Abstract
Aim
The aim of this systematic review was to assess the quality and outcomes of clinical trials investigating the effect of St John’s wort extracts on the metabolism of drugs by CYP3A.
Methods
Prospective clinical trials assessing the effect of St John’s wort (SJW) extracts on metabolism by CYP3A were identified through computer-based searches (from their inception to May 2005) of Medline, Cinahl, PsycINFO, AMED, Current Contents and Embase, hand-searches of bibliographies of relevant papers and consultation with manufacturers and researchers in the field. Two reviewers selected trials for inclusion, independently extracted data and recorded details on study design.
Results
Thirty-one studies met the eligibility criteria. More than two-thirds of the studies employed a before-and-after design, less than one-third of the studies used a crossover design, and only three studies were double-blind and placebo controlled. In 12 studies the SJW extract had been assayed, and 14 studies stated the specific SJW extract used. Results from 26 studies, including all of the 19 studies that used high-dose hyperforin extracts (>10 mg day−1), had outcomes consistent with CYP3A induction. The three studies using low-dose hyperforin extracts (<4 mg day−1) demonstrated no significant effect on CYP3A.
Conclusion
There is reasonable evidence to suggest that high-dose hyperforin SJW extracts induce CYP3A. More studies are required to determine whether decreased CYP3A induction occurs after low-dose hyperforin extracts. Future studies should adopt study designs with a control phase or control group, identify the specific SJW extract employed and provide quantitative analyses of key constituents.
Keywords: CYP3A, cytochrome P450, herb-drug interactions, Hypericum perforatum, St John’s wort, systematic review
Introduction
St John’s wort (SJW) extracts have become popular natural medicines for the treatment of mild-to-moderate depression. This popularity is in part a response to the outcomes of numerous randomized, controlled trials supporting their use in this condition. A number of these studies have demonstrated that SJW extracts are as effective as conventional medications for mild-to-moderate depression, but with a more favourable adverse event profile [1–5]. However, safety concerns have arisen over the concurrent use of SJW with other medications after the publication of numerous case studies implicating SJW extracts in pharmacokinetic drug interactions. These have included several cases involving cyclosporin resulting in threatened transplant rejection [6, 7]. Subsequently, SJW has become a focus for herb–drug interaction studies assessing its effect on the metabolism of many drugs.
Several systematic reviews have assessed the research on SJW-mediated drug interactions [8–11]. However, evaluation of the quality of the trial methodology has been neglected, with the exception of one systematic review that found trial quality in this area was poor [10]. The main aim of the reviews has been to assess the outcomes of SJW–drug interaction studies collectively, without considering the problem of generalizing the effects of individual extracts. Recent clinical trials [12, 13] employing SJW extracts that are low in hyperforin content suggest that such extracts may be less likely to interact with drugs than extracts that contain higher amounts of this compound. Hyperforin is a potentially important constituent involved in the antidepressant action of SJW [14].
The composition of SJW extracts varies between the different products on the market. In Europe, the hyperforin content was found to range from 0.2% to 4.0%[15]. In some extracts, such as Remotiv and Neuroplant 300, there was low variation between batches, including LI160, whereas that between others showed coefficients of variation of 20–25% and in one case 70%[15]. One systematic review of 22 studies found that only 15 presented data on the content of the SJW extract [10].
St John’s wort has been the subject of numerous clinical studies assessing its effect on several forms of cytochromes P450 as well as P-glycoprotein. Cytochrome P450 3A (CYP3A) is a major drug-metabolizing enzyme subfamily and is involved in the metabolism of up to 50% of pharmaceutical drugs [16]. Hence agents that affect CYP3A have the potential to alter the pharmacokinetics and, consequently, the clinical efficacy of many therapeutic agents.
Additional methodological issues in relation to SJW that have not been discussed in previous systematic reviews include: control of the intake of other substances that may affect the activity of CYP3A, stability of pharmacokinetic measurements over time through the undertaking of baseline assessments on more than one occasion, and compliance with SJW administration.
This systematic review aims to assess the quality and outcomes of clinical trials investigating the effect of SJW extracts on CYP3A. The primary question was ‘Do SJW extracts affect CYP3A?’ and the secondary question was ‘Do different SJW extracts affect CYP3A differently?’. A further aim was to assess key methodological issues that may influence the outcome of SJW studies.
Methods
Literature searches
The authors (D.L.W. and H.W.) performed a computer-based search of Medline (1966 to May 2005), Cinahl (1982 to May 2005), PsycINFO (1967 to May 2005), AMED (1985 to May 2005), Current Contents (1993 to May 2005) and Embase (1990 to May 2005) databases. The keyword search terms for St John’s wort (‘St John’s wort’, ‘Hypericum’, ‘Hypericum perforatum’, ‘herbal medicine’, ‘botanical medicine’, ‘complementary medicine’, ‘natural medicine’, ‘natural and complementary medicine’, ‘medicinal plant’, ‘plant, medicinal’, ‘phytotherapy’, ‘herb’, ‘plant extracts’, ‘herbal extract’) were combined, using the combination term AND, with keyword search terms for CYP3A (‘herb–drug interaction’‘CYP3A’, ‘CYP 3A’, ‘CYP3A4’, ‘CYP 3A4’, ‘CYP’, ‘cytochrome P450’, ‘cytochrome P450 enzyme system’, ‘mixed-function oxygenases’, ‘mixed-function oxidase’, ‘monooxygenases’, ‘P450IIIA’, ‘midazolam’, ‘cortisol’, ‘6beta-hydroxycortisol’, ‘6-beta-hydroxycortisol’, ‘6beta-hydroxylation’, ‘erythromycin’, ‘dapsone’, ‘erythromycin breath test’, ‘drug interactions’, ‘herb–drug interaction’). We also searched the Cochrane Controlled Trials Register (May 2005). Bibliographies of papers discussing SJW and drug interactions as well as previous systematic reviews were scrutinized for additional references. Manufacturers of major SJW extracts were contacted, as were researchers in the field. There were no language restrictions. If a study was published only as an abstract, an attempt was made to contact the principal author to obtain more details on the trial design and outcome.
Inclusion criteria and data abstraction
Study inclusion criteria were defined a priori. Outcomes, and the quality of the methods used were evaluated independently by two investigators (D.L.W. and H.W.). A third author resolved any discrepancies (J.A.H.). Studies were included if they were prospective clinical trials that assessed the effect of SJW on CYP3A-mediated metabolism. Case reports and retrospective studies were excluded.
Key data recorded for analysis were sample size, the drug/probe/endogenous marker employed, the SJW extract used, the hyperforin and hypericin content of the extract, SJW dosage regime, and the main outcomes. End-points included changes in pharmacokinetic parameters for CYP3A substrates or CYP3A-dependent metabolites.
Assessment of validity
Reports were assessed against the following criteria: (i) study design; (ii) analytical verification of the contents of the extract (or of the raw materials used to make the extract); (iii) quantification of the major constituents (hyperforin and hypericin) of SJW through independent assay; (iv) assessment of compliance with therapy (by participant diary entries, returned pill counts, serum assay, or computerized monitoring of tablet container opening); (v) restriction of intake of other substances that may alter the activity of CYP3A (including grapefruit, red wine, garlic and black pepper); and (vi) baseline pharmacokinetic measurements obtained on more than one occasion.
Results
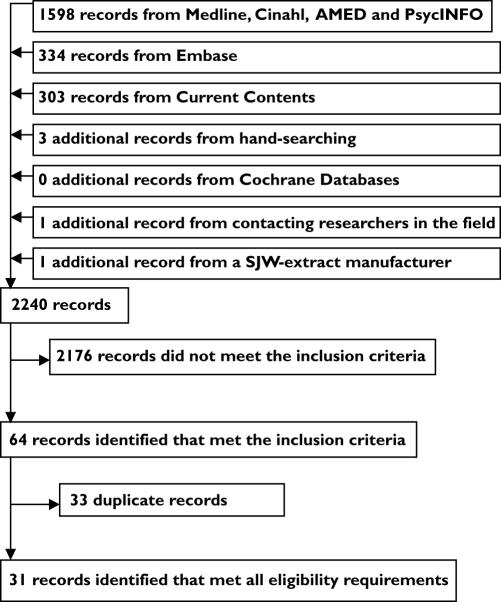
A total of 2235 records were found through database searches. A further three records were obtained from scrutinizing the bibliographies of relevant published articles. One record was obtained from a researcher in the field and one unpublished study from a distributor of SJW. Sixty-four of the records met the inclusion criteria. However, thirty-three of these were removed because they were duplicate studies. Thus, thirty-one studies were included in the analysis. Figure 1 shows the flow of records through the review process.
Figure 1.
The flow of records through the systematic review process
Out of these 31 studies, 15 used a previously described CYP3A probe or marker [17–20]. Five used the 24-h urinary 6β-hydroxycortisol/cortisol ratio, four used midazolam p.o. and i.v., two used midazolam p.o. only, three used alprazolam, one used simvastatin and one used the 14C-erythromycin breath test.
Fifteen studies assessed the pharmacokinetics of drugs that are not considered to be CYP3A probes but for which CYP3A contributes to their biotransformation. These included imatinib, tacrolimus, irinotecan, cyclosporin A, warfarin, indinavir, omeprazole, amitriptyline, theophylline, desogestrel and verapamil (Tables 1, 2 and 3).
Table 1.
Population characteristics, St John’s wort (SJW) dose and CYP3A-related outcomes for studies assessing high-hyperforin extracts
| Trial | Participants | Dosage regime for extract | Drug, probe or marker regime | CYP3A-related outcomes |
|---|---|---|---|---|
| LI160 extract | ||||
| Bauer 2002 [23] | 48, 48% female, age range 21–35 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | 24 h urinary 6BHC/C before and after SJW treatment | 6BHC/C increased from 9.9 to 14.3 (95% CI of difference 2.3, 6.5; P < 0.0001) |
| Bauer 2003 [24] | 11, renal transplant recipients, 18% female, age range 34–58 | 600 mg day−1 for 14 days (H 1.8 mg day−1) | Stable CSA prior to study, CSA pharmacokinetics tested before and after SJW treatment; CSA doses adjusted as required based on trough concentrations | Mean CSA AUC0–12 decreased by 46% (P < 0.005) and mean Ctrough decreased by 42% (P < 0.05) (corrected for additional CSA compensatory doses); the first CSA dose adjustment was required on day 3 of SJW coadministration |
| Dresser 2003 [25] | 21, 52% female, age range 20–55 | 300 mg t.d.s. for 12.5 days (H 2.7 mg day−1) | Midazolam i.v.1 mg, midazolam p.o. 4 mg, cyclosporin 2.5 mg kg−1 before and after SJW treatment | Midazolam i.v. clearance increased by 44% after SJW (P = 0.0001); midazolam p.o. clearance increased by 168%, midazolam p.o. Cmax decreased by 53% (P = 0.0001); cyclosporin clearance increased by 59% (P = 0.0001) |
| Dürr 2000 [26] | 8, 0% female, age range 23–35 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | Oral erythromycin breath test before and after SJW treatment | Demethylation of erythromycin increased by 44% (P = 0.08) |
| Frye 2004 [27] | 12, 50% female, age range 20–51 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | 24 h urinary 6BHC/C and imatinib 400 mg before and on day 12 of SJW treatment, blood samples drawn until day 14 of SJW treatment | 6BHC/C increased by 58% (P = 0.0058); imatinib mean AUC decreased by 30% after SJW (P < 0.0001) |
| Herbert 2004 [28] | 10, 80% female, age range 20–30 | 300 mg tds for 18 days (H 1.3 mg day−1) | Tacrolimus 0.1 mg kg−1 before and after 14 days SJW treatment; blood sampling continued alongside SJW treatment for 4 days | Mean decrease in tacrolimus AUC of 35% (P = 0.0004); mean oral clearance increased by 68% (P = 0.01) |
| Johne 2002 [29] | 12 individuals diagnosed with depression, 75% female, age range 24–59 | 900 mg day−1 for 14 days (H 2.7 mg day−1, HF 55.1 mg day−1) | Amitriptyline 75 mg b.d. for 13 days followed by SJW comedication for 14 days; pharmacokinetic test days before and after SJW treatment | Amitriptyline AUC0−12 decreased by 22% after SJW treatment (P = 0.03); four amitriptyline metabolites were also significantly reduced (between 19 and 41%) |
| Mai 2003 [30] | 10 renal transplant recipients, 40% females, age range 24–66 | 600 mg day−1 for 14 days (H 1.8 mg day−1) | Stable tacrolimus prior to study; pharmacokinetics tested before and after SJW treatment; tacrolimus doses adjusted as required based on trough concentrations | Mean decrease in tacrolimus AUC decrease by 59% (P = 0.005); trough levels decreased by 65% (P = 0.005) |
| Mai 2004 [12] (also assessed low HF, see Table 2) | 10 renal transplant recipients, 10% female, age range 35–65 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1, HF 42 mg day−1) | Stable CSA prior to study; CSA pharmacokinetics tested before and after SJW treatment, CSA doses adjusted as required, during and after SJW based on trough CSA concentrations | CSA AUC decreased by 52% (P < 0.05), CSA trough concentration fell by 54% (P < 0.05), CSA dose adjustments were necessary for longer than 2 weeks |
| Markowitz 2003 [31] | 12, 50% female, age range 22–38 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1, HF 4.2 mg day−1) | Alprazolam 2 mg before and after 14 days SJW treatment | Alprazolam AUC0–∞ decreased by 51% (P < 0.001); mean elimination half-life decreased by 52% (P < 0.001) |
| Pfrunder 2003 [32] | 17, 100% female, age range 20–35 | 300 mg b.d. (H 1.8 mg day−1) or t.d.s. (H 2.7 mg day−1) for 21 days | Ethinyloestradiol 0.02 mg and desogestral 0.150 mg from day 1 to 21 for three menstrual cycles, randomly combined with SJW 300 mg b.d. or SJW 300 mg t.d.s. during cycle 2 and 3; pharmacokinetic testing was performed on approx. day − 10 of each cycle; no washout interval between SJW-treatment cycles reported | Mean AUC of 3-ketodesogestrel (active CYP3A-dependent metabolite of desogestrel) decreased by 43.9% after SJW 300 mg b.d. (95% CI −49.3, − 38.5, P = 0.001) and by 41.7% after SJW 300 mg t.d.s. (95% CI −47.9, – 35.6, P = 0.001); intracyclic bleeding occurred in 35%, 76% and 88% of subjects during control, SJW 300 mg b.d. and SJW 300 mg t.d.s., respectively |
| Wenk 2004 [33] | 16, 50% female, age range 25–58 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | 24 h urinary 6BHC/C and dextromthorphan 25 mg before and after 14 days’ SJW treatment | 6BHC/C increased by 85%; there was a significant difference at baseline (P < 0.05) and after treatment (P < 0.005) between males and females; 3-methoxymophinan to dextromethorphan ratio increased significantly only in females (91% increase, P < 0.01) |
| St JohnSelect™ Indena S.p.A extract standardized to 0.3% hypericin and ≥4% hyperforin | ||||
| Smith 2004 [34] | 10, 30% female (age range N/A) | 300 mg t.d.s. for 14 days (H 2.7 mg day−1, HF ≥36 mg day−1) | Imatinib 400 mg before and after SJW treatment | Imatinib AUC0–∞ decreased by 32% (P < 0.05); Cmax decreased by 29% (P < 0.05) |
| Wang 2004 [35] | 12, 0% female, age range 18–25 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1, HF ≥36 mg day−1) | Omeprazole 20 mg after 14 days’ placebo and 14 days’ SJW (5-week interval between) | Omeprazole AUC0–∞ decreased by 43.9% (P = 0.011) and 37.9% (P = 0.014) in two different CYP219 genotypes; the AUC CYP3A-dependent metabolite omeprazole sulphone increased by 159% (P = 0.017) and 137% (P = 0.014) in two different CYP19 genotypes |
| Unknown SJW extract A, standardized to 0.3% hypericin | ||||
| Gurley 2002 [36] | 12, 50% female, age 25 ± 3.9 | 300 mg t.d.s. for 28 days (H 2.7 mg day−1, HF 12.2 mg day−1) | Midazolam p.o. 8 mg before and after SJW treatment | Mean 98% increase in 1-h 1-hydroxymidazolam/midazolam ratio (P < 0.0001); gender–SJW effect (P = 0.029) with females exhibiting a 74% greater increase in this ratio than males |
| Unknown SJW extract B, standardized to 0.3% hypericins | ||||
| Hall 2003 [37] | 12, 100% female, age 27 ± 7 | 300 mg t.d.s. for 56 days (H 3.3 mg day−1, HF 27 mg day−1) | Midazolam 0.05 mg kg−1 i.v. and 5 mg p.o. before and after 50 days SJW treatment; OCP (ethinyl oestradiol and norethindrone) for 22 out of 28 days for three menstrual cycles, SJW given during the 2nd and 3rd cycle; pharmacokinetic assessment of ethinyl oestradiol and norethindrone was performed on day −7 of the 1st and 3rd cycle | Oral clearance of midazolam increased by 53% (P = 0.007); systemic midazolam clearance was unchanged; oral clearance of norethindrone increased by 16% (P = 0.042); half-life of ethinyl oestradiol reduced by 48% (P = 0.023); breakthrough bleeding occurred in 16% and 58% of women in controlled phase and SJW phase, respectively |
| Wang 2001 [22] | 12, 42% female, age 29 ± 6 | 900 mg once 300 mg tds for 14–15 days (H 2.5 mg day−1, HF 33 mg day−1) | Midazolam i.v. 0.05 mg kg−1, midazolam p.o. 5 mg at baseline, after 1 day SJW and 2 weeks SJW treatment | No significant difference in pharmacokinetics of i.v. or p.o. midazolam after a single dose of SJW; however, there was a trend towards increased oral clearance; after 2 weeks’ SJW there was a 50% reduction in p.o. midazolam AUC (P < 0.05) and 109% increase in p.o. midazolam clearance (P < 0.05) |
| Unknown SJW extract C, standardized hypericin and hyperforin content | ||||
| Jiang 2004 [38] | 12, 0% female, age range 20–40 | Dried extract equivalent to 1 g of dry herb t.d.s. for 21 days (H 2.5 mg day−1, HF 37.5 mg day−1) | Rac-warfarin 25 mg following 14 days’ SJW treatment or no treatment; SJW continued for a further week after warfarin administration; treatment phases were separated by a 2-week washout period | R-warfarin AUC0–∞ ratio comparing SJW treatment with no treatment was 0.77 (95% CI 0.67, 0.87; P < 0.05), R-warfarin oral clearance ratio comparing SJW treatment to no treatment was 1.23 (95% CI 1.11, 1.37; P < 0.05) |
| Unknown SJW extract D, standardized to 3–6% hyperforin | ||||
| Tannergren 2004 [39] | 8, 0% female, age range 23–35 | 300 mg t.d.s. for 14 days (HF 27–45 mg) | Verapamil 400 mg before and after SJW treatment | R- and S-verapamil AUC0–∞ decreased by 78% and 80%, respectively (P < 0.0001); R- and S-norverapamil AUC decreased by 51% (P < 0.01) and 63% (P < 0.0001), respectively |
H, Hypericin; HF, hyperforin (italics, results from independent assay); CSA, cyclosporin A; 6BHC/C, 6-beta-hydroxycortisol/cortisol ratio; t.d.s., three times daily; b.d., twice daily; N/A, not available.
Table 2.
Population characteristics, St John’s wort (SJW) extract dose and CYP3A-related outcomes for studies assessing low-hyperforin extracts
| Trial | Subjects | Dosage regime for extract | Drug, probe or marker regime | CYP3A-related outcomes |
|---|---|---|---|---|
| Esbericum® | ||||
| Arold 2005 [14] | 28, 36% female, age range 22–49 | 120 mg b.d. for 10 days (H 1 mg day−1, HF 3.5 mg day−1) | Alprazolam 1 mg before and after 10 days’ SJW treatment | No significant difference in mean alprazolam AUC0−24 after SJW treatment |
| Ze 117 standardized to 0.2% total hypericins and < 1% hyperforin | ||||
| Brattström [40] | 16,100% female, age range 18–43 | 250 mg b.d. for 14 days (H 1 mg day−1, HF 1.5 mg day−1) | Ethinyl oestradiol 0.02 mg, desogestrel 0.15 mg for 3 months prior to study; during study cycle pharmacokinetic testing was done on day 7 and again on day 22 after 14 days of SJW treatment | No significant differences in ethinyl oestradiol or 3-ketodesgestrel (active metabolite and CYP3A substrate) after SJW treatment |
| Modified low-hyperforin LI160 | ||||
| Mai 2004 [12] (also assessed LI160, see Table 1) | 10 renal transplant recipients, 10% female, age range 35–65 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1, HF 0.6 mg day−1) | Stable CSA prior to study; CSA pharmacokinetics tested before and after SJW; CSA doses adjusted as required based on trough concentrations | There was a significant difference between the effects of the low-hyperforin extract and LI160 on CSA pharmacokinetics (P < 0.0001); there was no significant change to CSA AUC0−12,Ctrough and Cmax for the total patient sample after treatment with the low-hyperforin extract; however, the subgroup of patients that received the low-hyperforin extract during the first treatment period had a 15% decrease in CSA AUC0−12 (95% CI −9%, −21%; P < 0.05) and an 18% decrease in CSA Ctrough (95% CI −8.1, −27%); no CSA dose adjustment was required to offset these changes |
H, Hypericin; HF, hyperforin (italics, results from independent assay); CSA, cyclosporin A; 6BHC/C, 6-beta-hydroxycortisol/cortisol ratio; t.d.s., three times daily; b.d., twice daily.
Table 3.
Population characteristics, St John’s wort (SJW) dose and CYP3A-related outcomes for studies assessing extracts with unknown (or uncertain) hyperforin content
| Trial | Subjects | Dosage regime for extract | Drug, probe or marker regime | CYP3A-related outcomes |
|---|---|---|---|---|
| Early Indena S.p.A extract standardized to 0.3% hypericin | ||||
| Burstein 2000 [41] | 8, 38% female, age range 24–43 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | Carbamazepine for 22 days (dose built up to 400 mg over 7 days), followed by SJW comedication for 14 days; pharmacokinetic test days before and after SJW treatment | No significant change in Cmax, Tmax, AUC0−24 or oral clearance of carbamazepine or metabolite carbamazepine-10,11-epoxide |
| Piscitelli 2000 [42] | 8, 25% female, age range 29–50 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | Indinavir 800 mg t.d.s. on pharmacokinetic tests days (additional 800 mg given on the following day) before and after SJW treatment | Indinavir AUC0−8 decreased by 57% (P = 0.0008) |
| Roby 2000 [43] | 13, 69% female, age range 20–41 | 300 mg t.d.s. for 15 days (H 2.7 mg day−1) | 24 h 6BHC/C before and after SJW treatment | 6BHC/C increased by 83% (P = 0.003, range of change −25% to 259%) |
| Unknown SJW extract E, standardized to 0.3% hypericin | ||||
| Gurley 2005 [21] | 12, 50% female, age range 60–76 | 300 mg t.d.s. for 28 days (H 0.27 mg day−1, HF4.8 mg day−1) | Midazolam p.o. 8 mg before and after 27 days’ SJW | 141% increase in the mean 1-h 1-hydroxymidazolam/midazolam serum ratio (P < 0.001) with a presupplementation mean of 0.379 (95% CI 0.250, 0.507) and a postsupplementation mean of 0.389 (95% CI 0.633, 1.195) |
| Unknown SJW extract F, standardized to 0.3% hypericin | ||||
| Kawaguchi 2004 [44] | 13, 0% females, age 34 ± 6 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | 24 h urinary 6BHC/C and quazepam 15 mg after 14 days’ placebo and 14 days’ SJW treatment (4-week interval between) | The mean 6BHC/C ratio was 96% higher after SJW compared with after placebo (P < 0.05); quazepam mean AUC0−48 was 26% less and the Cmax was 29% less after SJW than after placebo (P < 0.05) |
| Sugimoto 2001 [45] | 8, 0% females, age range 26–44 | 300 mg t.d.s. for 14 days (H 2.7 mg day−1) | Simvastatin 10 mg after 14 days’ placebo and 14 days’ SJW treatment (4-week interval between) | AUC0−24 of pro-drug simvastatin lactone (metabolized to inactive metabolites by CYP3A) and simvastatin hydroxy acid (active metabolite) were 48% and 62% (P < 0.05) less, respectively, after SJW treatment compared with placebo |
| Morimoto 2004 [46] | 12, 0% females, age 25 ± 6 | 300 mg t.d.s. for 15 days (H 2.4 mg day−1) | Theophylline 400 mg after 14 days’ SJW or no treatment (19–35-day interval between) | No change in theophylline AUC curve was observed after 14 days’ SJW treatment (95% CI −9.3%, 23% change; P = 0.41); a trend towards a slight increase in 1-methyluric acid, a CYP1A2 and possibly a CYP3A metabolite |
| Unknown SJW extract G, standardized to 0.3% hypericin | ||||
| Markowitz 2000 [47] | 7, 43% female, age range 24–32 | 300 mg t.d.s. for 4 days (H 2.7 mg day−1) | Alprazolam (3 subjects 1 mg, 4 subjects 2 mg) before and after 3 days of SJW treatment; SJW treatment continued during pharmacokinetic testing for a further day | Alprazolam mean AUC0–∞ decrease of 41.3% (P = 0.14) |
| Unknown SJW extract H | ||||
| Mathijssen 2002 [48] | 5 cancer patients, 60% female, age range 54–66 | 300 mg t.d.s. for 18 days | I.v. irinotecan 350 mg m−2 given at 21-day intervals; subjects were randomly assigned to SJW 14 days prior (plus 4 days after) or no treatment 14 days prior | Active metabolite SN-38 AUC decreased by 42% (95% CI 14, 70; P = 0.033) |
| Unknown SJW extract I | ||||
| Xie 2005 [49] | 30, 26% female, age range 19–51 | 300 mg t.d.s. for 10 days | Oral midazolam 5 mg and i.v. midazolam 1 mg before and after SJW treatment | 56% increase in midazolam systemic clearance (P < 0.001); 45% decrease in absolute bioavailability of midazolam (P < 0.001), no difference between ethnic groups (black, White, Hispanic, Chinese, Indian, Malay) |
H, Hypericin; HF, hyperforin (italics, results from independent assay); CSA, cyclosporin A; 6BHC/C, 6-beta-hydroxycortisol/cortisol ratio; t.d.s., three times daily; b.d., twice daily.
The SJW extract was assayed in 12 of the studies (Table 4). Values of hypericin and hyperforin content were provided by 11 and six studies, respectively. Fourteen studies named the SJW extract tested. Of these, 12 used LI160 (Litchwer Pharma), one used Espericum® (Schaper & Brümmer) and one used Ze 117 (Bayer Vital). The extract used in two other studies was identified as St JohnSelect™ by the distributor (Indena S.p.A). In the remaining 15 studies the SJW extract was not identified.
Table 4.
Assessment of methodological issues in studies investigating the effect of St John’s wort (SJW) on CYP3A activity
| Trial | Design | Analysis of SJW extract performed | CYP3A potential inducers and inhibitors controlled for | Assessment of SJW compliance |
|---|---|---|---|---|
| Jarson LI160 standardized 0.03% hypericins | ||||
| Bauer 2002 [23] | BA | No | Not stated | No |
| Bauer 2003 [24] | BA | No | During study grapefruit juice | No |
| Dresser 2003 [25] | BA | No | 1 week prior to study ‘herbal and citrus products’, 3 days prior other medications | No |
| Dürr 2000 [26] | BA | No | 5 days prior citrus fruits | No |
| Frye 2004 [27] | BA | No | 48 h prior to pharmacokinetic testing grapefruit | Yes |
| Herbert 2004 [28] | BA | Yes | During study grapefruit | Yes |
| Johne 2002 [29] | BA | No | Prior to study urine/blood screen for cannabinoids, amphetamines, morphines, benzodiazepines, barbituates and cocaine | Yes |
| Mai 2003 [30] | BA | No | During study grapefruit | Yes |
| Mai 2004 [12] | R, CO | Yes | During study, grapefruit | No |
| Markowitz 2003 [31] | BA | Yes | During study grapefruit | No |
| Pfrunder 2003 [32] | R, CO | No | During study grapefruit | Yes |
| Wenk 2004 [33] | BA | No | During study grapefruit | No |
| St JohnSelect Indena S.p.A extract standardized to 0.3% hypericin and ≥4% hyperforin | ||||
| Smith 2004 [34] | BA | No | Not stated | No |
| Wang 2004 [35] | R, PC, CO | No | One month prior to study SJW, herbal medicines, OTC medicines | No |
| Unknown SJW extract A, standardized to 0.3% hypericin | ||||
| Gurley 2002 [36] | R, BA | Yes | 5 days prior to study probe drug administration and during study, grapefruit | No |
| Unknown SJW extract B, standardized to 0.3% hypericin | ||||
| Hall 2003 [37] | BA | Yes | 4 weeks prior to study SJW, 2 weeks prior grapefruit | Yes |
| Wang 2001 [22] | BA | Yes | 3 months prior to study marijuana, 4 weeks prior SJW, 2 weeks prior pharmaceutical drugs, grapefruit | No |
| Unknown SJW extract C, standardized hypericin and hyperforin content | ||||
| Jiang 2004 [38] | R, CO | Yes | 2 weeks prior to study all medications | No |
| Unknown SJW extract D, standardized to 3–6% hyperforin | ||||
| Tannergren 2004 [39] | BA | No | 3 days prior to pharmacokinetic testing grapefruit | No |
| Esbericum® | ||||
| Arold 2005 [14] | DB, R, PC, P | No | During study < 4 cups/day quinine containing beverages On pharmacokinetic test days (24 h before and after probe drug) grapefruit, quinine | Yes |
| Remotiv Ze 117 | ||||
| Brattstöm [40] | BA | No | Not stated | Yes |
| Modified low-hyperforin LI160 | ||||
| Mai 2004 [12] (also listed above) | R, CO | Yes | During study, grapefruit | No |
| Early Indina S.p.A extract standardized to 0.3% hypericin | ||||
| Burstein 2000 [41] | BA | Yes | 30 days prior to study SJW | Yes |
| Piscitelli 2000 [42] | BA | Yes | 30 days prior to study SJW | No |
| Roby 2000 [43] | BA | Yes | During study grapefruit, herbal dietary supplements and herbal tea | No |
| Unknown SJW extract E, standardized to 0.3% hypericin | ||||
| Gurley 2005 [21] | R, BA | Yes | During the study fruit juices, food medicine diary to document compliance | Yes |
| Unknown SJW extract F, standardized 0.3% hypericin | ||||
| Kawaguchi 2004 [44] | R, DB, PC, CO | No | During the study grapefruit, herbal and dietary supplements, and herbal tea | Yes |
| Sugimoto 2001 [45] | DB, CO | No | During the study grapefruit, herbal and dietary supplements, and herbal tea | No |
| Morimoto 2004 [46] | R, CO | Yes | 2 weeks prior to study and during the study grapefruit and SJW | No |
| Unknown SJW extract G, standardized to 0.3% hypericin | ||||
| Markowitz 2000 [47] | BA | No | Not stated | No |
| Unknown SJW extract H | ||||
| Mathijssen 2002 [48] | R, CO? | No | Not stated | No |
| Unknown SJW extract I | ||||
| Xie 2005 [49] | BA | No | 30 days prior to study ‘known enzyme-altering drugs or herbal products’ | No |
BA, Before-and-after; CO, crossover; R, randomized; DB, double-blind; P, placebo-controlled.
For the purpose of the present analysis, high-hyperforin extracts were defined as those containing ≥10 mg per daily dose and low-hyperforin extracts as those containing ≤4 mg hyperforin per daily dose. The low-hyperforin definition was based on the daily dose given in recent clinical trials claiming to have tested a ‘low-hyperforin’ extract [12, 13] and the high-hyperforin definition was based on the approximate content of 600–900 mg extract containing ≥2% hyperforin. Of the 31 studies analysed, 19 used a high-hyperforin and three used a low-hyperforin extract. One of these studies tested both a low- and a high-hyperforin extract. All studies employing the LI160 extract were classified as high-hyperforin based on published interbatch data, which indicated that the mean hyperforin content for a daily 900-mg dose was 22.5 mg with a coefficient of variation of 13.8%[15].
Ten studies used an extract of unknown hyperforin content. A recent study [21] was included in this group due to uncertainty over the reliability of the reported hypericin and hyperforin content of the extract.
The mean SJW treatment period was 15.8 days, with a range of 4–50 days (Tables 1, 2 and 3). One study also assessed midazolam clearance after a single 900-mg dose of SJW.
Twenty-six of the studies were conducted in healthy subjects, 3 in renal transplant recipients, 1 in patients with depression and 1 on cancer patients. The mean population size was 13.7 (SD 8.4, range 5–48). A mean of 40.1% of subjects were female (SD 30.9%, range 0–100%).
Twenty-six of the 31 studies had outcomes that were consistent with SJW extract-mediated induction of CYP3A (Tables 1, 2 and 3). All of the studies classified as using high-hyperforin extracts yielded results supporting CYP3A induction after a SJW treatment period of 12.5–21 days (Table 1). In one of these studies a single dose of SJW extract was given and found to have no significant effects on the pharmacokinetics of systemic or oral clearance of midazolam [22].
All three low-hyperforin extract studies reported no significant differences in pharmacokinetics after SJW treatment, with the exception of a subgroup in one study [12] (Table 2).
Seven out of the 10 studies on extracts with unknown hyperforin content reported results consistent with SJW-mediated CYP3A induction (Table 3).
Information on study design is outlined in Table 4. Two-thirds had a before-and-after design using subjects as their own controls. Eleven assessed compliance with SJW therapy. Measures to control for the intake of substances that may inhibit or induce CYP3A varied widely (Table 4). Only one study measured baseline pharmacokinetics on more than one occasion [21]. However, three studies assessed the effects of SJW in patients in whom steady-state concentrations of cyclosporin A or tacrolimus had been achieved.
Discussion
We included 31 studies in this systematic review of drug interaction trials involving SJW and CYP3A and its substrates. All studies using high-hyperforin extracts, including all 12 studies using the LI160 extract, were associated with significant changes in pharmacokinetic measurements consistent with CYP3A induction. Conversely, the three studies that assessed low-hyperforin extracts did not find any statistically significant changes in CYP3A activity (Table 2). The exception was one subgroup in one of these studies [12], but in this instance the statistically significant difference was not considered clinically relevant by the authors. This assessment was in accordance with the recommendations of the US Food and Drug Administration, which define an interaction as clinically significant when the interval of the AUC difference is completely outside the standard deviation 20% equivalence limit [50, 51]. Variable results were obtained in studies using SJW extracts with unknown hyperforin content. Key methodological issues pertinent to herb–drug interaction trials were not dealt with consistently.
Hyperforin may be a key constituent of SJW responsible for CYP3A induction. In vitro findings have shown that hyperforin activates the pregnane X receptor involved in the regulation of CYP3A expression [52, 53]. Cantoni et al. found [54] that the CYP3A-dependent erythromycin-N-demethylase activity of mice fed 300 mg kg−1 of Indena S.p.A SJW extract increased 2.2-fold. Furthermore, a hyperforin salt containing an equivalent amount of hyperforin to that in the extract increased erythromycin-N-demethylase activity by 1.8 times that of controls, suggesting that hyperforin plays an important role in the CYP3A-inducing effect of SJW, at least in mice [54]. The contrasting findings from human studies using low- and high-hyperforin extracts provide further evidence of the role of hyperforin in human CYP3A induction.
Two studies using borderline low-hyperforin extracts did have outcomes consistent with CYP3A induction [21, 31]. However, we believe it is valid to question the reliability of the reported hyperforin content for these studies for the following reasons: (i) hyperforin is unstable [55], and therefore its measurement is prone to inaccuracies; (ii) one of these studies [31] used the LI160 extract, which is characteristically hyperforin-rich, and according to an assessment of five different batches has low batch-to-batch variability [15]; and (iii) the reported hypericin content of the extract employed in the other study was a factor of 10 less than the label standardization claim [21], which could indicate that an assay, calculation or typographical error occurred in the reporting of the concentrations of both hypericin and hyperforin. On the other hand, the reported hyperforin content of these extracts may be correct, and therefore the outcomes of these studies may indicate the inconsistent effects of low-hyperforin extracts on CYP3A. It is also possible that differences in CYP3A inducibility between study populations (e.g. gender-based differences) or differences in hyperforin bioavailability between different extracts or dose forms could be responsible for these inconsistent results. The findings of Gurley et al.[21] could indicate that longer treatment (28 days vs. 10–14 days) with low-hyperforin extracts may result in CYP3A induction. Clearly, more human studies are needed to investigate further the role of hyperforin in CYP3A induction and assess the ability of low-hyperforin extracts to induce this and other important drug-metabolizing enzymes.
Hyperforin is reputed to be a key constituent responsible for the antidepressant activity of SJW [56] and it has been suggested that several manufacturers have responded to this by increasing the hyperforin content of their extracts [57]. However, studies employing low-hyperforin extracts have also demonstrated efficacy in the treatment of mild-to-moderate depression [2, 5, 58]. Furthermore, hyperforin is a relatively unstable hydrophobic compound and its presence in traditional extracts such as tinctures and water-based infusions is likely to be minimal [57].
Variable results were obtained in studies using SJW extracts with unknown hyperforin content. Of the 10 studies falling into this category, seven reported results consistent with CYP3A induction. One possible explanation for these variable outcomes is differences in the composition of the extracts employed. However, there are plausible alternative explanations relating to each of the three studies reporting negative reesults. Burstein et al. assessed the effects of a SJW extract on carbamazepine pharmacokinetics [41]. The authors suggested that the lack of a significant effect may have been due to the CYP3A autoinducing capabilities of carbamazepine and that SJW may not be potent enough to induce CYP3A further when this enzyme is already in an induced state. Morimoto et al., assessing the effects a SJW extract on theophylline, found no significant difference in the pharmacokinetics of this drug after SJW treatment for 14 days [46]. However, theophylline is predominantly metabolized by CYP1A2, with CYP2E1 and CYP3A4 being thought to play only minor roles. Malkowitz et al. conducted an early study prior to the publication of data suggesting that, contrary to in vitro findings, SJW may induce CYP3A. Hence this study, designed to detect CYP3A inhibition, not induction [47], found no significant effects of SJW.
Treatment with high-hyperforin extracts for periods of 14 days have clearly caused effects consistent with CYP3A induction. However, the ability of shorter treatment periods to initiate CYP3A induction has not been established. Wang et al. found no significant difference in midazolam systemic or oral clearance after a single dose of SJW, but after 14 days of treatment, oral clearance increased by 109%, suggesting that there was a time delay before induction began [22]. Both Bauer et al. and Mia et al. found that transplant recipients on previously stable cyclosporin A treatment required a dosage adjustment after 3 days’ coadministration with the LI160 extract of SJW [12, 24].
The duration of the washout period for the inductive effect of SJW on CYP3A has not been widely assessed. Bauer et al.[24] and Mai et al.[12] both found that it took longer than 2 weeks for baseline cyclosporin A doses to maintain trough cyclosporin A concentrations after cessation of 14 days’ LI160 treatment. Mai et al. also found there was a treatment-order effect in their randomized crossover study comparing LI160 with a modified low-hyperforin extract. There was a significant 15% decrease in cyclosporin A AUC0−12 and a 18% decrease in Ctrough (both P < 0.05) in participants who took the low-hyperforin SJW extract during the first treatment period. This effect was not regarded as clinically significant and no dosage adjustments were required. Changes in cyclosporin pharmacokinetics were not seen in those who took SJW during the second treatment period (Table 2). These findings suggest that the effect of the high-hyperforin extract on the inducibility of CYP3A may have persisted beyond the 27-day washout period, despite cyclosporin A dose requirements returning to baseline. Cyclosporin A is also a substrate for P-glycoprotein. Clinical studies suggest that P-glycoprotein is also induced by SJW [12, 59, 60] and hence these findings do not relate exclusively to CYP3A. However, the data do have implications for crossover studies and studies employing washout periods, and suggest that the effects of SJW treatment on CYP3A activity may persist for longer than the effect of SJW on drug pharmacokinetics.
The potential effects of compounds that modulate CYP3A activity during and after exposure to SJW raise questions of how best to control these factors in clinical studies. Whether such compounds should be withdrawn prior to the study, and how long this period of withdrawal should be, are unclear. If background intake of dietary and botanical CYP3A inducers and inhibitors remains constant throughout the study, these compounds may not interfere with the effect of SJW on CYP3A. Furthermore, if CYP3A is already in an induced state, it may not be susceptible to further induction. Additionally, if CYP3A activity is being inhibited, induction by SJW may not override this effect. Finally, exposure to these compounds may change on a daily basis.
The large variation in the measures instituted to control the intake of compounds that may affect CYP3A is perhaps a reflection of uncertainty about the most appropriate way to decrease the influence of these variables. In some studies such compounds were withdrawn 48 h prior to blood sampling, some for the whole treatment period and some for a washout period prior to and during the treatment period (Table 4). There are concerns with all of these strategies. The effect of some compounds may still be present after 48 h. For example, it may take longer than 3 days for the effect of grapefruit on CYP3A to subside [61] and exposure in the preceding 48 h is likely to be variable among participants and study phases. If baseline measurements are performed without a washout period, and subjects are fed a diet that is continued until after the study, a different environment will have been constructed for the baseline and after-treatment measurements, leading to the possibility of both Type I and Type II errors. Finally, there is the difficulty of determining the appropriate length of the washout period prior to the study and the uncertainty of whether unknown dietary inducers and inhibitors of CYP3A may also be present.
Conclusion
Most of the studies included in this review had an open-label, before-and-after design. Hence, their overall methodological rigour is less than that currently expected of therapeutic intervention studies. Whether this level of rigour is appropriate for herb–drug interaction studies requires further discussion. Key issues, such as control of diet and the duration of washout periods, need additional research in order to improve trial design. The identity of the extract of SJW employed should be explicitly stated and chemical analysis data on its key constituents should be included.
Our analysis suggest that there is reasonable evidence that high-hyperforin St John’s wort extracts induce CYP3A. More studies are required to investigate the possible decreased CYP3A induction potential of low-hyperforin extracts.
Competing interests: None declared.
References
- 1.Harrer G, Hubner W, Prodzuwent I. Effectiveness and tolerance of the hypericum extract LI 160 compared to maprotiline: a multicenter double-blind study. J Geriatr Psychiatry Neurol. 1999;7(Suppl.):524–8. doi: 10.1177/089198879400700108. [DOI] [PubMed] [Google Scholar]
- 2.Schrader E. Equivalence of St. John’s wort extract (Ze 117) and fluoxetine: a randomized, controlled study in mild-moderate depression. Int Clin Psychopharmacol. 2000;15:61–8. doi: 10.1097/00004850-200015020-00001. [DOI] [PubMed] [Google Scholar]
- 3.Szegedi A, Kohnen R, Dienel A, Kieser M. Acute treatment of moderate to severe depression with hypericum extracts WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine. BMJ. 2005;330:503. doi: 10.1136/bmj.38356.655266.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheatley D. LI 160, an extract of St. John’s wort, versus amitriptyline in mildly to moderately depressed outpatients—a controlled 6-week clinical trial. Pharmacopsychiatry. 1997;30(Suppl. 2):77–80. doi: 10.1055/s-2007-979523. [DOI] [PubMed] [Google Scholar]
- 5.Woelk H. Comparison of St John’s wort and imipramine for treating depression: randomised controlled trial. BMJ. 2001;321:536–9. doi: 10.1136/bmj.321.7260.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruschitzka F, Meier P, Turina M, Luscher T, Noll G. Acute heart transplant rejection due to Saint John’s Wort. Lancet. 2000;355:548–9. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 7.Turtoon-weeks S, Barone G, Gurley B, Ketel B, Lightfoot M, Abul-Ezz S. St John’s wort: a hidden risk for transplant patients. Prog Transplant. 2001;11:116–20. doi: 10.1177/152692480101100207. [DOI] [PubMed] [Google Scholar]
- 8.Henderson L, Yue Q, Bergquist C, Gerden B, Arlett P. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349–56. doi: 10.1046/j.1365-2125.2002.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzo A, Ernst E. Interactions between herbal medicines and prescription drugs. Drugs. 2001;61:2163–75. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mills E, Montori V, Wu P, Gallicano K, Clarke M, Guyatt G. Interaction of St John’s wort with conventional drugs: systematic review of clinical trials. BMJ. 2004;329:27–30. doi: 10.1136/bmj.329.7456.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammerbess P, Basch E, Ulbricht C, Barrette E, Foppa I, Basch S. St John’s wort: a systematic review of adverse effects and drug interactions for the consultation psychiatrist. Psychosomatics. 2003;44:271–82. doi: 10.1176/appi.psy.44.4.271. [DOI] [PubMed] [Google Scholar]
- 12.Mai I, Bauer S, Perloff E, Johne A, Uchleke B, Frank B, Budde K, Roots I. Hyperforin content determines the magnitude of the St John’s wort–cyclosporin drug interaction. Clin Pharmacol Ther. 2004;76:330–40. doi: 10.1016/j.clpt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Arold G, Donath F, Maurer A, Diefenbach K, Bauer S, Henneicke-von Zepelin H, Friede M, Roots I. No relevant interaction with alprazolam, caffeine, tolbutamide, and digoxin by treatment with a low-hyperforin St John’s wort extract. Planta Med. 2005;71:331–7. doi: 10.1055/s-2005-864099. [DOI] [PubMed] [Google Scholar]
- 14.Greeson J, Sanford B, St Monti D. St John’s wort (Hypericum perforatum) a review of the current pharmacological, toxicological and clinical literature. Psychopharmacology. 2001;153:402–14. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 15.Wurglics M, Westerhoff K, Kaunzinger A, Wilke A, Baumeister A, Dressman J, Schubert-Zsilavecz M. Comparison of German St. John’s Wort products according to hyperforin and total hypericin content. J Am Pharm Assoc. 2001;41:560–6. doi: 10.1016/s1086-5802(16)31280-3. [DOI] [PubMed] [Google Scholar]
- 16.Bertz R, Granneman G. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–58. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Watkins P. Noninvasive tests of CYP3A enzymes. Pharmacogen. 1994;4:171–84. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Streetman D, Bertino J, Nafziger A. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogen. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Bjornsson T, Callaghan D, Einoff H, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug–drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) Perspective. Drug Metab Dispos. 2003;31:815–32. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 20.Tucker G, Houston J, Huang S. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential—towards a consensus. Pharm Res. 2001;18:1071–80. doi: 10.1023/a:1010994022294. [DOI] [PubMed] [Google Scholar]
- 21.Gurley B, Gardner S, Hubbard M, Williams K, Gentry B, Cui Y, Ang C. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly. St John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22:525–39. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Gorski C, Hammam M, Huang S, Lesko L, Hall S. The effect of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317–26. [PubMed] [Google Scholar]
- 23.Bauer S, Stromer E, Kerb R, Johne A, Brockmoller J, Roots I. Differential effects of Saint John’s Wort (Hypericum perforatum) on the urinary excretion of d-glucaric acid and 6-beta-hydroxycortisol in healthy volunteers. Eur J Clin Pharmacol. 2002;58:581–5. doi: 10.1007/s00228-002-0527-5. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S, Stromer E, Johne A, Kruger H, Klemens B, Neumayer H, Roots I, Mai I. Alterations in cyclosporin A pharacokinetics and metabolism during treatment with St John’s wort in renal transplant patients. Br J Clin Pharmacol. 2003;55:203–11. doi: 10.1046/j.1365-2125.2003.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dresser G, Schwarz U, Wilkinson G, Kim R. Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- 26.Durr D, Stieger B, Kullak-Ublick G, Rentsch K, Steinert H, Meier P, Fattinger K. St John’s wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 27.Frye R, Fitzgerald S, Lagattuta T, Hruska M, Egorin M. Effect of St John’s wort on imatinib mesylate pharmacokinetics. Clin Pharmacol Ther. 2004;76:323–9. doi: 10.1016/j.clpt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Herbert M, Park J, Chen Y, Akhtar S, Larson A. Effects of St. John’s Wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2004;44:89–94. doi: 10.1177/0091270003261078. [DOI] [PubMed] [Google Scholar]
- 29.Johne A, Schmider J, Brockmoller J, Stradelmann A, Strormer E, Bauer S, Scholler G, Langheinrich M, Roots I. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St. John’s wort (Hypericum perforatum) J Clin Psychopharmacol. 2002;22:46–54. doi: 10.1097/00004714-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Mai I, Stormer E, Bauer S, Kruger H, Budde K, Roots I. Impact of St John’s wort treatment on the pharmacokinetics of tacrolimus and mycophenolic acid in renal transplant patients. Nephrol Dial Transplant. 2003;18:819–22. doi: 10.1093/ndt/gfg002. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz J, Donovan J, DeVane C, Taylor R, Ruan Y, Wang J, Chavin K. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290:1500–4. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 32.Pfunder A, Schiesser M, Gerber S, Haschke M, Bitzer J, Drewe J. Interaction of St John’s wort with a low-dose oral contraceptive therapy: a randomised controlled trial. Br J Clin Pharmacol. 2003;56:683–90. doi: 10.1046/j.1365-2125.2003.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenk M, Todesco L, Krahenbuhl S. Effect of St John’s wort on the activities of CYP1A2, CYP3A4, CYP2D6, N-acetyltransferase 2, and xantine oxidase in healthy males and females. Br J Clin Pharmacol. 2004;57:495–9. doi: 10.1111/j.1365-2125.2003.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith P, Bullock J, Booker B, Hass C, Berensn C, Jusko W. Induction of imatinib metabolism by Hypericum perforatum. Blood. 2004;104:1229–30. doi: 10.1182/blood-2004-04-1240. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Zhou G, Zhu B, Wu J, Wang J, El-Aty A, Li T, Liu J, Yang T, Wang D, Zhong X, Zhou H. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther. 2004;75:191–7. doi: 10.1016/j.clpt.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Gurley B, Gardner S, Hubbard M, Williams K, Gentry B, Cui Y, Ang C. Cytochrome P450 phenotypic ratios for predicting herb–drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–87. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 37.Hall S, Wang Z, Huang S, Hammas M, Vasavada N, Adigun A, Hilligoss J, Miller M, Gorksi C. The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74:525–35. doi: 10.1016/j.clpt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Williams K, Liauw W, Ammit A, Roufogalis B, Duke C, Day R, McLachlan A. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;59:425–32. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannergren C, Engman H, Knutson L, Hedeland M, Bondesson U, Kennernas H. St John’s wort decreases the bioavailability of R- and S-verapamil through induction of first-pass metabolism. Clin Pharmacol Ther. 2004;75:298–309. doi: 10.1016/j.clpt.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Brattstrom A. St John’s wort extract (Ze 117) does not alter the kinetics of a low dose oral contraceptive (unpublished data) 2005. [DOI] [PubMed]
- 41.Burstein A, Horton R, Dunn T, Alfaro R, Piscitelli S, Theodore W. Lack of effect of St John’s wort on carbamazepine pharmacokinetics in healthy volunteers. Clin Pharmacol Ther. 2000;68:605–12. doi: 10.1067/mcp.2000.111530. [DOI] [PubMed] [Google Scholar]
- 42.Piscitelli S, Burstein A, Chaitt D, Alfaro R, Falloon J. Indinavir concentrations and St John’s wort. Lancet. 2000;355:547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 43.Roby C, Anderson G, Kanton E, Dryer D, Burstein A. St John’s Wort: effect on CYP3A4 activity. Clin Pharmacol Ther. 2000;67:451–7. doi: 10.1067/mcp.2000.106793. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi A, Ohmori M, Tsufuoke S, Nishiki K, Harada K, Miyamori I, Yano R, Nakamura T, Masada M, Fujimura A. Drug interaction between St John’s wort and quazepam. Br J Clin Pharmacol. 2004;58:403–10. doi: 10.1111/j.1365-2125.2004.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, Arakawa M, Sakamoto K, Masada M, Miyamori I, Fujimura A. Different effects of St John’s wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2001;70:518–24. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto T, Kotegawa T, Tsutsumi K, Ohtani Y, Imai H, Nakano S. Effect of St John’s wort on the pharmacokinetics of theophylline in healthy volunteers. J Clin Pharmacol. 2004;44:95–101. doi: 10.1177/0091270003261496. [DOI] [PubMed] [Google Scholar]
- 47.Markowitz J, DeVane C, Boulton D, Carson S, Nahas Z, Risch S. Effect of St John’s wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Life Sci. 2000;66:133–9. doi: 10.1016/s0024-3205(99)00659-1. [DOI] [PubMed] [Google Scholar]
- 48.Mathijssen R, Verweoj J, Bruijn P, Loos W, Sparreboom A. Effects of St John’s wort on irinotecan metabolism. J Natl Cancer Inst. 2002;94:1247–9. doi: 10.1093/jnci/94.16.1247. [DOI] [PubMed] [Google Scholar]
- 49.Xie R, Tan L, Polasek E, Hong C, Teillol-Foo M, Gordi T, Sharma A, Nickens D, Arakawa T, Knuth D, Antal E. CYP3A and P-glycoprotein activity induction with St. John’s wort. J Clin Pharmacol. 2005;45:352–6. doi: 10.1177/0091270004273320. [DOI] [PubMed] [Google Scholar]
- 50.Mills E, Wu P, Johnston B, Gallicano K, Clarke M, Guyatt G. Natural health product–drug interactions: a systematic review of clinical trials. Ther Drug Monit. 2005;27:549–57. doi: 10.1097/01.ftd.0000170880.95267.90. [DOI] [PubMed] [Google Scholar]
- 51.Food and Drug Administration. In Vivo Drug Metabolism/Drug Interaction Studies–Study Design, Data Analysis, and Recommendations for Dosing and Labelling. Rockville, MD: FDA; 1999. [Google Scholar]
- 52.Moore B, Goodwin B, Jones S, Wisey G, Serabjit-Singh C, Wilson T, Collins J, Kliewer S. St John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wentworth J, Agostini M, Love J, Schwabe J, Chatterjee V. St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 54.Cantoni L, Rozio M, Mangolini A, Hauri L, Caccia S. Hyperforin contributes to the hepatic CYP3A-inducing effect of Hypericum perforatum extract in the mouse. Toxicol Sci. 2003;75:25–30. doi: 10.1093/toxsci/kfg174. [DOI] [PubMed] [Google Scholar]
- 55.Zanoli P. Role of hyperforin in the pharmacological activities of St. John’s wort. CNS Drug Rev. 2004;10:203–18. doi: 10.1111/j.1527-3458.2004.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greeson J, Sanford B, Monti D. St John’s wort (Hypericum perforatum): a review of the current pharmacological, toxicological and clinical literature. Psychopharmacology. 2004;153:402–14. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 57.Meier B. Comparing phytopharmaceuticals: the example of St. John’s wort. Adv Ther. 2001;18:35–46. doi: 10.1007/BF02850249. [DOI] [PubMed] [Google Scholar]
- 58.Kaufeler R, Meier B, Brattstrom A. Efficacy and tolerability of Ze 117 St. John’s wort extract in comparison with placebo, imipramine and fluoxetrine for the treatment of mild to moderate depression according to ICD-10. An overview. Pharmacopsychiatry. 2001;34(Suppl. 1):S49–S50. doi: 10.1055/s-2001-15457. [DOI] [PubMed] [Google Scholar]
- 59.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interactions of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–45. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Hammam M, Huang S, Lesko L, Hall S. Effect of St John’s wort on the pharmacokinetics of fexofenadine. Clin Pharmacol Ther. 2002;71:414–20. doi: 10.1067/mcp.2002.124080. [DOI] [PubMed] [Google Scholar]
- 61.Takanaga H, Ohnishi A, Murakami H, Matsuo H, Higuchi S, Urae A, Irie S, Furuie H, Matsukuma K, Kimura M, Kawano K, Orii Y, Tanaka T, Sawada Y. Relationship between time after intake of grapefruit juice and the effect on pharmacokinetics and pharmacodynamics of nisoldipine in healthy subjects. Pharmacokin Drug Dispos. 2000;67:201–14. doi: 10.1067/mcp.2000.104215. [DOI] [PubMed] [Google Scholar]