Abstract
Aims
Cyclophosphamide (CTX) is an established treatment of severe systemic lupus erythematosus (SLE). Cytotoxic CTX metabolites are mainly detoxified by multiple glutathione S-transferases (GSTs). However, data are lacking on the relationship between the short-term side-effects of CTX therapy and GST genotypes. In the present study, the effects of common GSTM1, GSTT1, and GSTP1 genetic mutations on the severity of myelosuppression, gastrointestinal (GI) toxicity, and infection incidences induced by pulsed CTX therapy were evaluated in patients SLE.
Methods
DNA was extracted from peripheral leucocytes in patients with confirmed SLE diagnosis (n = 102). GSTM1 and GSTT1 null mutations were analyzed by a polymerase chain reaction (PCR)-multiplex procedure, whereas the GSTP1 codon 105 polymorphism (Ile→Val) was analyzed by a PCR-restriction fragment length polymorphism (RFLP) assay.
Results
Our study demonstrated that SLE patients carrying the genotypes with GSTP1 codon 105 mutation [GSTP1*-105I/V (heterozygote) and GSTP1*-105 V/V (homozygote)] had an increased risk of myelotoxicity when treated with pulsed high-dose CTX therapy (Odds ratio (OR) 5.00, 95% confidence interval (CI) 1.96, 12.76); especially in patients younger than 30 years (OR 7.50, 95% CI 2.14, 26.24), or in patients treated with a total CTX dose greater than 1.0 g (OR 12.88, 95% CI 3.16, 52.57). Similarly, patients with these genotypes (GSTP1*I/V and GSTP1*V/V) also had an increased risk of GI toxicity when treated with an initial pulsed high-dose CTX regimen (OR 3.33, 95% CI 1.03, 10.79). However, GSTM1 and GSTT1 null mutations did not significantly alter the risks of these short-term side-effects of pulsed high-dose CTX therapy in SLE patients.
Conclusions
The GSTP1 codon 105 polymorphism, but not GSTM1 or GSTT1 null mutations, significantly increased the risks of short-term side-effects of pulsed high-dose CTX therapy in SLE patients. Because of the lack of selective substrates for a GST enzyme phenotyping study, timely detection of this mutation on codon 105 may assist in optimizing pulsed high-dose CTX therapy in SLE patients.
Keywords: cyclophosphamide, glutathione S-transferase, single nucleotide polymorphism, systemic lupus erythematosus, toxicity
Introduction
Cyclophosphamide (Cytoxan; CTX) is one of the most widely used alkylating agents in the treatment of haematological malignancies and a variety of solid tumours, including leukaemia, ovarian cancer, and small-cell lung cancer [1]. In addition, high-dose regimens of CTX are frequently used prior to bone marrow transplantation in patients with aplastic anaemia, leukaemia, or other malignancies for mobilization of haematopoietic progenitor cells from the bone marrow into peripheral blood [2, 3]. Moreover, CTX has been widely used as an immunosuppressive agent in the treatment of several autoimmune diseases, including systemic lupus erythematosus (SLE) [4] and rheumatoid arthritis [5]. In such cases, extended low-dose, pulse or high-dose regimens for CTX are often used with or without combination with other immuno-suppressants for SLE and rheumatoid arthritis [4]. SLE is a chronic autoimmune disease characterized by abnormalities of the immune system with unknown aetiology. This disease can affect any organs of the body, including the skin, joints, kidneys, brain, heart, lungs, and blood system [6]. Arthritis and cutaneous manifestations are most common, but renal, haematologic and neurologic manifestations contribute largely to the morbidity and mortality caused by SLE. The hallmark of SLE is the abnormalities in the immune system, manifested with the production of nonorgan specific auto-antibodies, most commonly antinuclear antibodies (ANA) and anti-DNA antibodies, generation of circulating immune complexes, and activation of the complement system [6]. Many immunosuppressant-based approaches aimed to attenuate the clinical symptoms and reduce organ injuries have been evaluated in SLE patients in the past 50 years. However, many result in disappointing clinical outcomes. Pulsed high-dose CTX regimen has become a well-established standard treatment for SLE patients, in particular, for those patients with diffuse proliferative lupus nephritis. A number of clinical studies using pulsed CTX therapy have provided convincing evidence supporting the long-term efficacy in reducing morbidity and mortality of SLE patients [7]. Despite its wide application in SLE patients, the mechanism of action of CTX as a potent immuno-modulating agent for SLE treatment has not been fully identified. Preliminary preclinical and clinical studies have indicated that CTX has modulating effects on both humoral and cell-mediated immunity [8], resulting in beneficial effects and improvements of clinical symptoms in SLE patients. Animal studies have demonstrated that this agent can deplete CD4+/CD25+ regulatory T cells but increases T lymphocyte proliferation and the number of T memory cells in vivo [9]. However, it can also decrease the number of activated T cells by 30–40% and dramatically decrease B cells for months [9]. In addition, CTX inhibits many cell-mediated immune responses, such as graft vs. host reactivity of lymphoid cells, cell-mediated cytotoxicity, mitogen- and antigen-stimulated blastogenesis, and production of soluble cytokines such as tumour necrosis factor-α and interleukin-1 [1]. These findings may provide some rationale for the use of CTX as an immunosuppressive agent in the treatment of autoimmune diseases such as SLE. Like all cytotoxic agents, the toxic metabolites of CTX gain entry to normal tissues including the GI tract and bone marrow, where they induce host organ injuries in many patients. CTX-based regimens for SLE patients often cause short-term toxicity, such as myelosuppression, GI symptoms (e.g. vomiting and diarrhoea), and infection due to marked suppression of the immune system. On the other hand, pulsed CTX therapy also often leads to severe long-term side-effects, such as gonadal toxicity, haemorrhagic cystitis, and secondary malignancy [1, 6]. The risk of secondary leukaemia and lymphoma appears to be very low. Premature ovarian failure, however, is a significant and common complication and occurs in up to 60% of women with SLE >30 years old [10, 11]. The usual dose-limiting toxicity for CTX is myelosuppression. However, at higher doses (2.4–26 g m−2) used prior to marrow transplantation, the dose-limiting toxicity is cardiac toxicity which may be fatal [12].
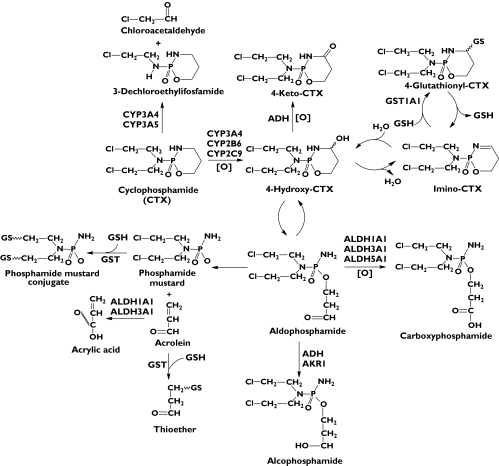
The pharmacokinetics and metabolism of CTX have been extensively studied [13]. As a prodrug, CTX requires bioactivation through multiple hepatic cytochrome P450s (CYP2B6, CYP3A4, and CYP2C9 to a lesser extent) to form 4-hydroxy-CTX which is finally converted to cytotoxic alkylating phosphoramide mustard (Figure 1) [13, 14]. The resultant 4-OH-CTX interconverts rapidly with its tautomer, aldophosphamide (an aldehyde intermediate) [15, 16]. Aldophosphamide degrades spontaneously by β-elimination, resulting in stoichiometric amounts of phosphoramide mustard and the toxic by-product acrolein, which is a highly electrophilic α, β-unsaturated aldehyde [16]. The resultant phosphoramide mustard is the therapeutically active compound responsible for alkylation and crosslinking with DNA double strands, while acrolein is responsible for the urotoxicity of CTX [17]. Furthermore, 4-OH-CTX undergoes reversible dehydration to generate immino-CTX, which is further conjugated with intracellular glutathione (GSH) by multiple glutathione S-transferases (GSTA1, GSTM1, GSTP1, and GSTT1), giving rise to nontoxic 4-glutathionyl-CTX [18, 19], a substrate for the multidrug resistance associated protein 2. Alternatively, 4-OH-CTX can be oxidized by a few cytosolic aldehyde dehydrogenases (ALDH1A, ALDH3A and ALDH5A), yielding nontoxic and unstable carboxyphosphamide (i.e. O-carboxyethyl-CTX mustard) and 4-keto-CTX [20, 21]. Competing with the 4-hydroxylation of CTX is a minor (∼10%) side chain oxidation pathway by CYP3A4 and CYP2B6, leading to N-dechloroethylation and the formation of inactive dechloroethyl metabolites and the neurotoxic by-product chloroacetaldehyde [22–24]. Notably, CTX, 4-OH-CTX, phosphoramide mustard, acrolein, and chloroacetaldehyde can all conjugate with intracellular GSH molecules by multiple cytosolic GSTs, including GSTA1, GSTM1, GSTT1 and GSTP1, resulting in various nontoxic GSH conjugates which are excreted into the bile and urine [18, 25, 26]. Since cytosolic GSTs are the principal conjugating (phase II) enzymes that detoxify CTX and its toxic metabolites, their expression and activities are important factors determining the efficacy and toxicity of CTX therapy [1].
Figure 1.
The metabolic pathways of cyclophosphamide (CTX) in humans. As a prodrug, CTX is activated by hepatic CYP2B6, CYP3A4 and CYP2C9 to form 4-OH-CTX. 4-OH-CPA is in equilibrium with its tautomer aldophosphamide which can decompose spontaneously by β-elimination to result in cytotoxic phosphoramide mustard and the toxic by-product acrolein. Acrolein is detoxified by glutathione S-transferases (GSTs). Alternatively, 4-OH-CTX is detoxified by aldehyde dehydrogenases (ALDH1A, ALDH3A1 and ALDH5A) to generate nontoxic carboxyphosphamide. 4-OH-CTX is also oxidized by alcohol dehydrogenase (ADH) to form nontoxic 4-keto-CTX. Furthermore, 4-OH-CTX undergoes reversible dehydration to result in immino-CTX that is further conjugated with intracellular GSH, giving rise to 4-glutathionyl-CTX, a substrate for multidrug resistance associated protein 2. Moreover, CTX can be converted to 3-dechloroethylifosfamide to a minor extent by CYP3A-catalysed side chain N-dechloroethylation with the production of the toxic by-product chloroacetaldehyde
GSTs (EC 2.5.1.18) comprise a superfamily of phase II conjugating enzymes, which are involved in the detoxification of a large number of hydrophobic and electrophilic compounds, including environmental carcinogens and many therapeutic agents [27, 28]. Human cytosolic GSTs include at least seven distinct classes, namely, α (A), µ (M), π (P), σ (Sigma), ζ (Zeta), ω (Omega) and θ (T), with 30–40% differences in amino acid sequences of these proteins [28, 29]. The α class family includes five functional genes (GSTA1-GSTA5) and seven pseudogenes [28]. There are two θ class genes, GSTT1 and GSTT2, and a pseudogene of GSTT2 has also been reported [28]. Five µ (GSTM1-GSTM5) genes have been identified. GSTM1 is abundantly expressed in the liver, while GSTM2-GSTM5 are mainly detected in extra-hepatic tissues, such as the brain, intestine and testis [30]. Furthermore, the GSTP1 gene has been mapped to chromosome 11q13 and GSTP1 has high and selective activity towards the carcinogenic epoxide of benzo(a)pyrene [31]. A number of genetic polymorphisms of GST genes have been reported (see http://www.pharmgkb.org), many of which have important clinical and toxicological implications [28]. For example, the GSTM1 null mutation represents an allele with a 15 kb deletion that spans the entire GSTM1 gene [32] and people homozygous for this allele lack expression of functional GSTM1 protein [33]. A common null polymorphism has also been identified at the GSTT1 locus [34] which may partially explain the phenotypic variation in GST-related detoxification of halomethanes by human erythrocytes [35]. In addition, a single nucleotide polymorphism (SNP) resulting in amino acid substitution at codon 105 (Ile→Val) at GSTP1 substantially diminishes the enzyme activity of the GSTP1 protein [28]. Due to the critical detoxifying role for a variety of potentially toxic compounds and drugs, deficiency in GST enzyme activity due to genetic polymorphisms could attenuate the ability of the body to eliminate chemotherapeutic agents such as CTX [36], ifosfamide [37] and busulphan [38] and their toxic metabolites which are substrates for GSTs. As such, patients carrying these genetic mutations may predispose to the toxicity of chemotherapy and may simultaneously undergo enhanced clinical response to chemotherapy.
To date, there are increasing numbers of clinical studies evaluating the association of GST genetic polymorphisms with the adverse side-effects (ADRs) and long-term effects (e.g. survival) of chemotherapy in patients [39, 40]. In a large-scale study with 5.3 years of follow-up, women who were homozygous for the variant GSTP1105Val allele had a 60% reduction in mortality risk compared with those who were homozygous for the 105Ile allele (Odds ratio (OR) 0.4, 95% confidence interval (CI) 0.2, 0.8) [39], in 1034 patients with invasive breast carcinoma receiving CTX-based chemotherapeutic regimen. In contrast, there was no association between the mortality risk and GSTM1 or GSTT1 mutations in this study population [39]. In another clinical study, it was found that inheritance of at least one GSTP1 Val105 allele, but not GSTM1 or GSTT1 mutation, conferred a significantly increased risk of developing chemotherapy-related secondary acute myeloid leukaemia (OR 1.81, 95% CI 1.11, 2.94) [40]. In a study with 52 patients with advanced gastric cancer, patients possessing the GSTP1*-105 V/V genotype showed a higher response rate compared with patients harbouring at least one GSTP1*105 Ile allele (63%vs. 21%, P = 0.038) [41]. Patients with the GSTP1*V/V (homozygotes) genotype had a significantly higher median survival time compared with patients with at least one GSTP1-*105Ile allele (15 vs. 6 months, P = 0.037) [41]. Furthermore, in a study involving 219 patients with primary epithelial ovarian cancer, it was found that patients with GSTM1 null were less likely to have disease progression (OR 0.65, 95% CI 0.43, 0.99) or to die (OR 0.68, 95% CI 0.45, 1.03) compared with patients with wildtype GSTM1 [42]. Patients with GSTM1 null mutation, GSTP1-*105I/V, or GSTP1-*105V/V genotype had a further reduction in risk of disease progression compared with patients with GSTM1 or GSTP1 wildtype (OR 0.42, 95% CI, 0.24, 0.75). A similar association was also suggested for overall survival (OR, 0.61, 95% CI 0.36, 1.05) [42]. Recently, Lu et al. [43] reported that patients with advanced-stage nonsmall cell lung carcinoma (n = 425) who had the GSTP1 exon 6 variant genotype (Ala/Val or Val/Val) had significantly better survival compared with patients who had the wildtype genotype (median survival: 16.1 vs. 11.4 months). Multivariate analysis revealed a reduced adjusted OR of death associated with the GSTP1 exon 6 variant genotype of 0.75 (95% CI 0.54, 1.05) [43]. This protective association was observed in patients younger than age 62 years (OR 0.59, 95% CI 0.57, 0.97) and in males (OR 0.64, 95% CI 0.41, 0.99). However, GSTP1*105I/V (heterozygote) on exon 5 was not associated with survival in these patients [43]. Moreover, a recent study has found that the GSTA1 mutation (GSTA1*A/B) caused significantly higher plasma busulfan concentrations in cancer patients [44]. These studies initially addressed the effects of GSTM1, GSTT1 and GSTP1 mutations on the long-term clinical outcomes such as survival [39, 43], chemotherapy-induced secondary leukaemia [40], and pharmacokinetic parameters of GST substrate drugs [44]. However, data on the relationship of GST genotypes and the short-term side-effects of chemotherapy are scanty. It is important to address this issue at an early stage of chemotherapy to minimize drug-induced toxicity by timely adjustment of the dosage and regimens. As such, we hypothesized that GSTM1, GSTT1 and GSTP1 genetic polymorphisms could affect the short-term ADRs of CTX therapy. To test this hypothesis, the present study was undertaken to investigate the relationships between common GSTM1, GSTT1 and GSTP1 mutations and the short-term ADR incidences of CTX in patients with SLE.
Methods
Patient selection
From January 2004 to April 2005, one hundred and two Han Chinese subjects (14 men and 88 women; mean age 29.8 ± 15.6 years, range 13–64 years) were recruited in this study and diagnosed with SLE by the Department of Rheumatology and Clinical Immunology at the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. This study was approved by the Ethics Committee of Sun Yat-Sen University for Clinical Studies (Guangzhou, China). Written informed consent was obtained from all participants.
Sample size is an important issue in high-quality pharmacogenetic studies. Before the start of the present study, we did a power analysis. The power of a study refers to the probability of detecting a ‘true’ effect of a factor. A proper sample size is always needed in clinical pharmacogenetic studies to detect such a positive effect and to avoid too high a study cost. The power in clinical risk (or odds) ratio studies is calculated used the following standard equations [45]:
| [1] |
where N is the sample size; π0 is the controls exposed to the risk factor, OR is the Odds ratio, π1 is the proportion of cases exposed to the risk factor calculated from equation 2 below, u is the one-side percentage point of the normal distribution corresponding to 100%– the power (e.g. if power = 0.9, u = 1.28) and v is the percentage point of the normal distribution corresponding to the two-sided significance level (e.g. if the significance level = 0.05, then v = 1.96).
| [2] |
| [3] |
Thus, the u value could be determined using equation 4:
| [4] |
As shown in equation 4, u is determined by the frequency of the risk factor (0–1), OR, number of cases exposed to the risk factor, and number of cases exposed to the risk factor in the control group. When we set the frequency of the risk factor, GSTP1 mutation, as 0.37, OR for myelotoxicity as 5.0, number of exposed cases as 41 out of 102, the number of control cases as 61 out of 102 (thus π0 = 0.598), the significance level as 0.05, and number of sides as 2, the calculated u was 6.21 and the power for discovering the effect of a risk factor (GSTP1 mutation) was 0.97 (97%). If we divided the study population (102) into two equal subgroups, the power was slightly decreased to 0.77 (77%).
If we set the frequency of the risk factor, GSTP1 mutation as 0.37, the OR for GI symptoms as 3.0, the number of cases as 41 out of 102, the number of control cases as 61 out of 102 (thus π0 = 0.598), the significance level as 0.05, and number of sides as 2, the resultant u was 3.71 and the power was 0.72 (72%). If we divided the study population (102) into two equal subgroups (then π0 = π1), the power was reduced to 0.44 (44%). However, for GSTM1 and GSTT1 null mutation, the OR values for ADRs of CTX were less than 2.0, thus the u values would be less than 1.75 and the power values (u) were less than 0.40 (40%).
From the above power analysis, a study population with a size of 102, as in our study, had enough power to discover the effect of GSTP1 mutation on important ADRs of CTX therapy, such as myelotoxicity and GI symptoms, although the subgroup analysis would lose some power. Not surprisingly, subgroup (stratification) analyses would lead to relatively wide 95% confidence intervals (CIs), but they still could provide some useful information on the potential effects of risk factors in SLE patients receiving pulsed CTX therapy.
The diagnosis of SLE in patients was confirmed based on the American College of Rheumatology (ACR) criteria published in 1997 [46]. The confirmation of SLE required a thorough history, a physical examination and laboratory tests, including a complete blood cell count, chemistry panel and urinalysis, and serologic tests (e.g. antinuclear antibodies, anti-Rho, anti-La, anti-RNP, anti-Smith, antidsDNA and antiphospholipid antibodies). Before a patient can be diagnosed with SLE, at least four of the following 11 disorders must be present: (a) malar rash, (b) siscoid rash, (c) photosensitivity, (d) oral ulcers, (e) arthritis, (f) serositis, (g) renal disorder, (h) neurologic disorder, (i) haematologic disorder, (g) immunologic disorder, and (k) antinuclear antibodies [46]. Rash occurs in 70–80% of SLE patients. Renal disease is manifested by hypertension, oedema of the lower extremities, retinal changes, and clinical manifestations associated with electrolyte abnormalities, nephrosis, or acute renal failure. Renal disease is more frequently observed in children than in adults [46].
Patients were excluded if (a) they were taking nonsteroidal anti-inflammatory drugs, (b) they were taking or took immunosuppressant drugs such as glucocorticoids, methotrexate and azathioprine or herbal immuno-modulators; or antimalarial agents (e.g. hydroxychloroquine) within 2 months, (c) they received a blood transfusion within 2 months, (d) they were pregnant or breast-feeding, (e) they had severe chronic heart, haematological (e.g. leucopenia, thrombocytopenia), renal, brain, liver or lung disease not due to SLE complications, (f) they were alcoholic, (g) they were taking or used phenobarbital, rifampin, or allopurinol within 2 months as these drugs may increase the toxicity of CTX due to drug interactions, (h) they had a recent vaccination and (i) they had received irradiation or cytotoxic therapy in the past 3 months. Patients were also excluded for final evaluation if they were lost to follow up during the period of clinical observation.
Drug administration schedule
Patients with newly diagnosed SLE who enrolled in this study received their initial pulsed high-dose CTX (provided by Bristol-Myers Squibb Oncology) therapy. CTX at 0.5–0.75 g m−2 was administered via intravenous (i.v.) bolus infusion with the total dose divided into two equal fractions and infused over 2 days. Each infusion was given along with i.v. hydration, an antinausea regimen, and mesna (sodium 2-mercaptoethane sulphonate) at a dose of three quarters that of the CTX dose. The latter was given to minimize bladder toxicity (haemorrhagic cystitis) associated with CTX therapy. In the kidney, mesna can react chemically with toxic CTX metabolites to cause their detoxification. The mean dose of pulsed CTX was 1.04 ± 0.20 g in these patients. All patients were supposed to receive the standard pulse regimen (0.5–0.75 g m−2 of CTX given as an i.v. infusion monthly for 6 months and then every 3 months thereafter for a total of 2 years).
Clinical monitoring
Before the first pulsed CTX therapy, a comprehensive physical (echocardiography and ultrasound for the kidneys), biochemical (electrolytes, glucose, creatinine, lipids, markers for hepatic and renal function), haematological (blood cell counting, particularly, total white blood cell count, neutrophil and platelet counts, and antibodies), and immunological examination (e.g. erythrocyte sedimentation rate, C-reactive protein, C1q, C2 and C4 complement, and anti-DNA and antinuclear antibodies) was conducted and recorded (i.e. baseline examination). Peripheral blood (∼20 ml) was drawn from all subjects for genotyping analysis.
During and after pulsed CTX therapy, patients were closely monitored with regard to the potential clinical responses and toxicities such as myelotoxicity (white blood cell and platelet count), GI symptoms (nausea, anorexia, vomiting, abdominal discomfort or pain, diarrhoea, haemorrhagic colitis, oral mucosal ulceration, and jaundice) and infection incidence (fever and coughs due to pneumonia) for 2 weeks after initiation of pulsed therapy. The toxicity caused by CTX therapy including diarrhoea, vomiting, infection due to significant suppression of the immune system and neutropenia, and myelosuppression were graded or scored based on the National Cancer Institute Common Toxicity Criteria (CTC) [47]. Nausea and vomiting were the most common side-effects of CTX therapy in patients with SLE. Less frequent, but of more concern were myelotoxicity and severe GI toxicity (e.g. diarrhoea). Recovery from leucopenia usually began 7–10 days after cessation of CTX therapy. If the total white blood cells were less than 1,500/mm3, CTX therapy was discontinued. Other toxicities such as bladder toxicity were also monitored and recorded in details.
Genotyping study of GSTM1, GSTT1 and GSTP1
Genomic DNA was extracted from peripheral blood leucocytes using the phenol-chloroform extraction method as described [48]. To detect deletions in the GSTM1 genes, the gene was co-amplified by using the primers as described [49] (G1–5′-GAA CTC CCT GAA AAG CTA AAG C-3′ and G2–5′GTT GGG CTC AAA TAT ACG GTG G-3′). A 268 bp fragment of the β-globulin gene was used as an internal standard using primers (G1–5′-CAA CTT CAT CCA CGT TCA CC3′ and G2–5′-GAA GAG CCA AGG ACA GGT C-3′).
A polymerase chain reaction (PCR)-based assay was used to detect the codon 105 mutation of GSTP1 on exon 5 as described [49]. The sequences of the forward and reverse primers for GSTP1 exon 5 were 5′-GAG GAA ACT GAG ACC CAC TGA G-3′) and 5′-AGC CCC TTT CTT TGT TCA GCC-3′. The primer was designed by Oligo 6.0 software. Typical PCR reaction was performed in a 25 µl volume containing 1 × PCR buffer, 3.0 mm MgCl2, 0.25 mm dNTPs, 1.5 units of Taq polymerase, and 0.3 mm of primers of GSTP1 exon 5. The DNA chains were denatured by incubation at 94 °C for 5 min, followed by 35 cycles of chain reaction (94 °C for 30 s, then 60 °C for 30 s, and 72 °C for 30 s) followed by a final extension step at 72 °C for 5 min. A 424 bp DNA fragment was amplified for GSTP1 exon 5 and followed by 3 h digestion with 4 units of BsmAI for exon 5 (New England Biolabs, Beverly, MA). The fragments were separated on a 3.0% agarose gel stained with ethidium bromide. In the wild-type (I/I), heterozygous genotype (I/V), and homozygous genotype (V/V) of GSTP1, two bands (292 and 132 bp), four bands (292, 222, 132 and 70 bp), and three bands (222, 132 and 70 bp) were observed after electrophoresis, respectively. Automated sequencing of the PCR fragments confirmed that the expected sequence was amplified with these primers and the mutations of interest.
Statistical analysis
Data analysis was performed with the computer software SPSS for Windows (Version 11.0). Whenever appropriate, the observed number of each genotype was compared with that expected for a population in the Hardy–Weinberg equilibrium by using a goodness of fit χ2 test. For clinical outcomes, differences in the incidences of ADRs between different genotype groups were compared by χ2 test, or Fisher’s exact test. OR and 95% CI values were calculated to estimate the risks of myelotoxicity, GI symptoms and infection in SLE patients associated with a specific genetic polymorphism in comparison with those patients carrying the non-null genotype for GSTM1 and GSTT1 or the wild-type for GSTP1 (Ile/Ile).
Stratification analysis was further performed to reveal the effects of different genotype groups on the ADRs of pulsed CTX therapy when age, gender, and total CTX dose were considered as the additional modifying factors. Age was an important factor affecting the onset, disease severity, prognosis and toxicity of pulsed CTX therapy in SLE patients [50–53]. We set an age of 30 years as the cutoff point based on previous studies where an age of around 25–35 years appeared to be the critical age range for the onset of SLE and an important factor affecting the clinical outcomes of CTX therapy. Bell et al. [50] reported that female SLE patients with older age of onset (mean 48 years, range 38–60 years) showed an increased frequency of HLA-DR3 compared with gender and age matched controls, while those SLE patients with early onset at a younger age (mean 21.6 years, range 12–32 years) showed no significant increase in any HLA antigens. Alba et al. [51] also reported that patients with lupus nephritis were significantly younger than the controls without lupus nephritis at the time of SLE diagnosis (mean ± SD 25.6 ± 8.8 vs. 33.7 ± 12.5 years, P < 0.001). In addition, Evangelopoulos et al. [52] found that SLE patients with mitral valve prolapse were younger (33.6 ± 12.4 years) than those without this complication (41.1 ± 12.9, P < 0.05). When the duration of the disease was taken into account (8.44 ± 6.33 years), the mean age of onset of SLE in these patients with mitral valve prolapse was about 30 years [52]. Importantly, age was also a determining factor for the prognosis of SLE patients treated with CTX. Ioannidis et al. [53] reported that in women with SLE aged ≥ 32 years, there was a substantially increased risk of sustained amenorrhea even with very short intravenous CTX therapy, while in younger women (<32 years) the risk of sustained amenorrhea was markedly smaller, especially in those women with a shorter disease duration (<5 years) without the presence of anti-Ro/SSA and anti-U1RNP antibodies [53]. Moreover, Boumpas et al. [10] revealed that intermittent pulse CTX therapy in SLE patients was associated with sustained amenorrhea. In this study, among the eight patients who were ≥31 years, five (62%) developed amenorrhea compared with 12% of women with a younger age (<31 years). Based on the previous findings, we set 30 years of age as the cutoff point in our stratification analysis.
In addition, logistic regression analysis of variance was used to examine the effects of the GSTM1, GSTT1 and GSTP1 genotypes and other covariates such as age and gender on the incidences of myelotoxicity, GI symptoms and infection caused by CTX therapy. A P value of <0.05 was considered statistically significant.
Results
Demographic information and clinical characteristics
All 102 Han Chinese patients with newly diagnosed SLE were included in the study. Their mean body weight was 51.5 kg (range 33.5–72.0 kg). During the 2 weeks from the initiation of pulsed CTX therapy, myelotoxicity occurred in 41 patients (seven males and 34 females), GI symptoms occurred in 32 patients (four males and 28 females), and infection occurred in 16 patients (three males and 13 females). There were no statistical differences in the frequency of ADRs to CTX therapy between males and females in this study population (P > 0.05).
There were 56, 53, 64, 34 and 4 patients carrying the GSTM1(-), GSTT1(-), GSTP1(I/I), GSTP1*-105I/V (heterozygote) and GSTP1-*105V/V (homozygote) genotypes, respectively. There were no statistical differences in GST genotype frequencies between males and females (P > 0.05). Also, there were no statistical differences (P > 0.05) in age, gender, body weight and total CTX dose between the GSTM1(+) and GSTM1(-) genotype groups, the GSTT1(+) and GSTT1(-) genotype groups, and the GSTP1 wildtype genotype and GSTP1-*105I/V, or GSTP1-*105V/V genotype groups, as shown in Table 1.
Table 1.
Demographic data of the patients with SLE grouped according to GSTM1, GSTT1 and GSTP1 genotypes
| Gender | ||||||
|---|---|---|---|---|---|---|
| Genotype | n (%) | Male n (%) | Female n (%) | Age (years) | Body weight (kg) | Total dose (g) |
| GSTM1 | ||||||
| GSTM1(+) | 46 (45.1) | 5 (4.9) | 41 (40.2) | 30.8 ± 11.7 | 51.0 ± 8.2 | 1.03 ± 0.22 |
| GSTM1(-) | 56 (54.9) | 9 (8.8) | 47 (46.1) | 28.9 ± 10.1 | 51.8 ± 10.3 | 1.04 ± 0.20 |
| GSTT1 | ||||||
| GSTT1(+) | (48.0) | (8.8) | (39.2) | 29.9 ± 10.9 | 53.2 ± 9.3 | 1.04 ± 0.20 |
| GSTT1(-) | 53 (52.0) | 5 (4.9) | 48 (47.1) | 29.6 ± 10.9 | 49.9 ± 9.2 | 1.03 ± 0.20 |
| GSTP1 | ||||||
| GSTP1 (I/I) | 64 (62.7) | 8 (7.8) | 56 (54.9) | 28.5 ± 10.2 | 50.1 ± 7.6 | 1.04 ± 0.18 |
| GSTP1 (I/V or V/V) | 38 (37.3) | 6 (5.9) | 32 (31.4) | 31.9 ± 11.7 | 53.7 ± 11.5 | 1.03 ± 0.23 |
| Total | 102 (100) | 14 (13.7) | 88 (86.3) | 29.8 ± 10.8 | 51.5 ± 9.4 | 1.04 ± 0.20 |
Relationships between GST genotypes and the risk of ADRs to CTX
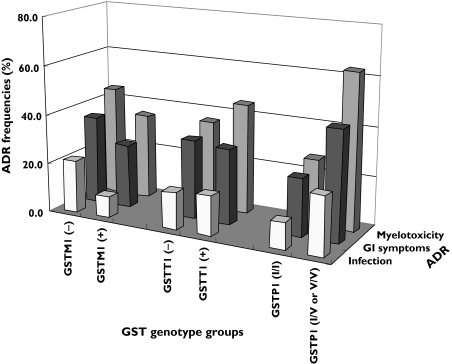
The ADR frequencies following pulsed CTX therapy among different GST genotypes are shown in Figure 2. There were no significant differences in myelotoxicity, GI symptom and infection between patients carrying the GSTM1(+) and GSTM1(-) genotypes, and patients carrying GSTT1(+) and GSTT1(-) genotypes (P > 0.05). The incidence of myelotoxicity and GI symptoms was significantly higher in patients with GSTP1-*105I/V, or GSTP1-*105V/V genotypes, than in patients with the GSTP1 wildtype genotype (P < 0.001 and P = 0.025, respectively). In contrast, no significant difference in infection rate was observed between these two genotype groups (GSTP1-*105I/V or GSTP1-*105V/V vs. GSTP1 *105I/I (wildtype), P = 0.087).
Figure 2.
The incidence of short-term (2-week) side-effects, including myelotoxicity, gastrointestinal (GI) symptoms, and infection with different GSTM1, GSTT1 and GSTP1 genotypes in SLE patients receiving pulsed high-dose (1.0 g) cyclophosphamide (CTX) therapy. The incidence of myelotoxicity and GI symptoms was significantly higher in patients with the GSTP1-*105I/V or GSTP1-105 V/V genotype than in patients with the GSTP1 wildtype genotype (P < 0.001 and P < 0.05, respectively)
Combined effects of GST genotypes and gender, age or CTX dose on the ADRs to pulsed CTX therapy in SLE patients
Table 2 shows the results of stratification analysis on the relationships of ADRs to CTX treatment with genotypes of GSTM1, GSTT1 and GSTP1 with regard to gender, age and total CTX dosage. Among females, the frequency of myelotoxicity in patients with the GSTP1*-105I/V or GSTP1*-105 V/V genotype was significantly higher than those with the wildtype genotype (OR 5.00, 95% CI 1.96, 12.76). As for age, the frequency of myelotoxicity in patients with the GSTP1-*105I/V or GSTP1-*105V/V genotype was significantly higher than those with the wildtype genotype (OR 7.50, 95% CI 2.14, 26.24) in subjects aged <30 years, but this increased risk of myelotoxicity was not observed in patients aged ≥30 years. In addition, in patients receiving higher CTX doses (>1.0 g), the frequency of myelotoxicity in GSTP1-*105I/V or GSTP1-*105V/V genotype groups was significantly higher than those in the wildtype genotype group (OR 12.88, 95% CI 3.16, 52.57). However, the frequency of GI symptoms in the lower CTX dose group (≤1.0 g) in patients with the GSTP1*105I/V, or GSTP1-105V/V genotype was significantly higher than in those with wildtype genotype (OR 3.33, 95% CI 1.03, 10.79). However, there were no significant differences for the incidence of infection induced by pulsed CTX therapy between these different GST genotypes tested (P > 0.05).
Table 2.
The effects of GSTM1, GSTT1 and GSTP1 genotypes on the incidences of short-term adverse drug reactions (myelosuppression, GI toxicity and infection event) induced by pulsed high-dose cyclophosphamide (CTX) therapy in SLE patients stratified by gender, age, and total CTX dose
| Myelotoxicity | GI toxicity | Infection event | |||||
|---|---|---|---|---|---|---|---|
| Factor | Genotype | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Gender (n) | |||||||
| Male (14) | GSTM1(-) vs. (+) | 1.88 | 0.20, 17.27 | 0.56 | 0.31, 1.00 | 0.67 | 0.42, 1.06 |
| GSTT1(-) vs. (+) | 0.53 | 0.06, 4.91 | 0.50 | 0.04, 6.68 | 0.88 | 0.06, 12.98 | |
| GSTP1(I/I) vs. (I/V or V/V) | 3.33 | 0.36, 30.70 | 7.00 | 0.50, 97.75 | 3.50 | 0.24, 51.90 | |
| Female (88) | GSTM1(-) vs. (+) | 1.43 | 0.60, 3.40 | 1.25 | 0.50, 3.08 | 2.19 | 0.62, 7.74 |
| GSTT1(-) vs. (+) | 0.74 | 0.31, 1.76 | 1.17 | 0.47, 2.88 | 0.97 | 0.30, 3.15 | |
| GSTP1(I/I) vs. (I/V or V/V) | 5.00* | 1.96, 12.76 | 2.33 | 0.93, 5.88 | 2.33 | 0.71, 7.68 | |
| Age (n) | |||||||
| <30 (years) (56) | GSTM1(-) vs. (+) | 2.09 | 0.66, 6.65 | 1.31 | 0.42, 4.07 | 7.04 | 0.82, 60.82 |
| GSTT1(-) vs. (+) | 0.73 | 0.24, 2.19 | 1.17 | 0.39, 3.55 | 2.27 | 0.51, 10.18 | |
| GSTP1(I/I) vs. (I/V or V/V) | 7.50* | 2.14, 26.24 | 2.80 | 0.87, 9.04 | 1.89 | 0.44, 8.09 | |
| ≥30 years (46) | GSTM1(-) vs. (+) | 0.92 | 0.30, 2.82 | 1.92 | 0.52, 7.12 | 1.40 | 0.28, 7.12 |
| GSTT1(-) vs. (+) | 0.61 | 0.19, 1.96 | 0.97 | 0.27, 3.52 | 0.28 | 0.05, 1.62 | |
| GSTP1(I/I) vs. (I/V or V/V) | 2.83 | 0.85, 9.46 | 2.80 | 0.74, 10.52 | 4.00 | 0.69, 23.30 | |
| Total dose (n) | |||||||
| ≤1 g (55) | GSTM1(-) vs. (+) | 1.39 | 0.45, 4.34 | 2.00 | 0.59, 6.76 | 3.17 | 0.34, 29.23 |
| GSTT1(-) vs. (+) | 0.54 | 0.18, 1.61 | 1.23 | 0.40, 3.78 | 0.82 | 0.15, 4.44 | |
| GSTP1(I/I) vs. (I/V or V/V) | 1.80 | 0.58, 5.61 | 3.33* | 1.03, 10.79 | 4.53 | 0.75, 27.50 | |
| >1 g (47) | GSTM1(-) vs. (+) | 1.72 | 0.53, 5.57 | 1.09 | 0.30, 3.92 | 3.83 | 0.85, 17.30 |
| GSTT1(-) vs. (+) | 0.90 | 0.28, 2.89 | 0.86 | 0.24, 3.09 | 1.06 | 0.26, 4.27 | |
| GSTP1(I/I) vs. (I/V or V/V) | 12.88* | 3.16, 52.57 | 1.83 | 0.48, 6.95 | 1.64 | 0.40, 6.70 | |
CI Confidence interval; G, gastrointestinal; OR = Odds ratio.
P < 0.05.
Risk factors associated with ADRs to pulsed CTX therapy in SLE patients
When multiple logistic regression analysis was conducted to evaluate the risk factors associated with ADRs to pulsed CTX therapy in SLE patients, we found that the risk of myelotoxicity and GI toxicity in GSTP1-*105I/V or GSTP1*-105V/V carriers, was 5.7-fold (OR 5.75, 95% CI 2.25, 14.70) and 3.8-fold higher (OR 3.83, 95% CI 1.45, 10.08) than that in patients with the GSTP1 wildtype genotype.
Following adjustment for other potential risk factors, including gender, age, total CTX dose and GSTP1 genotype for myelotoxicity and GI symptoms, it appeared that the GSTM1 and GSTT1 null mutations did not affect the incidence of ADRs to pulsed high-dose CTX therapy in our study population. In addition, all risk factors (e.g. age, gender and GST genotypes) evaluated in this study were not associated with an infection rate caused by pulsed CTX therapy, as shown in Table 3.
Table 3.
Relationships between the short-term side-effects (myelosuppression, gastrointestinal toxicity and infection event) of pulsed high-dose cyclophosphamide (CTX) therapy and various covariates including gender, age, body weight, total CTX dose, and GSTM1, GSTT1 and GSTP1 genotypes in SLE patients by logistic regression analysis
| Factor | Myelotoxicity | GI symptoms | Infection | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.02 | 0.98, 1.06 | 0.96 | 0.92, 1.01 | 1.00 | 0.95, 1.06 |
| Gender | 0.47 | 0.12, 1.88 | 0.78 | 0.18, 3.37 | 0.84 | 0.18, 4.07 |
| Body weight | 0.95 | 0.90, 1.01 | 0.95 | 0.90, 1.00 | 1.01 | 0.95, 1.08 |
| Total dose | 1.07 | 0.13, 9.00 | 0.34 | 0.04, 3.18 | 3.05 | 0.20, 47.75 |
| GSTM1 | 1.70 | 0.70, 4.16 | 1.57 | 0.63, 3.93 | 2.91 | 0.84, 10.05 |
| GSTT1 | 0.55 | 0.22, 1.37 | 0.82 | 0.33, 2.08 | 0.94 | 0.30, 2.96 |
| GSTP1 | 5.75* | 2.25, 14.70 | 3.83* | 1.45, 10.08 | 2.48 | 0.80, 7.64 |
CI Confidence interval; GI gastrointestinal; OR = Odds ratio.
P < 0.05.
Discussion
Pharmacogenetics is the study of how genetic variations affect drug responses in individual patients [54]. Because CTX chemotherapy is relatively nonspecific and has narrow therapeutic indices, there is great potential for pharmacogenetics to improve treatment outcomes by increasing efficacy while reducing toxicity [55]. Because multiple enzymes are involved in the bioactivation and detoxification of CTX, detailed pharmacogenetic studies are needed to explore the role of mutations in genes encoding GSTs, CYPs, ALDHs and other relevant proteins/enzymes involved in the pharmacokinetic and pharmacodynamic variability in patients treated with CTX. Many other genes may also affect the response and toxicity of CTX therapy and their role is unclear. Pharmacogenetics can provide insights into genetic factors influencing the pharmacokinetics, pharmacodynamics and toxicology of CTX and make individualized medication possible for chemotherapy in SLE patients. Much information on the mechanism of action, resistance, toxicity, pharmacokinetics, and pharmacogenetics of CTX has been gained from a large number of preclinical and clinical studies [13, 56]. However, the interplay between the pharmacokinetics, pharmacodynamics and pharmacogenetics of CTX, in particular, the dose–response and dose-genetic factor relationships, has not been fully elucidated. The lack of such important information makes it difficult to optimize the dose and treatment regimens when CTX is used in SLE patients.
Pulsed high-dose CTX therapy has become one of the most effective approaches in improving the clinical symptoms and long-term efficacy (e.g. survival) of SLE patients with major organ injuries [6]. However, pulsed CTX therapy is often associated with an increased risk of short-term ADRs, such as myelotoxicity, GI symptoms and infection, and long-term ADRs, including ovarian failure, urotoxicity, and secondary tumours [40, 57]. Because of these severe ADRs, especially short-term ADRs, the dosage of pulsed CTX therapy has to be markedly reduced or even is discontinued in some patients [58]. Since CTX and its toxic metabolites are mainly detoxified by multiple GSTs, including GTSM1, GSTA1, GSTT1 and GSTP1, we hypothesized that the genetic polymorphisms of GSTM1, GSTT1 and GSTP1 might lead to increased ADRs to CTX therapy in SLE patients due to reduced detoxification of the parental drug and its toxic metabolites. This study indicated that GSTP1-*105I/V and GSTP1-*105V/V genotypes are risk factors for short-term toxicity, including myelotoxicity and GI symptoms, to a pulsed high-dose CTX regimen in SLE patients. The data also indicated that patients who were younger than 30 years old or who received a higher dose of CTX (>1.0 g) had higher risk of short-term ADRs. Dose intensity is an important determinant of clinical outcomes (efficacy and toxicity) in the treatment of patients with severe SLE when they receive pulsed CTX therapy. Attempts to increase dose-intensity of CTX in clinical practice are always limited by haematologic toxicity (mainly myelosuppression) and other organ toxicities in many patients. Therefore, close clinical monitoring of ADRs, especially severe myelosuppression, is always required in patients receiving pulsed CTX therapy.
GSTs play an important role in the detoxification of CTX in vivo. Particularly the GSTP1 enzyme exhibits specific and high activity in the conjugation of CTX and its toxic metabolites [18, 59]. Studies have shown that the GSTP1 protein encoded by GSTP1 codon 105 mutation (Ile→Val) has significantly lower catalytic activity towards its substrates and lower enzyme thermal stability compared with the wildtype protein [60, 61]. Compared with the wildtype GSTP1 protein, the GSTP1*-105V/V protein has a 2- and 7.5-fold lower catalytic efficiency to thiotepa and chlorambucil, respectively [60, 61]. Our results suggest that the mutations of GSTP1 codon 105 resulted in reduced enzyme activity towards CTX and its toxic metabolites (e.g. phosphoramide mustard and acrolein), and subsequently these toxic metabolites would accumulate in the body, leading to increased short-term ADRs to pulsed CTX therapy in SLE patients.
In the present study, we evaluated the association of the common GSTT1, GSTM1 and GSTP1 genotypes with short-term ADRs to pulsed CTX therapy. The results indicated that the GSTP1-*105I/V and GSTP1-*105V/V mutation, but not the GSTM1 or GSTT1 null mutation, significantly increased the likelihood of short-term ADRs (myelosuppression and GI toxicity) to pulsed CTX therapy in SLE patients. Thus, GSTP1*105I/V and GSTP1-*105V/V mutations may be considered good predictors for the short-term toxicity of CTX therapy in SLE patients. Because the determination and monitoring of toxic metabolites of CTX is difficult due to their instability and short half-lives in vivo [13], the evaluation of the effects of GSTM1, GSTT1 and GSTP1 genotypes on the susceptibility to ADRs to CTX therapy has important clinical implications. By exploring the sensitive genotypes for short-term ADRs or efficacy of CTX therapy, we can identify important genetic information. Such information is useful for the improvement of efficacy and minimization of short-term ADRs by optimization of pulsed CTX therapy in SLE patients. As such, some special groups of SLE patients with specific GST genotypes have to reduce the dose or stop the pulsed high-dose CTX regimen because of severe myelotoxicity and/or GI toxicity and an alternative therapeutic regimen has to be considered. In SLE patients receiving pulsed CTX therapy, the maximum tolerated doses (0.5–1.0 g m−2 body surface area) are always given to obtain maximum clinical efficacy, but this always leads to significant organ toxicity, which may be life-threatening. Thus, a complete risk-benefit assessment and close clinical monitoring are often needed in these patients and dosage and treatment regimens should be adjusted when necessary.
On the other hand, previous studies have shown that patients with the GSTP1-*105V mutation experienced more long-term toxicity from chemotherapy than patients without this mutation [39, 40]. Allan et al. [40] reported that patients who inherited the GSTP1-*105V mutation had a significantly increased risk of developing secondary chemotherapy-related acute myeloid leukaemia, a devastating complication of long-term cancer survival, after chemotherapy. However, to date, there are limited studies concerning the impact of GST genotypes on the short-term ADRs to CTX-based chemotherapy. Similar to the results from previously published reports addressing the long-term efficacy (survival) or toxicity (secondary leukaemia) of chemotherapeutic agents [39, 40], we found that GSTM1 and GSTT1 null mutations did not alter the short-term ADRs to pulsed high-dose CTX therapy in SLE patients.
In this study, we did not closely monitor the clinical efficacy or evaluate its relationship with GST genotype because of the nature of this study (only 2 weeks close monitoring of toxicity). Our results suggested that the GSTP1-*105V mutation is likely to enhance the efficacy of CTX due to increased concentrations of cytotoxic phosphoramide mustard. Previous clinical studies investigating the association of GST genotype with the clinical outcomes after CTX-based chemotherapy demonstrated that individuals with the GSTP1-*105I/V and GSTP1-*105V/V genotypes responded better to chemotherapy than those patients carrying the wildtype GSTP1 gene. Sweeney et al. [62] reported that patients with the GSTP1-*105V/V (homozygote) mutation have a significantly better prognosis than those with the wildtype gene when treated with CTX in combination with adriamycin for advanced breast cancer. Both CTX and adriamycin are GSTP1 substrates [13]. Yang et al. [39] also reported that patients with advanced breast cancer who were homozygous for the variant GSTP1-*105V allele had a 60% reduction in mortality risk compared with patients who were homozygous for the Ile allele (wildtype), while no association was found with respect to GSTM1 or GSTT1 mutation. These findings indicate that GSTP1 codon 105 mutation also enhances the efficacy of CTX-based chemotherapy while the organ toxicity was reduced. On the other hand, some GST mutations may confer drug resistance to CTX-based chemotherapy when the mutations result in increased enzyme activity [56].
In paediatric patients with a steroid-sensitive nephrotic syndrome, a GSTM1 null polymorphism due to gene depletion gave a significantly better rate of sustained remission after CTX therapy than in patients with the heterozygous or homozygous GSTM1 wild type [63]. In contrast, children with a GSTP1 codon 105 heterozygous or homozygous polymorphism had a significantly lower rate of sustained remission compared with the homozygous wild type [63]. The GSTT1 genotype did not alter the outcome of CTX treatment. Fifty % of children with the combination of GSTM1 null and GSTP1 wild type did not relapse, compared with 6% in other children. These findings indicate that the genetic polymorphisms of GSTM1 and GSTP1 have a significant impact on the long-term remission rate of CTX treatment in children with steroid-sensitive nephrotic syndrome. GSTM1 null mutation increased the efficacy of CTX, whereas GSTP1 polymorphism seemed to be related to enhanced susceptibility to further relapses [63]. The GSTM1 null mutation results in lack of expression of the corresponding protein, probably generating more accumulation of the cytotoxic and immuno-suppressive 4-OH-CTX that is a substrate for GSTM1 protein.
However, a clinical study in patients with ovarian cancer indicated that GSTA1 and GSTP1 enzyme levels in the tumour tissue were not associated with the clinical response to CTX treatment in combination with carboplatin [64]. Furthermore, concomitant expression of GSTP1 with MRP1 failed to enhance resistance to 4-OH-CTX [65]. 4-OH-CTX does not appear to be a substrate for GSTP1. These clinical findings indicate that the polymorphism of GSTs may or may not affect the metabolism and subsequently the clinical response to CTX, depending on many factors associated with the patients, disease and combined drugs. Further studies are warranted to explore the relative contribution of various GST enzymes in the conjugation of CTX and its metabolites and the clinical importance in CTX-based chemotherapy when the activity of these enzymes is altered.
Our stratification analysis indicated that patients with the GSTP1 codon 105 mutation (both heterozygous and homozygous) more readily experienced myelotoxicity in several subgroups of SLE patients. Patients younger than 30 years with the GSTP1-*105I/V and GSTP1-*105V/V genotypes had a significantly higher rate of myelotoxicity compared with those patient with the wildtype GSTP1 genotype. This is probably due to the fact that the progenitor cells in bone marrow of young people are more sensitive to CTX than those of older age patients. When the protection of the GST enzymes by detoxification of CTX and its toxic metabolites by the progenitor cells in bone marrow decreases in SLE patients with GSTP1-*105I/V and GSTP1-*105V/V genotypes, myelotoxicity would increase after CTX treatment. In addition, the present study demonstrated that SLE patients with the GSTP1-*105I/V, or GSTP1-*105V/V genotype, treated with a higher CTX dose (>1.0 g) had significantly higher risk of myelotoxicity, compared with those with the GSTP1 wildtype genotype. This is probably due to reduced GSTP1 enzyme activity resulting from codon 105 mutation and thus toxic CTX metabolites accumulate in the body, leading to an increased rate of short-term ADRs such as myelosuppression. Interestingly, the frequency of GI toxicity in SLE patients treated with lower CTX doses (≤1.0 g) with GSTP1-*105I/V or GSTP1-*105V/V genotype was significantly higher than those with the wildtype GSTP1 genotype. The reason for this is unclear, but may be related to the intestinal disposition of CTX and its toxic metabolites. CTX is primarily (∼70%) excreted in urine in the form of conjugated and oxidized metabolites and to a less extent in the faeces [13, 66]. However, only ∼10–20% is excreted in an unchanged form in the urine and only about 4% is excreted in the bile after CTX administration [1, 67]. Since the pharmacokinetics of CTX are nonlinear (dose-dependent), administration of a lower dose of CTX in SLE patients with the GSTP1-*105I/V or GSTP1-*105V/V genotype may result in higher biliary excretion of toxic metabolites than after a high CTX dose. Therefore, a lower dose of CTX may cause a higher incidence of GI toxicity than a high dose of CTX.
In the present study, there was evidently a difference in the incidence of SLE between males and females (1 : 6.3). Because of the small number of male subjects, we did not find marked differences between the different genotype groups of GSTM1, GSTT1 and GSTP1 in males after stratification by gender.
The current study revealed that certain GST genotypes (e.g. GSTP1 codon 105 mutation) could predict the incidence of short-term ADRs after pulsed CTX therapy in SLE patients. SLE patients with the GSTP1 codon 105 mutation, especially those who are younger than 30 years or who are administered more than 1.0 g CTX, should receive intensive clinical monitoring for severe myelotoxicity and other side-effects. Proper dosage adjustment is needed for some subgroups of SLE patients. Therapeutic drug monitoring plays a critical role in the optimal and rational use of CTX in SLE patients [68]. Recently, pharmacogenetic-orientated therapeutic drug monitoring has been developed and the oxazaphosphorines including CTX and its analogue ifosfamide have been proposed as potential candidates for this novel and efficient approach [68]. Based on the knowledge of the pharmacokinetics, pharmacodynamics, toxicology and pharmacogenetics of CTX and effective use of therapeutic drug monitoring, optimal use with individualized dose regimens will become possible for SLE patients.
Careful study design is always needed in pharmacogenetic studies as genetic factors can interact with a number of factors associated with the patients (e.g. age, gender and smoking) and drugs administered (e.g. dosage, regimen, and duration of therapy). The subjects in our study all suffered from SLE and were treated with a similar pulsed CTX regimen with relatively narrow dosage ranges. Therefore, this could facilitate the evaluation of the effects of the genetic mutations of GSTM1, GSTT1 and GSTP1 on the short-term ADRs, including myelotoxicity, GI symptoms and the incidence of infection following pulsed intravenous CTX therapy over 2 weeks. However, the effects of other factors such as smoking, alcohol consumption, occupation and radiotherapy on the clinical outcomes of CTX therapy and their interplay with the genetic mutations of GSTT1, GSTM1 and GSTP1 genes should be investigated in SLE patients in future clinical trials.
A number of proteins/enzymes such as GSTs, CYPs and ALDHs are involved in the metabolism and disposition of CTX. A number of synonymous and nonsynonymous SNPs have been found in the genes encoding these proteins [1]. Up to date, more than 1.4 million SNPs have been identified in the initial sequencing of the human genome, with over 60 000 of them in the coding region of genes [69]. A single gene may have multiple SNPs. A SNP of an important gene such as GTSP1 or CYP2B6 may alter the metabolism and elimination of CTX, but haplotypes (combinations of genetic polymorphisms) may be more important in determining interindividual variability in disposition and response to CTX. Thus, haplotype structure is often a better predictor of phenotypic consequences (e.g. ADRs and clinical efficacy) than are individual polymorphisms. It would be desirable to develop simple but robust molecular methods to determine the haplotype structure of GSTM1, GSTT1 and GSTP1 genes and other relevant genes in SLE patients treated with a pulsed CTX regimen. The resultant genetic information would be useful for optimizing CTX therapy in these patients.
In conclusion, the GSTP1 codon 105 mutation conferred increased susceptibility to myelotoxicity, especially when carriers were younger than 30 years or the total CTX dose was >1.0 g in SLE patients. This mutation also increased the risk of GI symptoms in SLE patients after pulsed high-dose CTX treatment. However, the frequencies of GI symptoms in the lower CTX dose group (≤1.0 g) in SLE patients with the GSTP1-*105I/V (heterozygote) or GSTP1-*105V/V (homozygote) genotype were significantly higher than those with the wildtype GSTP1 genotype. In contrast, GSTM1 and GSTT1 null mutations did not affect short-term ADR susceptibility in SLE patients receiving pulsed CTX therapy. Further studies are needed to explore the effects of common and rare GSTT1, GSTM1 and GSTP1 mutations on the short-term and long-term outcomes (toxicity and efficacy) of pulsed CTX therapy in SLE patients.
Acknowledgments
The authors appreciate the support by the National Nature Science Fund of China (No. 30572231) and the National University of Singapore Academic Research Funds (Nos. R-148-000-047-101 and R-148-000-054-112).
References
- 1.Zhang J, Tian Q, Zhou SF. Clinical pharmacology of cyclophosphamide and ifosfamide. Curr Drug Ther. 2006;1:104–68. [Google Scholar]
- 2.Demirer T, Buckner CD, Appelbaum FR, Bensinger WI, Sanders J, Lambert K, Clift R, Fefer A, Storb R, Slattery JT. Busulfan, cyclophosphamide and fractionated total body irradiation for autologous or syngeneic marrow transplantation for acute and chronic myelogenous leukemia: phase I dose escalation of busulfan based on targeted plasma levels. Bone Marrow Transplant. 1996;17:491–5. [PubMed] [Google Scholar]
- 3.Majado MJ, Gonzalez C, Marin L, Morales A, Moya MR, Candel R. Second mobilization of peripheral blood progenitor cells in patients with poor first mobilization. Transplant Proc. 2003;35:2027–8. doi: 10.1016/s0041-1345(03)00710-3. [DOI] [PubMed] [Google Scholar]
- 4.Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limon JM, Fuentes de la Mata J, Clark P, Vargas F, Alocer-Varela J. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:620–5. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verburg RJ, Sont JK, van Laar JM. Reduction of joint damage in severe rheumatoid arthritis by high-dose chemotherapy and autologous stem cell transplantation. Arthritis Rheum. 2005;52:421–4. doi: 10.1002/art.20859. [DOI] [PubMed] [Google Scholar]
- 6.Petri M. Systemic lupus erythematosus: 2006 update. J Clin Rheumatol. 2006;12:37–40. doi: 10.1097/01.rhu.0000200420.67262.04. [DOI] [PubMed] [Google Scholar]
- 7.Houssiau FA. Cyclophosphamide in lupus nephritis. Lupus. 2005;14:53–8. doi: 10.1191/0961203305lu2060oa. [DOI] [PubMed] [Google Scholar]
- 8.Lindemann A, Hoffken K, Schmidt RE, Diehl V, Kloke O, Gamm H, Hayungs J, Oster W, Bohm M, Kolitz JE. A phase-II study of low-dose cyclophosphamide and recombinant human interleukin-2 in metastatic renal cell carcinoma and malignant melanoma. Cancer Immunol Immunother. 1989;28:275–81. doi: 10.1007/BF00205237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4 (+) 25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 10.Boumpas DT, Austin HA, rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119:366–9. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Katsifis GE, Tzioufas AG. Ovarian failure in systemic lupus erythematosus patients treated with pulsed intravenous cyclophosphamide. Lupus. 2004;13:673–8. doi: 10.1191/0961203304lu2012oa. [DOI] [PubMed] [Google Scholar]
- 12.Peters WP, Stuart A, Klotman M, Gilbert C, Jones RB, Shopall EJ, Gockerman J, Bast RC, Jr, Moore JO. High-dose combination cyclophosphamide, cisplatin, and melphalan with autologous bone marrow support. A clinical and pharmacologic study. Cancer Chemother Pharmacol. 1989;23:377–83. doi: 10.1007/BF00435840. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Tian Q, Chan SY, Li SC, Zhou S, Duan W, Zhu YZ. Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metab Rev. 2005;37:611–703. doi: 10.1080/03602530500364023. [DOI] [PubMed] [Google Scholar]
- 14.Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–37. [PubMed] [Google Scholar]
- 15.Ren S, Yang JS, Kalhorn TF, Slattery JT. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res. 1997;57:4229–35. [PubMed] [Google Scholar]
- 16.Zon G, Ludeman SM, Brandt JA, Boyd VL, Ozkan G, Egan W, Shao KL. NMR spectroscopic studies of intermediary metabolites of cyclophosphamide. A comprehensive kinetic analysis of the interconversion of cis- and trans-4-hydroxycyclophosphamide with aldophosphamide and the concomitant partitioning of aldophosphamide between irreversible fragmentation and reversible conjugation pathways. J Med Chem. 1984;27:466–85. doi: 10.1021/jm00370a008. [DOI] [PubMed] [Google Scholar]
- 17.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 18.Dirven HA, van Ommen B, van Bladeren PJ. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res. 1994;54:6215–20. [PubMed] [Google Scholar]
- 19.Dirven HA, Venekamp JC, van Ommen B, van Bladeren PJ. The interaction of glutathione with 4-hydroxycyclophosphamide and phosphoramide mustard, studied by 31P nuclear magnetic resonance spectroscopy. Chem Biol Interact. 1994;93:185–96. doi: 10.1016/0009-2797(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 20.Yule SM, Price L, McMahon AD, Pearson AD, Boddy AV. Cyclophosphamide metabolism in children with non-Hodgkin’s lymphoma. Clin Cancer Res. 2004;10:455–60. doi: 10.1158/1078-0432.ccr-0844-03. [DOI] [PubMed] [Google Scholar]
- 21.Moreb JS, Gabr A, Vartikar GR, Gowda S, Zucali JR, Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J Pharmacol Exp Ther. 2005;312:339–45. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 22.Bohnenstengel F, Hofmann U, Eichelbaum M, Kroemer HK. Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol. 1996;51:297–301. doi: 10.1007/s002280050201. [DOI] [PubMed] [Google Scholar]
- 23.Kaijser GP, Korst A, Beijnen JH, Bult A, Underberg WJ. The analysis of ifosfamide and its metabolites (review) Anticancer Res. 1993;13:1311–24. [PubMed] [Google Scholar]
- 24.Kaijser GP, Beijnen JH, Jeunink EL, Bult A, Keizer HJ, de Kraker J, Underberg WJ. Determination of chloroacetaldehyde, a metabolite of oxazaphosphorine cytostatic drugs, in plasma. J Chromatogr. 1993;614:253–9. doi: 10.1016/0378-4347(93)80316-v. [DOI] [PubMed] [Google Scholar]
- 25.Tacka KA, Dabrowiak JC, Goodisman J, Souid AK. Kinetic analysis of the reactions of 4-hydroperoxycyclophosphamide and acrolein with glutathione, mesna, and WR-1065. Drug Metab Dispos. 2002;30:875–82. doi: 10.1124/dmd.30.8.875. [DOI] [PubMed] [Google Scholar]
- 26.Crook TR, Souhami RL, Whyman GD, McLean AE. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986;46:5035–8. [PubMed] [Google Scholar]
- 27.Richard CS, Peter WJ, Anthony AF. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett. 2000;112–113:357–63. doi: 10.1016/s0378-4274(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 28.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 29.Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61:154–66. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1–1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–6. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Wang Y, Roe B, Pearson WR. Characterization of the human class Mu glutathione S-transferase gene cluster and the GSTM1 deletion. J Biol Chem. 1998;273:3517–27. doi: 10.1074/jbc.273.6.3517. [DOI] [PubMed] [Google Scholar]
- 33.Siedegard J, Vorachek WR, Pero RW, Pearson WR. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci USA. 1988;85:7293–7. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pemble S, Scroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterisation of a genetic polymorphism. Biochem J. 1994;300:271–6. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruhn C, Brockmoller J, Kerb R, Roots I, Borchert HH. Concordance between enzyme activity and genotype of glutathione S-transferase theta (GSTT1) Biochem Pharmacol. 1998;56:1189–93. doi: 10.1016/s0006-2952(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 36.Yuan ZM, Smith PB, Brundrett RB, Colvin M, Fenselau C. Glutathione conjugation with phosphoramide mustard and cyclophosphamide. A mechanistic study using tandem mass spectrometry. Drug Metab Dispos. 1991;19:625–9. [PubMed] [Google Scholar]
- 37.Dirven HA, Megens L, Oudshoorn MJ, Dingemanse MA, van Ommen B, van Bladeren PJ. Glutathione conjugation of the cytostatic drug ifosfamide and the role of human glutathione S-transferases. Chem Res Toxicol. 1995;8:979–86. doi: 10.1021/tx00049a012. [DOI] [PubMed] [Google Scholar]
- 38.Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R. Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos. 2001;29:264–7. [PubMed] [Google Scholar]
- 39.Yang G, Shu XO, Ruan ZX, Cai QY, Jin F, Gao YT, Zheng W. Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer. 2005;103:52–8. doi: 10.1002/cncr.20729. [DOI] [PubMed] [Google Scholar]
- 40.Allan JM, Wild CP, Rollinson S, Willett EV, Moorman AV, Dovey GJ, Roddam PL, Roman E, Cartwright RA, Morgan GJ. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc Natl Acad Sci U S A. 2001;98:11592–7. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goekkurt E, Hoehn S, Wolschke C, Wittmer C, Stueber C, Hossfeld DK, Stoehlmacher J. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS) – novel predictors for response and survival in gastric cancer patients. Br J Cancer. 2006;94:281–6. doi: 10.1038/sj.bjc.6602891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beeghly A, Katsaros D, Chen H, Fracchioli S, Zhang Y, Massobrio M, Risch H, Jones B, Yu H. Glutathione S-transferase polymorphisms and ovarian cancer treatment and survival. Gynecol Oncol. 2006;100:330–7. doi: 10.1016/j.ygyno.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Spitz MR, Zhao H, Dong Q, Truong M, Chang JY, Blumenschein GR, Jr, Hong WK, Wu X. Association between glutathione S-transferase pi polymorphisms and survival in patients with advanced nonsmall cell lung carcinoma. Cancer. 2006;106:441–7. doi: 10.1002/cncr.21619. [DOI] [PubMed] [Google Scholar]
- 44.Kusama M, Kubota T, Matsukura Y, Matsuno K, Ogawa S, Kanda Y, Iga T. Influence of glutathione S-transferase A1 polymorphism on the pharmacokinetics of busulfan. Clin Chim Acta. 2006. in press. [DOI] [PubMed]
- 45.Kirkwood BR, Sterne JAC. Medical Statistics. 2. Malden, MA, USA: Blackwell Science; 2003. [Google Scholar]
- 46.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 47.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W. Common toxicity criteria: Version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 48.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 49.Zhong SL, Zhou S, Chen X, Huang M. Rapid determination of common mutations in glutathione S-transferase gene by PCR-based methods in healthy Chinese. Clin Chim Acta. 2006;364:205–8. doi: 10.1016/j.cccn.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Bell DA, Rigby R, Stiller CR, Clark WF, Harth M, Ebers G. HLA antigens in systemic lupus erythematosus: relationship to disease severity, age at onset, and sex. J Rheumatol. 1984;11:475–9. [PubMed] [Google Scholar]
- 51.Alba P, Bento L, Cuadrado MJ, Karim Y, Tungekar MF, Abbs I, Khamashta MA, D’Cruz D, Hughes GR. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis. 2003;62:556–60. doi: 10.1136/ard.62.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evangelopoulos ME, Alevizaki M, Toumanidis S, Sotou D, Evangelopoulos CD, Koutras DA, Stamatelopoulos SF, Mavrikakis M. Mitral valve prolapse in systemic lupus erythematosus patients: clinical and immunological aspects. Lupus. 2003;12:308–11. doi: 10.1191/0961203303lu314oa. [DOI] [PubMed] [Google Scholar]
- 53.Ioannidis JP, Katsifis GE, Tzioufas AG, Moutsopoulos HM. Predictors of sustained amenorrhea from pulsed intravenous cyclophosphamide in premenopausal women with systemic lupus erythematosus. J Rheumatol. 2002;29:2129–35. [PubMed] [Google Scholar]
- 54.Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 55.Petros WP, Evans WE. Pharmacogenomics in cancer therapy: is host genome variability important? Trends Pharmacol Sci. 2004;25:457–64. doi: 10.1016/j.tips.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Tian Q, Chan SY, Duan W, Zhou S. Insights into oxazaphosphorine resistance and possible approaches to its circumvention. Drug Resist Updat. 2005;8:271–97. doi: 10.1016/j.drup.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Park MC, Park YB, Jung SY, Chung IH, Choi KH, Lee SK. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13:569–74. doi: 10.1191/0961203304lu1063oa. [DOI] [PubMed] [Google Scholar]
- 58.Katsifis GE, Tzioufas AG, Vlachoyiannopoulos PG, Voulgarelis M, Moutsopoulos HM, Ioannidis JP. Risk of myelotoxicity with intravenous cyclophosphamide in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2002;41:780–6. doi: 10.1093/rheumatology/41.7.780. [DOI] [PubMed] [Google Scholar]
- 59.Devi A, Devaraj H. Induction and expression of GST-Pi foci in the liver of cyclophosphamide-administered rats. Toxicology. 2006;217:120–8. doi: 10.1016/j.tox.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Srivastava SK, Singhal SS, Hu X, Awasthi YC, Zimniak P, Singh SV. Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys. 1999;366:89–94. doi: 10.1006/abbi.1999.1217. [DOI] [PubMed] [Google Scholar]
- 61.Pandya U, Srivastava SK, Singhal SS, Pal A, Awasthi S, Zimniak P, Awasthi YC, Singh SV. Activity of allelic variants of Pi class human glutathione S-transferase towards chlorambucil. Biochem Biophys Res Commun. 2000;278:258–62. doi: 10.1006/bbrc.2000.3787. [DOI] [PubMed] [Google Scholar]
- 62.Sweeney C, McClure GY, Fares MY, Stone A, Coles BF, Thompson PA, Korourian S, Hutchins LF, Kadlubar FF, Ambrosone CB. Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res. 2000;60:5621–4. [PubMed] [Google Scholar]
- 63.Vester U, Kranz B, Zimmermann S, Buscher R, Hoyer PF. The response to cyclophosphamide in steroid–sensitive nephrotic syndrome is influenced by polymorphic expression of glutathion-S-transferases-M1 and – P1. Pediatr Nephrol. 2005;20:478–81. doi: 10.1007/s00467-004-1759-7. [DOI] [PubMed] [Google Scholar]
- 64.Tanner B, Hengstler JG, Dietrich B, Henrich M, Steinberg P, Weikel W, Meinert R, Kaina B, Oesch F, Knapstein PG. Glutathione, glutathione S-transferase alpha and pi, and aldehyde dehydrogenase content in relationship to drug resistance in ovarian cancer. Gynecol Oncol. 1997;65:54–62. doi: 10.1006/gyno.1996.4593. [DOI] [PubMed] [Google Scholar]
- 65.Morrow CS, Smitherman PK, Townsend AJ. Combined expression of multidrug resistance protein (MRP) and glutathione S-transferase P1–1 (GSTP1-1) in MCF7 cells and high level resistance to the cytotoxicities of ethacrynic acid but not oxazaphosphorines or cisplatin. Biochem Pharmacol. 1998;56:1013–21. doi: 10.1016/s0006-2952(98)00240-8. [DOI] [PubMed] [Google Scholar]
- 66.Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet. 2000;38:291–304. doi: 10.2165/00003088-200038040-00001. [DOI] [PubMed] [Google Scholar]
- 67.Dooley JS, James CA, Rogers HJ, Stuart-Harris R. Biliary elimination of cyclophosphamide in man. Cancer Chemother Pharmacol. 1982;9:26–9. doi: 10.1007/BF00296757. [DOI] [PubMed] [Google Scholar]
- 68.de Jonge ME, Huitema AD, Schellens JH, Rodenhuis S, Beijnen JH. Individualised cancer chemotherapy: strategies and performance of prospective studies on therapeutic drug monitoring with dose adaptation: a review. Clin Pharmacokinet. 2005;44:147–73. doi: 10.2165/00003088-200544020-00002. [DOI] [PubMed] [Google Scholar]
- 69.Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL, Hunt SE, Cole CG, Coggill PC, Rice CM, Ning Z, Rogers J, Bentley DR, Kwok PY, Mardis ER, Yeh RT, Schultz B, Cook L, Davenport R, Dante M, Fulton L, Hillier L, Waterston RH, McPherson JD, Gilman B, Schaffner S, Van Etten WJ, Reich D, Higgins J, Daly MJ, Blumenstiel B, Baldwin J, Stange-Thomann N, Zody MC, Linton L, Lander ES, Altshuler D. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–33. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]