulating tumor necrosis factor alpha is elevated in male but not in female patients with type II diabetes mellitus. Horm Metab Res. 1997;29:111–4. doi: 10.1055/s-2007-979001. [DOI] [PubMed] [Google Scholar]
- 43.Winkler G, Cseh K, Baranyi E, Melczer Z, Speer G, Hajos P, Salamon F, Turi Z, Kovacs M, Vargha P, Karadi I. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Res Clin Pract. 2002;56:93–9. doi: 10.1016/s0168-8227(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 44.Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–8. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 45.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 46.Tan GD, Fielding BA, Currie JM, Humphreys SM, Desage M, Frayn KN, Laville M, Vidal H, Karpe F. The effects of rosiglitazone on fatty acid and triglyceride metabolism in type 2 diabetes. Diabetologia. 2005;48:83–95. doi: 10.1007/s00125-004-1619-9. [DOI] [PubMed] [Google Scholar]