Abstract
Background
The association between thiopurine methyltransferase (TPMT) deficiency and myelosuppression with azathioprine is well recognized. Two cases are presented that illustrate the very different outcomes that may occur with azathioprine in patients with TPMT deficiency, which affects 0.3–0.6% of caucasians.
Case 1
The first patient's TPMT deficiency was identified following hospitalization for pancytopenia attributed to azathioprine.
Case 2
The second patient was identified as deficient early in treatment and myelosuppression was avoided by treating with a greatly decreased dose (25 mg per week).
Conclusions
Testing for TPMT ideally should be performed in every patient commencing a thiopurine drug.
Keywords: azathioprine, pancytopenia, pharmacogenetics, thiopurine methyltransferase
Introduction
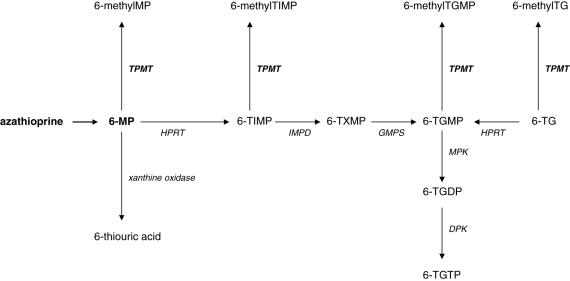
Thiopurine methyltransferase (TPMT) is an enzyme critical to the metabolism of azathioprine and its metabolite, 6-mercaptopurine (6-MP). Amongst caucasians, there is a trimodal distribution of TPMT enzyme activity, with 0.3–0.6% having very low (negligible) activity and approximately 10% having decreased activity compared with the remaining population [1, 2]. Severe TPMT deficiency leads almost inevitably to life-threatening bone marrow depression if ‘standard’ thiopurine doses are used [3, 4]. This myelotoxicity is thought to be due to elevated concentrations of the cytotoxic metabolites, 6-thioguanine nucleotides (6-TGNs), which occur when 6-MP is unable to be metabolized by TPMT to 6-methylmercaptopurine (6-MMP) (Figure 1) [5].
Figure 1.
The metabolism of azathioprine. Abbreviations: DPK, Diphosphate kinase; GMPS, guanosine monophosphate synthetase; HPRT, hypoxanthine phosphoribosyltransferase; IMPD, inosine monophosphate dehydrogenase; MPK, monophosphate kinase; TG, thioguanine; TGMP, TGDP and TGTP, thioguanine mono-, di- and triphosphate; TIMP, thioinosine monophosphate; TXMP, thioxanthosine monophosphate
We present two patients with low TPMT activity and widely different outcomes with azathioprine treatment. The first patient was identified as TPMT deficient retrospectively, after being hospitalized with pancytopenia due to azathioprine. The second patient was identified prospectively and bone marrow depression avoided by treating with a greatly decreased dose.
Case 1
A 65-year-old man was admitted to hospital with pancytopenia (haemoglobin 66 g l−1, white cell count 1.6 × 109 l−1 (nadir 0.6), neutrophils 0.7 × 109 l−1, platelets 39 × 109 l−1). Eight weeks earlier he had commenced azathioprine 100 mg per day (∼1.4 mg kg−1 day−1) for Crohn's colitis that was inadequately controlled with mesalazine 4 g and prednisone 10 mg daily. He had a complex medical history including Type 2 diabetes mellitus, hypertension, ischaemic heart disease and paraplegia secondary to a motor vehicle accident. Concurrent drug therapy was extensive and comprised amlodipine, aspirin, atenolol, bendrofluazide, carbamazepine, paracetamol, paroxetine, quinapril, temazepam, steroid and bronchodilator inhalers, iron, potassium and glyceryl trinitrate spray.
A bone marrow aspirate was not diagnostic because the sample was haemodiluted, but hypoplastic and dysplastic features were seen, consistent with immunosuppressive therapy. Azathioprine was stopped and over the next 17 days the patient required barrier nursing, transfusion with 10 units of resuspended red blood cells and 4 units of platelets, granulocyte–colony-stimulating factor for the pancytopenia, broad-spectrum antibiotics for neutropenic sepsis, and antifungal and antiviral agents to treat or prevent opportunistic infections. His haematological indices improved (haemoglobin 113 g l−1, white cell count 10.0 × 109 l−1, neutrophils 6.8 × 109 l−1, platelet count 65 × 109 l−1) and he was discharged. Seven months later, he was inadvertently rechallenged with azathioprine 100 mg daily and again developed pancytopenia (haemoglobin 71 g l−1, white cell count 1.9 × 109 l−1, neutrophils 0.9 × 109 l−1, platelet count 52 × 109 l−1) within 4 weeks, resolving on cessation of the drug. The Naranjo algorithm [6] for assessment of adverse reactions was applied and a score of 10 points was achieved indicating that azathioprine ‘definitely’ caused the pancytopenia. Subsequently, when the methods for measuring erythrocyte TPMT enzyme activity and genotype became available locally, he was found to have low TPMT enzyme activity (0.6 IU ml−1 red blood cells; ‘normal’ range 9.3–17.6) and to be homozygous for the TPMT*3 mutation. Enzyme activity and genotype were determined using a radiochemical method and multiplexed amplification refractory mutation system assay, respectively [7].
Case 2
A 19-year-old man was tested for TPMT enzyme activity on the same day as he commenced azathioprine 50 mg daily (0.64 mg kg−1 day−1) for ulcerative colitis. Results were available on the fourth day of dosing and he was identified as having low TPMT enzyme activity (0.9 IU ml−1 red blood cells) and was subsequently found to be homozygous for TPMT*3 mutant alleles. Thus, he would almost certainly have developed bone marrow depression if continued on conventional doses of azathioprine. The drug was stopped immediately and a blood sample was taken for erythrocyte 6-TGN concentrations which were already elevated (576 pmol per 8 × 108 red blood cells; local target range 235–450) after a total dose of 200 mg of azathioprine. 6-MMP, a product of TPMT, was undetectable, consistent with TPMT deficiency. 6-TGN concentrations declined slowly over more than 42 days with a half-life of approximately 14 days. This compares with an average half-life of 5 days in those without TPMT deficiency [8]. Ongoing symptoms of diarrhoea and rectal bleeding from his ulcerative colitis resulted in further treatment with azathioprine but at a lower dose 6 months later. The initial dose of 12.5 mg twice weekly (0.3 mg kg−1 week−1 or 0.05 mg kg−1 day−1) was based on limited experience with dose reductions to approximately 10% in patients with low TPMT activity treated for acute lymphoblastic leukaemia [9, 10] and inflammatory bowel disease [4]. Six months after being maintained on this dose, the patient has achieved clinical improvement, with 6-TGN concentrations of 250–400 pmol per 8 × 108 red blood cells. These are within the range associated with efficacy [11] and less toxicity [12]. 6-TGN and 6-MMP concentrations were determined using high-performance liquid chromatography [7].
Discussion
The above cases demonstrate the beneficial effect of testing, and the consequences of not testing, for TPMT in patients treated with azathioprine or 6-MP [13]. We and others [14] advocate that all patients should be screened for TPMT deficiency prior to commencing these drugs. However, it is recognized that these tests are not readily available in all centres [15]. Alternative options may include delaying treatment with the thiopurine until the results can be obtained via a central specialist laboratory. For a condition such as inflammatory bowel disease, a small delay in treatment is unlikely to have a significant influence on the control of disease. In countries that do not have this test available, azathioprine can be started at a ‘low’ dose with cautious dose escalation if tolerated. Unfortunately, this may not always avoid the occurrence of profound myelosuppression in TPMT-deficient patients, and what constitutes a ‘low’ dose has yet to be defined. Furthermore, TPMT testing will not avoid the requirement for vigilant monitoring of blood counts since most cases of myelosuppression with thiopurines occur in individuals with TPMT activity within the ‘normal’ range. The main reason for prescreening at present is to detect the individuals with virtually absent TPMT activity. In these patients, azathioprine and 6-MP should be avoided completely, or extremely low doses used (e.g. 10% of usual doses).
TPMT can be assessed by two main methods, both of which involve a single blood test. One involves testing for the more common variants of the TPMT gene that are associated with decreased enzyme activity. The other test measures TPMT enzyme activity in red blood cells. A recent survey has demonstrated that clinicians prefer to determine TPMT activity (i.e. phenotype) rather than genotype [15], presumably because it reflects more than one cause of variation in enzyme activity (such as the inhibitory effect of concurrent use of 5-aminosalicylic acid derivatives). TPMT activity varies widely amongst those with a functioning enzyme, and in patients with a high activity a greater thiopurine dose should be considered. However, measurement of TPMT enzyme activity may be affected by factors such as blood transfusions [16] and uraemia [17]. Genotyping may provide a useful adjunct in these circumstances, although it is possible that rare mutations may not be detected with standard screening methods [18]. The results of pharmacoeconomic studies in inflammatory bowel disease and rheumatoid arthritis also support the use of these tests [19, 20]. We estimated that the cost of the 17-day hospital stay for our first patient was in excess of NZ$25 000 [∼ £9100], which would cover the cost of at least 430 tests for TPMT enzyme activity (NZ$57.75 or ∼£21 each).
Monitoring of 6-TGN concentrations is more controversial, although there is some evidence to suggest an increased likelihood of response in inflammatory bowel disease if concentrations are above 235 pmol per 8 × 108 red blood cells [11]. The upper limit of 6-TGN concentrations used by us is based on limited evidence that suggests that an increased risk of leucopenia exists with concentrations above 500 pmol per 8 × 108 red blood cells [12]. These data suggest a preliminary therapeutic range of 235–500 pmol per 8 × 108 red blood cells, although an association between 6-TGN and response to thiopurine drugs has not been demonstrated in all studies [21]. Therapeutic monitoring of 6-TGN may be useful in circumstances such as TPMT deficiency to confirm that smaller doses of azathioprine or 6-MP are appropriate. However, such testing must be used together with standard methods of monitoring thiopurine treatment such as regular full blood counts. The clinical value of measuring 6-MMP is less well defined. Very low concentrations could suggest noncompliance if coupled with low 6-TGN concentrations, whereas very high concentrations have been associated with hepatotoxicity [11, 22].
Competing interests: None declared.
References
- 1.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Schaeffeler E, Fischer C, Brockmeier D, Wernet D, Moerike K, Eichelbaum M, Zanger UM, Schwab M. Comprehensive analysis of thiopurine S-methyltransferase phenotype–genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14:407–17. doi: 10.1097/01.fpc.0000114745.08559.db. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JB, Szumlanski C, Weinshilboum RM, Schmiegelow K. Pharmacokinetics, dose adjustments, and 6-mercaptopurine/methotrexate drug interactions in two patients with thiopurine methyltransferase deficiency. Acta Paediatrica. 1998;87:108–11. doi: 10.1080/08035259850158001. [DOI] [PubMed] [Google Scholar]
- 4.Kaskas BA, Louis E, Hindorf U, Schaeffeler E, Deflandre J, Graepler F, Schmiegelow K, Gregor M, Zanger UM, Eichelbaum M, Schwab M. Safe treatment of thiopurine S-methyltransferase deficient Crohn's disease patients with azathioprine. Gut. 2003;52:140–2. doi: 10.1136/gut.52.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–54. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 7.Gearry RB, Barclay ML, Roberts RL, Harraway J, Zhang M, Pike LS, George PM, Florkowski CM. Thiopurine methyltransferase and 6-thioguanine nucleotide measurement: early experience of use in clinical practice. Intern Med J. 2005. in press. [DOI] [PubMed]
- 8.Derijks LJJ, Gilissen LPL, Engels LGJB, Bos LP, Bus PJ, Lohman JJHM, Curvers WL, Van Deventer SJ, Hommes DW, Hooymans PM. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease. Ther Drug Monit. 2004;26:311–8. doi: 10.1097/00007691-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Lennard L, Gibson BE, Nicole T, Lilleyman JS. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1993;69:577–9. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatrics. 1991;119:985–9. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- 11.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Theoret Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–13. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 12.Schütz E, Gummert J, Mohr FW, Armstrong VW, Oellerich M. Should 6-thioguanine nucleotides be monitored in heart transplant recipients given azathioprine? Ther Drug Monit. 1996;18:228–33. doi: 10.1097/00007691-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lennard L. TPMT in the treatment of Crohn's disease with azathioprine. Gut. 2002;51:143–6. doi: 10.1136/gut.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidman EG. Clinical use and practical application of TPMT enzyme and 6-meraptopurine metabolite monitoring in IBD. Rev Gastroenterol Dis. 2003;3(Suppl 1):S30–S38. [PubMed] [Google Scholar]
- 15.Gardiner SJ, Begg EJ. Pharmacogenetics testing for drug metabolizing enzymes — is it happening in practice? Pharmacogen Genomics. 2005;15:365–9. doi: 10.1097/01213011-200505000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Cheung ST, Allan RN. Mistaken identity. misclassification of TPMT phenotype following blood transfusion. Eur J Gastroenterol Hepatol. 2003;15:1245–7. doi: 10.1097/01.meg.0000085479.12407.b8. [DOI] [PubMed] [Google Scholar]
- 17.Pazmino PA, Sladek SL, Weinshilboum RM. Thiol S-methylation in uremia: erythrocyte enzyme activities and plasma inhibitors. Clin Pharmacol Ther. 1980;28:356–67. doi: 10.1038/clpt.1980.174. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson J, Ansari A, Marinaki T, Duley J. Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem. 2004;41:294–302. doi: 10.1258/0004563041201455. [DOI] [PubMed] [Google Scholar]
- 19.Marra CA, Esdaile JM, Anis AH. Practical pharmacogenetics: the cost effectiveness of screening for thiopurine s-methyltransferase polymorphism in patients with rheumatological conditions treated with azathioprine. J Rheumatol. 2002;29:2507–12. [PubMed] [Google Scholar]
- 20.Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593–9. doi: 10.1111/j.1365-2036.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowry PW, Franklin CL, Weaver AL, Pike MG, Mays DC, Tremaine WJ, Lipsky JJ, Sandborn WJ. Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut. 2001;49:665–70. doi: 10.1136/gut.49.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther. 2004;75:274–81. doi: 10.1016/j.clpt.2003.12.001. [DOI] [PubMed] [Google Scholar]