Abstract
Aims
To describe the pharmacokinetic–pharmacodynamic (PK–PD) characteristics of the direct thrombin inhibitor dabigatran in hip replacement patients by assessing coagulation parameters activated partial thromboplastin time (aPTT) and ecarin clotting time (ECT), interindividual variability and factors affecting PD responses.
Methods
BISTRO I patients received oral dabigatran etexilate postsurgery for 6–10 days. Dabigatran plasma concentrations and aPTT/ECT were measured on the day of surgery, on subsequent days and at steady state. PK–PD characteristics of the dabigatran–aPTT/ECT relationships were evaluated using NONMEM V.
Results
The dabigatran concentration–aPTT relationship was described combining a linear and an Emax model. Mean baseline aPTT was 33.4 s and Emax (maximum increase in aPTT contributed by the Emax model) was 26.9 s. The dabigatran concentration needed to attain 50% of maximum effect (EC50) was 94.7 ng ml−1 and the mean slope of the linear concentration–response relationship (SLOP) was 0.0509 s ng−1 ml−1. Baseline aPTT and Emax were highest following surgery and declined with time. The dabigatran concentration–ECT relationship fitted a linear model. Mean baseline ECT was 28 s and decreased with time; 50% of the maximum effect was observed after 2.9 days. SLOP decreased from 0.38 to 0.27 s ng−1 ml−1 with a half-life of 1.1 day, indicating greater PD effects on the day of surgery. Interindividual and residual variability was low. Covariates could not explain variability of this model.
Conclusions
aPTT and ECT prolongation were directly correlated with dabigatran concentrations. Blood coagulation prolongation was most pronounced following surgery. Data suggest that ECT provides a more precise description of the anticoagulant effect than aPTT.
Keywords: coagulation time, dabigatran etexilate, direct thrombin inhibitor, total hip replacement
Introduction
Thrombin, a trypsin-like serine protease, plays a central role in the processes of thrombosis and haemostasis. It is a key enzyme in the blood coagulation cascade, exhibiting both pro- and anticoagulant properties, and a major factor in the initiation and propagation of thrombotic disease through the catalysis of fibrin formation from fibrinogen and by stimulating platelet aggregation. It also activates coagulation factors V, VIII, XI and XIII of the coagulation cascade. Given the key role of thrombin in thrombotic events, thrombin inhibition represents a therapeutic target for numerous thromboembolic diseases [1, 2].
Dabigatran etexilate (Boehringer Ingelheim Pharma, Ingelheim, Germany) is the orally available prodrug of dabigatran, a synthetic, direct, reversible inhibitor of thrombin. The active principal, dabigatran, selectively inhibits thrombin with a Ki of 4.5 nm and has been shown to exhibit potent anticoagulant and antithrombotic activities in a number of animal models of thrombosis [3, 4]. The prodrug, dabigatran etexilate, is rapidly and completely converted to the active principal dabigatran by hydrolysis catalysed by unspecific, ubiquitous esterases [2]. No specific enzymes, e.g. CYP450 reductases, are involved in dabigatran etexilate prodrug conversion.
Dabigatran etexilate is under development as an orally active anticoagulant for the prevention and treatment of thromboembolic events. Dabigatran etexilate has been administered to healthy male subjects in a series of Phase I clinical studies [5–7]. The linear pharmacokinetic (PK) profile of dabigatran is characterized by maximum plasma concentrations reached within 2 h after administration and by a bi-exponential disposition and elimination phase. The terminal half-life is 14–17 h after multiple-dose administration; steady state is achieved on the third day of twice-daily (bid) treatment. The drug is mainly eliminated unchanged by renal excretion; after intravenous administration of dabigatran, urinary recovery amounts to approximately 80% of the dose. Dabigatran is not metabolized by cytochrome P450 isoenzymes. Small concentrations of pharmacodynamically active dabigatran glucuronide conjugates are present in plasma. The concentrations of the acylglucuronides were quantified in plasma samples from several clinical studies and were in the range of 7–24% of total dabigatran in plasma. Absolute bioavailability of dabigatran administered as the prodrug, dabigatran etexilate, is about 5%. The interindividual variability in elderly healthy subjects was shown to be low. Interindividual coefficients of variation (CV) of the maximum plasma concentrations and area under the plasma concentration–time curves were usually < 30%. The within-subject variability was very low (6–14% CV) [7]. In orthopaedic patients, interindividual variability of PK parameters were high, with CVs > 60%[8]. The increased variability of dabigatran pharmacokinetics in orthopaedic patients might be rationalized by surgical effects on drug absorption, comedications, e.g. opioids causing gastroparesis, and higher variations in patients’ kidney function.
In healthy volunteers, close correlations have been observed between dabigatran plasma concentrations and blood coagulation times, as assessed by prolonged activated partial thromboplastin time (aPTT), ecarin clotting time (ECT), thrombin time (TT) and prothrombin time [expressed as International Normalized Ratio (INR)][5, 6]. Toxicology studies have shown that bleeding is the dose-limiting event without specific target organ toxicity.
A dose-finding Phase IIa study (BISTRO I) [9] has been performed to determine the safe therapeutic range for dabigatran etexilate following total hip or knee replacement surgery. In this multicentre, open-label, dose-escalating study, patients received oral doses of dabigatran etexilate [12.5, 25, 50, 100, 150, 200 or 300 mg bid, or 150 mg or 300 mg once daily (qd)] for 6–10 days after surgery. All patients in a given dose group received only that dose of study medication. Primary efficacy outcomes included venographic deep vein thrombosis (DVT), symptomatic DVT and pulmonary embolism during the treatment period; the primary safety outcome was major bleeding. The overall incidence of DVT was 12.4% (28/225 patients) and there was no consistent relationship between dabigatran dose and incidence of DVT; the highest incidence of DVT in any patient subgroup was 20.8% (5/24 patients) in the 12.5-mg dose group. No major bleeding events were observed in any group; however, two patients with reduced renal function receiving the highest dose (300 mg bid) suffered bleeding from multiple sites associated with prolonged pharmacodynamic (PD) effects. A dose–response was demonstrated for all major and minor bleeding events.
The BISTRO I study indicated that dabigatran etexilate demonstrates an acceptable safety profile when administered within the therapeutic range (12.5–300 mg bid). Furthermore, the low number of venous thromboembolic (VTE) events within each treatment group indicates a satisfactory antithrombotic potential, although the BISTRO I study was not powered to determine efficacy [9]. Oral administration of dabigatran etexilate, commenced early in the postoperative period, was shown to be effective and safe across a range of doses in 1949 patients treated in the BISTRO II study [10].
The primary objective of the current population PD analysis was to describe the PK–PD characteristics of dabigatran in patients undergoing elective hip replacement surgery in the BISTRO I study by assessing the blood coagulation parameters, aPTT and ECT. The analysis also aimed to investigate the effects of patient demographics (age, weight, gender, creatinine clearance) and treatment effects (e.g. fed/fasted condition, concomitant medications) on PD model parameters. Inter- and intraindividual variability in blood coagulation parameters in orthopaedic patients were also studied.
Methods
Study design
Data were obtained from the multicentre, open-label, dose-escalation study, BISTRO I [8]. In the study, 289 patients received dabigatran etexilate orally at doses of 12.5, 25, 50, 100, 150, 200 or 300 mg bid, or 150 mg or 300 mg qd. The first dose was administered 4–8 h after total hip replacement surgery and dosing was continued for 6–10 days. Between 20 and 46 patients were treated in each of the nine dosing subgroups (Table 1). Of the 289 patients, 262 completed the study and 27 discontinued treatment prior to study completion. Available data from these 27 patients were included in the analysis. Two patients for whom plasma concentration values were not available were excluded. The protocol was approved by National Ethics Committees and written informed consent was obtained from all patients prior to inclusion.
Table 1.
Baseline characteristics of all patients receiving dabigatran treatment within the nine dosage subgroups following total hip replacement surgery in BISTRO I
| Dose of dabigatran etexilate (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic N* | 12.5 bid 27 | 25 bid 28 | 50 bid 30 | 100 bid 40 | 150 bid 29 | 200 bid 28 | 300 bid 20 | 150 qd 41 | 300 qd 46 | All 289 |
| Age (years) | ||||||||||
| Mean | 68 | 65 | 67 | 67 | 67 | 67 | 70 | 66 | 67 | 67 |
| Range | 50–88 | 35–85 | 52–86 | 45–80 | 49–79 | 55–82 | 47–82 | 44–82 | 50–84 | 35–88 |
| Weight (kg) | ||||||||||
| Mean | 78 | 80 | 76 | 80 | 81 | 76 | 75 | 80 | 77 | 78 |
| Range | 58–130 | 49–117 | 54–102 | 55–127 | 57–120 | 55–106 | 56–128 | 53–105 | 50–109 | 49–130 |
| Female sex | ||||||||||
| Number | 11 | 12 | 17 | 24 | 12 | 16 | 14 | 20 | 26 | 152 |
| Percentage | 40.7 | 42.9 | 56.7 | 60.0 | 41.4 | 57.1 | 70.0 | 48.8 | 56.5 | 52.6 |
| BMI (kg m−2) | ||||||||||
| Mean | 26 | 27 | 26 | 28 | 27 | 26 | 26 | 27 | 27 | 27 |
| Range | 22–36 | 19–37 | 21–31 | 21–40 | 21–39 | 21–32 | 21–40 | 20–34 | 18–36 | 18–40 |
N, Number of patients receiving surgery and dabigatran treatment within each treatment group; BMI, body mass index.
Sample collection
Dabigatran plasma concentrations and aPTT and ECT values were measured at the following approximate time points in patients in each dosage subgroup: before and 4 h after the first dose of dabigatran on the day of surgery; before drug administration and 2 h after each dose on subsequent days; and by frequent sampling at steady state – usually on day 4 after surgery. Blood samples were collected predose within 2–4, 4–8 and 8–12 h after drug administration. In a subpopulation of 21 patients, a more conventional PK–PD sampling was also performed, in which seven blood samples were collected predose and at 0.5, 1, 2, 4, 8 and 12 h after administration of the morning dose on day 4.
Determination of plasma dabigatran concentrations
Total concentrations of dabigatran in plasma representing free, nonconjugated dabigatran and dabigatran released after alkaline cleavage of dabigatran conjugates were determined.
Quantitative measurement of plasma dabigatran concentrations was performed using a liquid chromatography-tandem mass spectroscopy (LC-MS/MS) method. Eighty-microlitre sample volumes of patient plasma were analysed using a Sciex API 3000 (PerkinElmer, Boston, MA, USA) LC-MS/MS system. An electrospray ion source with atmospheric pressure ionization was used for measurements performed in the positive ionization mode. Separation was achieved by direct injection onto a precolumn and subsequent transfer by column switching onto a high-performance liquid chromatography (HPLC) column. Monitored ions were 472.2→289.5 (dabigatran), 478.2→295.6 (internal standard) and 648.2→288.9 (dabigatran glucuronide). The lower limit of quantification (LOQ) was 1.0 ng ml−1. The linearity of the method, i.e. the mean correlation coefficient of the standard curves, was 0.99953. At the lower limit of quantification, the precision of the analytical method was 6.67% CV and accuracy (bias) was − 2.66%.
Sample stability was demonstrated for at least 6 months at − 20 °C. Samples were shipped from study sites on a regular basis for continuous analysis during the study term.
APTT and ECT analysis of plasma samples
aPTT and ECT values were determined in two separate central laboratories, according to Good Laboratory Practice regulations. Blood samples were drawn in citrated tubes and plasma was separated. ECT was measured using a Biomatic B10 coagulometer (Desaga, Wiesloch, Germany) in a central laboratory and aPTT was measured in a second central laboratory employing an STA Compact coagulation analyser (Roche Diagnostics, Basel, Switzerland).
Data analysis
aPTT and ECT data were fitted separately. All the analyses were performed under the population modelling approach with NONMEM version V using the First Order Conditional Estimation (FOCE) method [11, 12], in accordance with the recommendations on population PK analysis from the Food and Drug Administration [13]. Interindividual variability was modelled exponentially and a proportional error model was used to account for the residual variability.
Model selection was based on the visual inspection of the goodness-of-fit plots and the precision of model parameter estimates. The minimum value of the objective function (MOFV) provided by NONMEM was also used as a guide during the model-building process. A decrease in MOFV of 3.84 and 10.83 points between two nested models is significant at the 5 and 0.1% levels, respectively.
Population model development
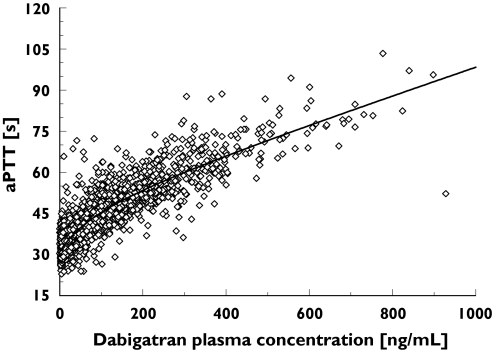
As a first step, a model describing the data adequately without incorporating covariates (basic population model) was selected. A preliminary exploration of the observed effects (aPTT and ECT) vs. the observed total (conjugated plus unconjugated) dabigatran concentrations in plasma (C) revealed that counter-clockwise or clockwise hysteresis loops were not present in the data, suggesting a direct relationship between drug in plasma and response (see Figures 1, 4 and 5). Such a direct relationship has been observed in the past for two other oral thrombin inhibitors, inogatran and melagatran [14, 15, 16].
Figure 1.
Activated partial thromboplastin time (aPTT) values (s) with increasing dabigatran plasma concentrations (ng ml−1) in individual patients following administration of dabigatran etexilate on day 4 (steady state) following orthopaedic surgery in BISTRO I
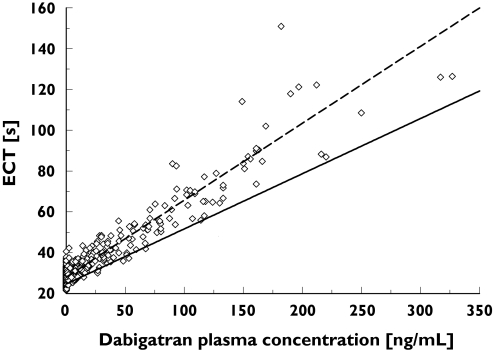
Figure 4.
The relationship between patient ecarin clotting time (ECT) values and dabigatran plasma concentrations 0–12 h after first administration of dabigatran etexilate following orthopaedic surgery. Individual patient ECT values are indicated by empty squares. The dashed black line represents the fit of the linear ECT model to the early (0–12 h post surgery) data; the solid grey line represents the fit of the model to the late (>100 h post surgery) data. SLOP and BASE at time 0 h (- - -), individual values ≤ 12 h (⋄), SLOP and BASE at time 150 h (——)
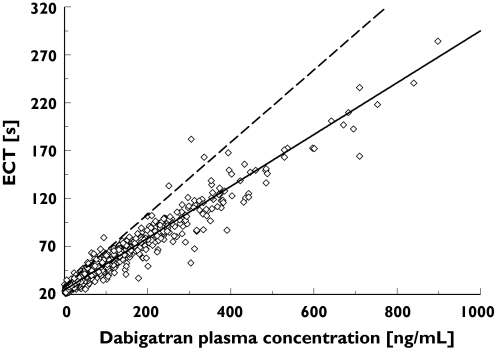
Figure 5.
The relationship between patient ecarin clotting time (ECT) values and dabigatran plasma concentrations > 100 h after first administration of dabigatran etexilate (steady state) following orthopaedic surgery. Individual patient ECT values are indicated by empty squares. The dashed black line represents the fit of the linear ECT model to the early (0–12 h post surgery) data; the solid grey line represents the fit of the model to the late (>100 h post surgery) data. SLOP and BASE at time 0 h (- - -), individual values > 100 h (⋄), SLOP and BASE at time 150 h (——)
The PD profiles shown in Figure 1 suggest that the relationship between aPTT and dabigatran in plasma can be described with a combination of a linear and an Emax model, as was the case for inogatran [14].
| 1 |
E0 is aPTT at baseline; Emax and EC50 are the maximum increase in aPTT and the dabigatran plasma concentration eliciting half of Emax corresponding to the nonlinear part of the model, respectively. SLOP is the slope of the linear relationship.
In Figures 4 and 5, the ECT vs. dabigatran in plasma data show a linear profile:
| 2 |
E0 in Equation 2 is ECT at baseline and SLOP is the slope of the linear relationship.
Other models, such as the sigmoidal Emax and power models, were also fitted to the data. Since dabigatran was administered during a 2-week period, the possibility of time effects on the PD model parameters was also investigated. For this purpose, linear Emax or exponential models were used.
The general additive modelling (GAM) [17] approach was performed with S-PLUS using the Xpose [18] program to preselect potential covariates. The Akaike information criterion is the selection criterion of this GAM approach. Covariates selected during the GAM approach were evaluated individually in NONMEM and those that showed significance on a level of P = 0.05 were then tested and incorporated one at a time until the full covariate model was obtained (forward inclusion). Following the forward inclusion, a backward elimination was then performed on a significance level of P = 0.001.
The selected model was validated internally [13] computing the bias and precision in the model parameters and simulated response values obtained from 100 datasets, including the same study design characteristics of the original data and simulated data based on the fixed- and random-effect parameters estimated in the selected model. The median performance error (MPE) and the median of the absolute performance error (MAPE) were calculated and used as a measure of bias and precision, respectively.
Results
Patient demographics
In total, 4854 parallel PK and PD observations were employed for the development of the aPTT model and 5060 observations were employed for the ECT model. The performance of these observations yielded approximately 17 samples per patient for the 287 patients included in the analysis. Both patients and PK–PD observations were uniformly distributed across the nine dosage groups. The greatest proportion of samples (23%) was collected at steady state on day 4; only 2.7% of the samples were taken after day 7. Demographic data from the study subjects, according to dosage subgroup, are shown in Table 1.
APTT model
Overall, the prolongation of aPTT increased in a nonlinear manner with increasing concentrations of dabigatran (Figure 1). A combined Emax and linear model (Equation 1) best describes the relationship between dabigatran plasma concentration (C) and aPTT at steady state (depicted by a solid line in Figure 1).
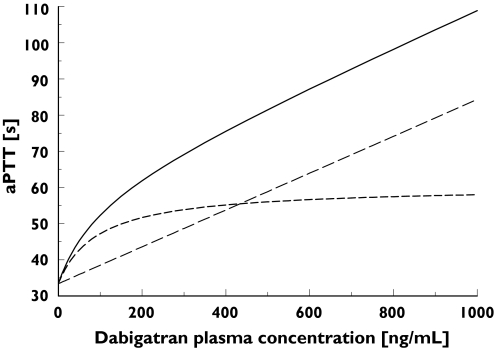
Figure 2 shows the contributions of both the linear and nonlinear parts of the dabigatran plasma concentration–aPTT relationship to the overall PK–PD response. Up to a dabigatran plasma concentration of approximately 200 ng ml−1, the PK–PD response was dominated by the nonlinear, Emax-type correlation, whereas at higher dabigatran concentrations the PK–PD relationship became linear. The resulting combined Emax and linear model provided a good fit for the patient data (Figure 1, open symbols). E0 and Emax were found to be dependent on the amount of time elapsed after surgery. Decreases in E0 and Emax values over time (T) were modelled using proportional inhibitory models:
| 3 |
| 4 |
Figure 2.
Contributions of the Emax-type (nonlinear) and linear parts of the dabigatran plasma concentration–activated partial thromboplastin time (aPTT) relationship to overall pharmacokinetic–pharmacodynamic responses in the treated patient population at time 0 (time of administration of the first dose) on the day of surgery in BISTRO I. Linear and Emax + baseline (——), linear part + baseline (––), Emax part + baseline (- - -)
In the first model, BAS0 represents the E0 value at time 0 (the time of the first administration of dabigatran etexilate) on the day of surgery, EMBA is the maximum decrease in E0, and ET50 is the time point at which E0 decreases by 50%. In the latter model, EMAO represents the initial Emax value at time 0, EMMX is the maximum decrease in Emax, and ET50 is the time point at which Emax decreases by 50%.
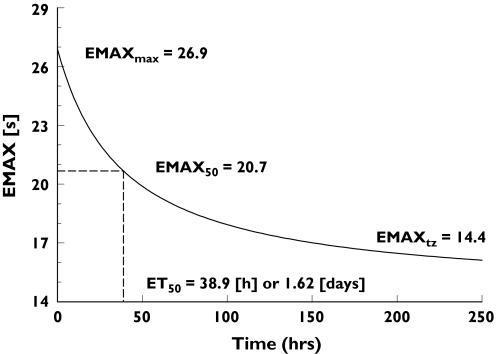
Figure 3 shows the change over time after surgery in the maximum aPTT response. The initial Emax of 26.9 s at the time of surgery diminished with a half-life of 1.62 days. At an infinite time after surgery, the maximum prolongation of aPTT contributed by the sigmoidal part of the concentration–aPTT relationship was estimated to be 14.4 s, representing a 46% decrease in Emax (Table 2).
Figure 3.
Changes in Emax over time in patients receiving dabigatran etexilate following orthopaedic surgery based on calculations from the concentration–response model for activated partial thromboplastin time
Table 2.
Mean estimates, standard error, interindividual variability and descriptions of component parameters in the activated partial thromboplastin time (aPTT) concentration–response model based on data from the BISTRO I patient population
| Parameter | Parameter estimate | RSE (%)* | IIV (%)† | RSE of IIV (%)* | Description |
|---|---|---|---|---|---|
| BAS0 (s) | 33.4 | 0.63 | 8.7‡ | 10.51§ | Baseline aPTT at time 0 on the day of surgery |
| EMA0 (s) | 26.9 | 12.45 | 19.9‡ | 33.92§ | Emax at time 0 on the day of surgery |
| EMBA | 0.102 | 14.41 | NE | NE | Constant describing the maximum decrease in baseline aPTT over time |
| EC50 (ng ml−1) | 94.7 | 17.11 | 38.5 | 40.41§ | Plasma concentration at which the effect of the non linear part of the model is 50% that of Emax |
| SLOP (s ng−1 ml−1) | 0.0509 | 6.68 | 15.2 | 45.22§ | Constant describing the slope of the linear part of the line of regression |
| EMMX | 0.463 | 12.68 | NE | NE | Constant describing the maximum decrease in Emax over time |
| ET50 (days) | 1.62 | 15.99 | NE | NE | Time point at which baseline aPTT and Emax decreases by 50% and the effect is half that of EMBA and EMMX |
| Residual error (σ) | 7.55* | 3.53 | NE | NE | Proportional error reported as coefficient of variation (CV %) |
RSE, Residual standard error. Percentage standard error for the parameter estimates was calculated according to percentage RSE: 100% · SE/parameter estimate.
IIV, Inter-patient variability. Estimates of variance components (ω and σ) were converted into standard deviations by taking their square root. These are reported as coefficients of variation (% CV) after multiplying them by 100.
Interpatient variability is based on values for E0 and Emaxrather than BAS0(initial E0at time 0) or EMA0(initial Emax at time 0).
Percentage SE is given on the variance scale; NE, not estimated.
The dabigatran plasma concentration required to achieve 50% of the maximum effect, EC50, was 94.7 ng ml−1. Baseline aPTT (33.4 s) showed a similar decline over time, with 50% of the maximum effect observed after 1.62 days and a baseline value of 30.0 s estimated at an infinite time after surgery. Parameter estimates from the concentration–aPTT response model based on the treated patient population, together with standard error and interindividual variability calculations, are provided in Table 2.
A covariate analysis was performed in order to identify patient or treatment variables that might influence the aPTT and ECT responses. The major covariates included comedications (e.g. diuretics, opioids, nonsteroidal anti-inflammatory drugs, drugs accelerating the gastrointestinal transit time, acetaminophen, cytochrome 3A4 inhibitors), patient demographics (gender, age, height, body mass index) and standard clinical laboratory parameters. None of the covariates exhibited relevant effects on any of the aPTT model parameters.
ECT model
A linear model adequately described the relationship between dabigatran plasma concentration and prolongation of ECT (Equation 2).
In contrast to the aPTT model, there was a simple linear relationship between dabigatran plasma concentration and the prolongation of ECT. However, due to mis-specifications observed in the goodness-of-fit plots during early model development, it was found that the slope of the line of regression varied with time after surgery. To illustrate this effect, the ECT/dabigatran plasma concentration dataset can be separated into data obtained within 12 h after surgery and data obtained at 100 h after surgery.
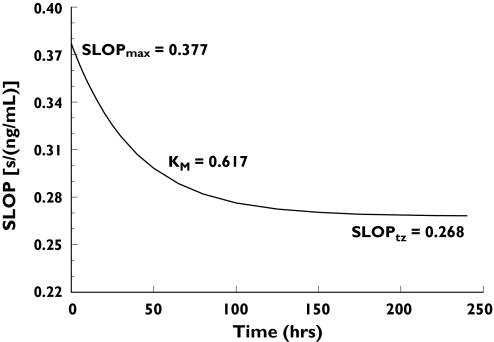
Early data revealed a steeper slope of the regression line compared with late data (>100 h) indicating greater sensitivity in the ECT response shortly after surgery (Figures 4 and 5). The exponential decay in SLOP from time zero to infinity (Figure 6) was modelled by combining two exponential terms:
| 5 |
Figure 6.
Change over time after surgery in SLOP – the slope of the linear part of the equation describing the relationship between dabigatran plasma concentration and prolongation of ecarin clotting time (ECT). The effect of dabigatran administration on ECT prolongation was greatest shortly after surgery and declined with time
SLO0 is the initial slope value at time 0 (time of first administration, usually on the day of surgery), SLOF is the final slope value at time infinity, and KM describes the rate of decrease in the slope which can be expressed as a half-life with a value of 27 h. The initial slope of 0.377 s ng−1 ml−1 decreased to 0.268 s ng−1 ml−1 at time infinity. Immediately after surgery, a 10 ng ml−1 increase in dabigatran plasma concentrations prolonged the ECT by 3.8 s, whereas at later time points (e.g. > 5 days after surgery), the same dabigatran concentrations increased the ECT by 2.7 s (Figure 6).
As with aPTT, baseline ECT was found to vary with time after surgery, and again, the change in baseline over time was successfully modelled using a proportional inhibitory Emax model, equal to the one used in the case of aPTT responses (Equation 3).
Baseline ECT decreased from 28 s at the time of surgery to 23.1 s with half the maximum effect at 2.9 days after surgery. Parameter estimates obtained with the final ECT model are summarized in Table 3.
Table 3.
Mean estimates, standard error, interindividual variability and descriptions of component parameters in the ecarin clotting time (ECT) concentration–response model based on data from the BISTRO I patient population
| Parameter | Parameter estimate | RSE (%)* | IIV (%)† | RSE of IIV (%)* | Description |
|---|---|---|---|---|---|
| BAS0 (s) | 28.0 | 0.49 | 8.2‡ | 8.98§ | Baseline ECT at time 0 on the day of surgery |
| SLO0 (s ng−1 ml−1) | 0.377 | 2.18 | 13.7‡ | 13.76§ | Initial slope value (SLOP) at time 0, on the day of surgery |
| SLOF (s ng−1 ml−1) | 0.268 | 1.49 | NE | NE | Final slope value at time infinity |
| KM (h−1) | 0.617 | 13.55 | NE | NE | Constant describing the exponential rate of decline in the slope from 0 h to the final value |
| EMBA | 0.175 | 6.46 | NE | NE | Constant describing the maximum decrease in baseline ECT over time |
| ET50 (days) | 2.86 | 13.50 | NE | NE | Time point at which the baseline ECT has declined to half the EMBA |
| Residual error | 6.63* | 6.83 | NE | NE | Proportional error reported as coefficient of variation (CV %) |
RSE, Residual standard error. Percentage standard error for the parameter estimates was calculated according to percentage RSE: 100% · SE/parameter estimate.
IIV, Interindividual variability. Estimates of variance components (ω and σ) were converted into standard deviations by taking their square root. These are reported as coefficients of variation (CV %) after multiplying them by 100.
Interindividual variability is based on values for E0and SLOP rather than BAS0(initial Emaxat time 0) or SLO0(initial SLOP at time 0).
Percent SE is given on the variance scale; NE, not estimated.
As with aPTT, covariate analysis revealed that none of the investigated covariates (comedications, patient demographics, serum creatinine clearance, standard clinical laboratory parameters) exerted relevant effects on any of the model parameters.
Model validation
The MPE for fixed effects in the aPTT model ranged from −4.8% to 0.8%, while the MPE for random effects was in the range −12.50 to 6.33%, indicating that the aPTT values predicted by the model were accurate. The MAPE for fixed effects ranged between 0.6% and 11.7%, with the MAPE for random effects ranging between 6.2% and 31.7%. MPE and MAPE values from the ECT model were much smaller than those from the aPTT model. Bias and imprecision of the aPTT values were 0.9% and 11.6%, respectively, while those of the ECT values were 0.8% and 9.9%, respectively.
Discussion
aPTT is a commonly used tool for measuring the degree of anticoagulation in patients receiving anticoagulant medications. It has long been used to monitor treatment with heparin [19] and has been employed more recently in the clinical evaluation of direct thrombin inhibitors (DTIs), such as hirudin, hirulog, argatroban, inogatran and melagatran [20–26]. Therapeutic ranges for aPTT have been established empirically for heparin in various indications [27], but difficulties exist in the useful application of such ranges due to a lack of standardization in measurement methods between laboratories.
ECT is a biomarker that is being used increasingly to measure levels of anticoagulation [28, 29]. ECT offers some advantages in comparison with aPTT due to its higher sensitivity and its linearity even at high plasma concentrations.
In the BISTRO I dose-escalation study [8], plasma concentrations of the oral DTI dabigatran etexilate and the blood coagulation parameters, aPTT and ECT, were analysed in order to evaluate the relationship between dabigatran plasma concentrations and the degree of anticoagulation achieved in patients following total hip replacement surgery. Maximum prolongation of both aPTT and ECT occurred approximately 2 h after oral drug administration and displayed an increase with rising doses of dabigatran etexilate. Maximum aPTT, measured at steady state, was 1.9-fold longer than baseline levels (predose aPTT), while maximum ECT was prolonged by approximately fivefold.
The objective of the population modelling of aPTT/ECT data from the study was to provide PK–PD models for both blood coagulation parameters. An in-depth analysis of the PD response to dabigatran etexilate was considered important to enable appropriate dose selection for subsequent Phase III clinical trials evaluating the benefits of dabigatran in the prevention of VTE after orthopaedic surgery. However, further investigations of the relationship between aPTT and ECT prolongation and clinical outcome data, i.e. incidence of VTE and bleeding events, are required for dosing recommendations.
In contrast to warfarin, dabigatran etexilate is an oral anticoagulant that is not metabolized by the cytochrome P450 isoenzymes (CYP450). In vitro studies indicated that dabigatran does not induce or inhibit CYP450 enzymes. In a Phase I clinical trial the absence of any relevant drug–food interactions has been shown [8]. Therefore, its PD response is likely to be more predictable than that of warfarin and dabigatran etexilate might be administered without the requirement for coagulation monitoring. An assessment of the status of anticoagulation may be appreciated for the safe and effective management of patients undergoing emergency surgery and those who experience bleeding events following anticoagulation. The aPTT and ECT models developed in this study provide an insight into the PD characteristics of dabigatran, contributing to rational dose selection for this new therapeutic agent that may be adopted to enable its effective application in thrombosis prophylaxis.
aPTT model
Prolongation of aPTT in orthopaedic patients following the administration of dabigatran etexilate was characterized by a curvilinear increase, which was best described by a combined Emax-type/linear model. This model provided a significantly better fit than a power model generated for aPTT data from healthy volunteers in Phase I studies of the drug [6].
Similar nonlinear aPTT relationships have recently been shown for other DTIs. Inogatran displayed a curvilinear aPTT correlation, which has also been modelled using a combined Emax-type/linear model [14]. The addition of melagatran to pooled human plasma prolonged the aPTT in a concentration-dependent manner but was also associated with a nonlinear concentration–effect response that was flattened at higher concentrations [16]. The model used to describe the PK–PD characteristics of melagatran employed linear least squares regression of the aPTT ratios vs. the square root of the melagatran plasma concentrations. The authors concluded that, due to the low sensitivity of the assay, aPTT may be more suitable to provide a qualitative indication of anticoagulant activity than for precise quantification of the anticoagulant effect. The same conclusion might be drawn regarding the aPTT response to dabigatran. The lack of standardization of aPTT assays and the use of a large variety of assay reagents with different sensitivities between laboratories [29], in conjunction with the low precision and sensitivity of this assay, prohibit its use as a quantitative tool for monitoring DTIs.
In the current study, population PD modelling of the aPTT response in patients undergoing hip replacement surgery who received treatment with dabigatran etexilate for VTE prophylaxis provided insight into the time course of the aPTT response. Sensitivity and baseline aPTT were higher shortly after surgery than at later time points (e.g. 6–10 days after treatment). The PD model adequately describes the varying sensitivity of aPTT over time and provides estimates of the magnitude of these effects. The time to 50% of the maximum aPTT value (ET50) was approximately 1.6 days for both Emax and baseline aPTT, with a maximum decline of approximately 46% for Emax and 10% for baseline aPTT. The clinical relevance of this observation remains to be elucidated; however, it may be explained by dilution and draining effects caused by transfusions and/or perioperative bleeding. The higher sensitivity of the aPTT response early after surgery may compensate for the approximately 12% lower bioavailability of dabigatran following oral administration on day of surgery compared with drug exposure at steady state [8].
ECT model
The relationship between dabigatran plasma concentrations and ECT in the current study was described by a linear function over the full range of concentrations. Even at very high concentrations, no flattening of the ECT response was observed. The sensitivity of ECT to dabigatran was greater than that of aPTT, with a maximum ECT prolongation at the upper end of the clinically relevant dose range of approximately fivefold, compared with the twofold maximum prolongation of aPTT. The precision of the ECT assay was also superior to that of aPTT, as indicated by low interindividual variability (CV %) in ECT values at baseline (8%) and in the slope of the line of regression (14%). These findings suggest that, compared with aPTT, the ECT assay may provide a more accurate quantitative measure of anticoagulation in patients receiving a DTI. However, the sensitivity of the ECT assay varies between laboratories, depending on the ecarin reagent used, and a standardized ECT test is not yet available for routine use in clinical laboratories.
As with aPTT, the mean population values for ECT at baseline and slope changed with time after surgery. Baseline ECT was 28 s and declined by approximately 12.6% within 1 week after surgery. The slope of the plasma concentration–ECT relationship decreased from 0.38 s ng−1 ml−1 at the time of first administration to 0.27 s ng−1 ml−1 at time infinity, indicating higher sensitivity early after surgery. As with aPTT, the clinical significance of these effects remains unclear.
Conclusions
The PK–PD relationship between dabigatran plasma concentrations and the blood coagulation parameters, aPTT and ECT, has been fully characterized in orthopaedic patients receiving oral dabigatran etexilate for thromboprophylaxis following hip replacement surgery. As with the other DTIs, the aPTT response was nonlinear, less precise and of relatively low sensitivity. In contrast, the PK–PD relationship between dabigatran and ECT remained linear over the full plasma concentration range. Compared with aPTT, the ECT assay provided a greater degree of sensitivity and accuracy, enabling more precise description of the anticoagulant effect of dabigatran.
This is the first report to demonstrate that both aPTT and ECT responses in orthopaedic patients vary with time after surgery. aPTT and ECT responses are highest immediately after surgery and both decline with time. The mechanism underlying this finding remains unclear; however, perioperative effects, such as the transfusion of large volumes of fluids, may contribute to this effect.
Conflict of interest
K-H.L., H.G.S., C.T. and J.S. are employees of Boehringer Ingelheim. I.F.T. is a consultant for Boehringer Ingelheim. B.I.E. was principal investigator of the BISTRO I study and has declared no conflict of interest with regard to this publication.
References
- 1.Becker RC. Understanding the dynamics of thrombin in cardiovascular disease. Pathobiology and biochemistry for the clinician. Am Heart J. 2005;149:S2–8. doi: 10.1016/j.ahj.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Ries UJ, Wienen W. Serine proteases as targets for antithrombotic therapy. Drugs Future. 2003;28:355–70. [Google Scholar]
- 3.Wienen W, Nar H, Ries UJ. Effects of the direct thrombin inhibitor BIBR 953 ZW and its orally active prodrug BIBR 1048 MS on experimentally induced clot formation and template bleeding time in rats. Thromb Haemost. 2001:P761. (Suppl.) Abstract. [PubMed] [Google Scholar]
- 4.Wienen W, Nar H, Ries UJ. Antithrombotic effects of the direct thrombin inhibitor BIBR 953 ZW and its orally active prodrug BIBR 1048 MS in a model of venous thrombosis in rabbits. Thromb Haemost. 2001:OC853. (Suppl.) Abstract. [Google Scholar]
- 5.Stangier J, Rathgen K, Gansser D, Kohlbrenner V, Stassen JM. Pharmacokinetics of BIBR 953 ZW, a novel low molecular weight direct thrombin inhibitor in healthy volunteers. Thromb Haemost. 2001:OC2347. (Suppl.) Abstract. [Google Scholar]
- 6.Stassen JM, Rathgen K, Zimmermann R, Wienen W, Stangier J. Pharmacodynamics of the synthetic direct thrombin inhibitor BIBR 953 ZW in healthy subjects. Thromb Haemost. 2001:OC160. (Suppl.) Abstract. [Google Scholar]
- 7.Stangier J, Stähle H, Rathgen K, Fuhr R, Roth W. Pharmacokinetics and pharmacodynamics of the direct thrombin inhibitor dabigatran in elderly healthy subjects. J Thromb Haemost. 2005;3(Suppl. 1):P2207. [Google Scholar]
- 8.Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Rathgen K, Svard R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:538–46. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Dahl OE, Ahnfelt L, Kalebo P, Stangier J, Nehmiz G, Hermansson K, Kohlbrenner V. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost. 2004;2:1573–80. doi: 10.1111/j.1538-7836.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo M-L, Ahnfelt L, Piovella F, Stangier J, Kälebo P, Reilly P for the BISTRO II Study Group. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: The BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–11. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 11.Beal SL, Boeckman AJ, Sheiner LB, editors. Part VI. PREDPP Guide. San Francisco: NONMEM Project Group, University of California; 1992. NONMEM Users Guide. [Google Scholar]
- 12.Beal SL, Sheiner LB, editors. Part VII. Conditional Estimation Methods. San Francisco: NONMEM Project Group, University of California; 1998. NONMEM Users Guide. [Google Scholar]
- 13.US Department of Health and Human Services Food and Drug Administration. Population Pharmacokinetics. Washington DC: US Department of Health and Human Services Food and Drug Administration; 1999. Guidance for Industry. [Google Scholar]
- 14.Carlsson SC, Mattson C, Eriksson UG, Sarich TC, Wahlander K, Eliasson A, Karlson BW, Sheth SB, Held P. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res. 2005;115:9–18. doi: 10.1016/j.thromres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, a thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol. 2001;51:71–9. doi: 10.1046/j.0306-5251.2001.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson UG, Mandema JW, Karlsson MO, Frison L, Gileskog PO, Wählby U, Hamrén B, Gustafsson D, Eriksson BI. Pharmacokinetics of melagatran and the effect on ex vivo coagulation time in orthopaedic surgery patients receiving subcutaneous melagatran and oral ximelagatran. Clin Pharmacokinet. 2003;42:687–701. doi: 10.2165/00003088-200342070-00006. [DOI] [PubMed] [Google Scholar]
- 17.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–28. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson EN, Karlsson MO. Xpose: An Splus based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 19.Kher A, Dieri RA, Hemker HC, Béguin S. Laboratory assessment of antithrombotic therapy: what tests and if so why? Haemostasis. 1997;27:211–8. doi: 10.1159/000217459. [DOI] [PubMed] [Google Scholar]
- 20.Antman EM for the TIMI 9A Investigators. Hirudin in acute myocardial infarction. Safety report from the thrombolysis and thrombin inhibition in myocardial infarction (TIMI) 9A trial. Circulation. 1994;90:1624–30. doi: 10.1161/01.cir.90.4.1624. [DOI] [PubMed] [Google Scholar]
- 21.Clark RJ, Mayo RN, Fitzgerald GA, Fitzgerald DJ. Combined administration of aspirin and a specific thrombin inhibitor in man. Circulation. 1991;83:1510–8. doi: 10.1161/01.cir.83.5.1510. [DOI] [PubMed] [Google Scholar]
- 22.Conrad KA. Clinical pharmacology and drug safety: lessons from hirudin. Clin Pharmacol Ther. 1995;58:123–6. doi: 10.1016/0009-9236(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 23.Fox I, Dawson A, Loynds P, Eisner J, Findlen K, Levin E, Hanson D, Mant T, Wagner J, Maraganore J. Anticoagulation activity of Hirulog™, a direct thrombin inhibitor, in humans. Thromb Haemost. 1993;69:157–63. [PubMed] [Google Scholar]
- 24.Gusto IIa Investigators. Randomized trial of intravenous heparin versus recombinant hirudin for acute coronary syndromes. Circulation. 1994;90:1631–7. doi: 10.1161/01.cir.90.4.1631. [DOI] [PubMed] [Google Scholar]
- 25.Gusto IIb Investigators. A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med. 1996;335:775–82. doi: 10.1056/NEJM199609123351103. [DOI] [PubMed] [Google Scholar]
- 26.Lee V. Initial experience with hirudin and streptokinase in acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) 6 trial. Am J Cardiol. 1995;75:7–13. doi: 10.1016/s0002-9149(99)80517-7. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh J. Heparin. N Engl J Med. 1991;324:1565–74. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 28.Buch E, Nowack G. The Ecarin Coagulation Time (ECT), the first specific method suited for quick determination of direct acting thrombin inhibitors in whole blood, plasma and body fluids. Int Angiol. 1995;174:4. Abstr. 65. [Google Scholar]
- 29.Fenyvesi T, Jörg I, Harenberg J. Monitoring of anticoagulant effect of direct thrombin inhibitors. Semin Thromb Hemost. 2002;28:361–8. doi: 10.1055/s-2002-34305. [DOI] [PubMed] [Google Scholar]