Abstract
What is already known about this subject
The concentration of protease and non-nucleoside reverse transcriptase inhibtors in plasma has been related to both efficacy and toxicity.
Most antiretroviral concentration data come from selected populations of patients undergoing therapeutic drug monitoring programmes, which may overestimate interindividual variability.
What this study adds
Our study has demonstrated the large interindividual variability in antiretroviral drug concentrations in an unselected population of patients during routine clinical practice.
These results may provide interesting information to clinicians for the management of antiretroviral therapy in HIV-infected patients.
Aims
The objective of this study was to assess interindividual variability in trough concentrations of plasma of non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI) among HIV-infected adults in a routine outpatient setting.
Methods
One hundred and seventeen patients who attended our clinic for routine blood tests, and who were receiving antiretroviral therapy which included NNRTI or PI were studied. Patients were not informed that drug concentrations were going to be measured until blood sampling. The times of the last antiretroviral dose and of blood sampling were recorded. Drug concentrations were considered optimal if they were above the proposed minimum effective value. In addition, efavirenz, nevirapine and atazanavir concentrations were considered potentially toxic if they were >4.0 mg l−1, >6.0 mg l−1 and >0.85 mg l−1, respectively.
Results
Overall, interindividual variability of NNRTI and PI concentrations in plasma was approximately 50%, and only 68.4% of the patients had drug concentrations within the proposed therapeutic range. Poor adherence explained only 35% of subtherapeutic drug concentrations.
Conclusion
Interindividual variability in trough concentrations of NNRTI and PI among HIV-infected adults is large in routine clinical practice, with drug concentrations being outside the therapeutic window in a significant proportion of patients. These findings provide further evidence that therapeutic drug monitoring may be useful to guide antiretroviral therapy in clinical practice.
Keywords: antiretroviral agents, clinical practice, drug concentrations, HIV infection, interindividual variability
Introduction
Current highly active antiretroviral therapy (HAART) can successfully suppress HIV replication and its widespread use has resulted in a marked decrease in HIV-related morbidity and mortality [1]. However, despite the initial favourable response to HAART, it fails to maintain complete viral suppression in the long term in a significant proportion of HIV-infected subjects [2–4]. Treatment failure may be due to poor adherence on the part of the patients, the development of viral resistance, or to pharmacokinetic issues.
There is growing evidence of a relationship between non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI) concentrations in plasma and the efficacy and toxicity of these drugs [5]. In this regard, small decreases in plasma concentrations of antiretroviral drugs can render them unable to maintain complete viral suppression, promoting treatment failure and evolution of viral resistance. On the other hand, excessively high drug concentrations may contribute, at least in part, to the appearance of antiretroviral therapy-related adverse events [6–18].
Marked differences in NNRTI and PI concentrations in plasma have been reported among HIV-infected subjects [5, 13, 15, 19, 20]. Moreover, a large proportion of patients had trough PI concentrations lower than the proposed minimum effective concentration (MEC) in previous studies [19–22]. This interindividual variability may be explained by differences in drug absorption, distribution, metabolism and elimination. In addition, poor adherence to treatment, concomitant disease and drug–drug or food–drug interactions may further enhance this variability.
Most of the currently available information regarding interindividual variability in the concentration of antiretroviral agents comes from clinical trials and therapeutic drug monitoring (TDM) units. However, results obtained in such settings may substantially differ from those observed in routine clinical practice with unselected patient populations. Thus, the objective of the present study was to assess the interindividual variability in trough NNRTI and PI concentrations in plasma among HIV-infected adults in a routine outpatient setting.
Patients and methods
HIV-infected subjects aged ≥18 years, who attended consecutively our clinic during a 2-week period for routine outpatient blood tests, were studied. To be eligible, patients had to be receiving an NNRTI- or a PI-based HAART for at least 4 weeks. Patients on treatment with more than one PI (ritonavir was not considered as a second PI when given at boosting doses) or with combinations of PI with NNRTI, were excluded. Informed consent was obtained from all patients at the time of blood sampling, and the protocol was approved by the ethics committee of our institution.
The primary end-point of the study was assess the interindividual variability in trough NNRTI and PI concentrations in plasma among HIV-infected adults. The relationship between drug concentrations and virological outcome or drug-related toxicity was also explored.
Demographic and clinical variables including age, sex, time since HIV diagnosis, hepatitis C virus coinfection, concomitant medication, treatment adherence, HIV-1 RNA load evolution and drug-related toxicity were recorded for each subject. Treatment adherence was self-reported as the number of doses taken out of those prescribed within the week before the sample collection. Viral load was recorded at the time of sampling and every 12 weeks thereafter up to 48 weeks of follow-up. Virological failure was defined as the presence of an HIV-1 RNA load >50 copies ml−1 in two consecutive determinations after at least 24 weeks on HAART or if previously undetectable. Specific drug-related toxicities were recorded in those patients on treatment with drugs for which an upper limit of the therapeutic window has been proposed. Thus, hypersensitivity reactions or liver enzyme elevations were registered in patients taking nevirapine, central nervous system disorders in those taking efavirenz, or total bilirubin or jaundice in those patients receiving atazanavir.
In an attempt to avoid modifying their normal adherence to antiretroviral therapy, subjects were not informed that drug concentrations were going to be measured until blood sampling. The time of the last dose of antiretroviral treatment and that of blood sampling were recorded for each patient. Because trough concentrations have been proposed as the most suitable pharmacokinetic parameter for routine clinical monitoring [5], only blood samples collected between 10 and 13 h or between 21 and 25 h after the last treatment intake were used in patients taking antiretroviral drugs twice or once daily, respectively. In the case of patients taking efavirenz once daily at bedtime, samples were collected during the day, at least 8 h after the last dose [15].
Blood samples were collected into potassium and ethylenediamine tetraacetic acid (EDTA)-containing tubes. Plasma was isolated by centrifugation (1200 g for 15 min) and stored at −20 °C until analysis. Drug concentrations were determined by using a high-performance liquid chromatograph with a PDA detector (2996 Waters, Barcelona, Spain). Concentrations of nevirapine were measured using a NovaPak® C18 3.9 × 150 mm analytical column and a NovaPak® C18 guard column (Waters). The method involved precipitation of proteins with perchloric acid and injecting the supernatant by isocratic elution with phosphate buffer acetonitrile containing 0.1% of triethylammine (pH 6). The method was linear over a concentration range of 0.1–10 mg l−1. Efavirenz concentrations were determined using solid-phase extraction, according to the method described by Sarasa-Nacenta et al. [23]. The analytical column was a NovaPak® C8, 4.6 × 150 mm and the guard column was a NovaPak® C8 (Waters). The method was validated over the range of 0.1–10 mg l−1. Concentrations of lopinavir, nelfinavir, saquinavir, amprenavir, atazanavir and indinavir were quantified simultaneously. The analytical column used was a NovaPak® C18 3.9 × 150 mm and the guard column was a NovaPak® C18 (Waters). The method involved liquid–liquid extraction of the six drugs from plasma with tert-butyl methyl ether after basification and a second wash with hexane. Gradient elution using a mobile phase of phosphate buffer acetonitrile (pH 6.70) was performed. The method was linear over the range of 0.05–20 mg l−1 for lopinavir, amprenavir, 0.042–17 mg l−1 for nelfinavir, 0.044–17.5 mg l−1 for saquinavir and atazanavir and 0.04–16 mg l−1 for indinavir. The intraday and interday coefficients of variation of each method were lower than 8.2% and 8.7%, respectively. Our laboratory is subjected to the KKGT quality assurance programme organized by the Dutch Association for Quality Assessment in Therapeutic Drug Monitoring and Clinical Toxicology of the Radbound University Medical Centre Nijmen, with 36 laboratories being involved in 2004 [24]. Plasma HIV-1 RNA was quantified by the Amplicor® Ultrasensitive Assay (Roche Amplicor HIV-1 Monitor assay, v1.5), with a limit of detection of 50 copies ml−1.
Based on previously published data [18, 25], drug concentrations were considered optimal if they were above the proposed MEC. Thus, target trough concentrations were 3.4 mg l−1 for nevirapine, 1.0 mg l−1 for efavirenz, 0.8 mg l−1 for nelfinavir, 0.1 mg l−1 for indinavir and for saquinavir, 0.15 mg l−1 for atazanavir, 1.0 mg l−1 for lopinavir (4.0 mg l−1 in patients who previously failed on PI therapies) and 0.4 mg l−1 for amprenavir (1.2 mg l−1 in patients who previously failured on PI therapies). In addition, efavirenz, nevirapine and atazanavir concentrations were considered potentially toxic if they were higher than 4.0 mg l−1, 6.0 mg l−1 and 0.85 mg l−1, respectively [18, 25, 26].
Statistical analysis was performed using SPSS Version 11.5 software (SPSS Inc., Chicago, IL, USA). Data with a normal distribution were presented as means (SD), whereas medians [interquartile range (IQR)] were presented for variables not normally distributed. Interindividual variability in drug concentrations was assessed only for those agents administered to at least five patients. The coefficient of variation (CV) was calculated as the quotient of the SD divided by the mean. Proportions were compared by the χ2 or the Fisher’s exact test, where appropriate.
Results
One hundred and ninety-one patients receiving NNRTI or PI therapy attended our clinic for routine blood tests. Of these, 117 patients were eligible for the study and were included. Median (IQR) length of time on the current antiretroviral regimen was 20 (8.5–39) months. Overall, compliance with antiretroviral therapy was high and 82% of the patients stated that they had taken all the prescribed doses within the previous week. Table 1 summarizes the demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics of the 117 patients studied
| n (%) | |
|---|---|
| Gender | |
| Male | 79 (67.5) |
| Female | 38 (32.5) |
| Age (years)* | 41.1 (9.4) |
| HCV | 50 (42.7) |
| Years since HIV diagnosis* | 10.5 (5.2) |
| Months on ARV therapy† | 20.0 (8.5–39.0) |
| Current ARV treatment | |
| NNRTI | 60 (51.3) |
| PI | 57 (48.7) |
| Missing doses within the previous week | |
| 0 | 96 (82.1) |
| 1 | 16 (13.7) |
| ≥ 2 | 5 (4.2) |
Data are expressed as n (%), except where noted. HCV, Hepatitis C virus; ARV, antiretroviral; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Mean (SD).
Median (interquartile range).
Sixty patients were receiving one NNRTI and 57 subjects one PI when blood samples were collected (Table 1). Forty-two patients were receiving efavirenz (600 mg once daily) and 18 were using nevirapine (200 mg twice daily). Forty-three subjects were taking lopinavir/ritonavir (400/100 mg twice daily), nelfinavir (1250 mg twice daily) was being used by seven patients, saquinavir/ritonavir (1000/100 mg twice daily) by one, amprenavir/ritonavir (600/100 mg twice daily) by two, atazanavir (400 mg once daily) by three and indinavir (1200 mg twice daily) by one patient. No one was receiving ritonavir as single PI when samples were collected.
The mean (SD) sampling time was 11.3 (0.8) h after the last dose for subjects on a twice-daily antiretroviral regimen (n = 72), 22.0 (1.1) h for patients on a once-daily regimen at breakfast time (n = 3) and 10.2 (1.1) h for patients receiving efavirenz once daily at bed-time (n = 42).
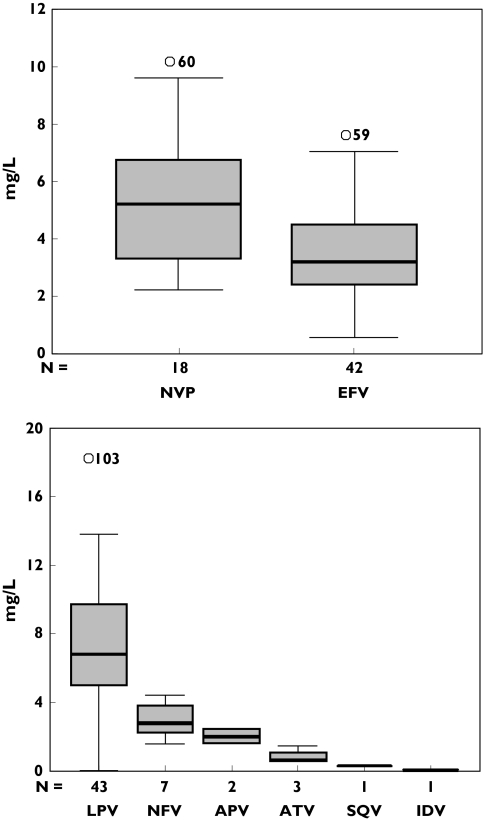
The plasma concentration data are presented in Figure 1. One sample, taken from a patient receiving lopinavir, was below the lower limit of quantification. Overall, interindividual variability in plasma NNRTI and PI concentrations was approximately 50%. In addition, 12% of the patients showed drug concentrations below the MEC, with only 68% of NNRTI or PI concentrations being within the proposed therapeutic range (Table 2).
Figure 1.
Trough plasma concentrations of non-nucleoside reverse transcriptase inhibitor and protease inhibitor. The box plot provides a five-point summary of the data: minimum, 1st quartile, median, 3rd quartile and maximum. NVP, Nevirapine; EFV, efavirenz; LPV, lopinavir; NFV, nelfinavir; APV, amprenavir; ATV, atazanavir; SQV, saquinavir; IDV, indinavir
Table 2.
Distribution of the plasma concentrations of non-nucleoside reverse transcriptase inhibitors and protease inhibitors
| Antiretroviral drug | Number | Concentration (mg l−1)* | CV (%) | Optimal n (%) |
|---|---|---|---|---|
| Nevirapine | 18 | 5.31 (2.13) | 40.1 | 5 (27.8) |
| Efavirenz | 442 | 3.62 (1.70) | 46.9 | 26 (61.9) |
| Lopinavir | 43 | 7.26 (3.91) | 53.8 | 35 (81.4) |
| Nelfinavir | 7 | 3.02 (1.11) | 36.7 | 7 (100) |
| Saquinavir/rtv | 1 | 0.350 | 1 (100) | |
| Amprenavir/rtv | 2 | 2.06 (1.66–2.45)† | 2 (100) | |
| Atazanavir | 3 | 0.67 (0.62–1.52)† | 3 (100) | |
| Indinavir | 1 | 0.10 | 1 (100) | |
| Total | 117 | 80 (68.4) |
CV, Coefficient of variation; rtv, ritonavir.
Data are expressed as mean (SD), except where noted.
Median (interquartile range).
When drug class was considered, drug concentrations were optimal in only 52% of the patients treated with NNRTIs, compared with 86% receiving PIs (odds ratio 0.17; 95% confidence interval 0.06, 0.46; P < 0.001). Out of the 18 patients on nevirapine therapy, drug concentrations were subtherapeutic in five (28%), potentially toxic in eight (44%) and optimal in only five subjects (28%). Similarly, efavirenz concentrations were within the therapeutic range in 62% of the patients who were receiving this drug. Although only one patient on efavirenz therapy had drug concentrations <1.0 mg l−1, concentrations were found to be potentially toxic in 15 (36%) of the patients being treated with this drug. The proportion of patients with PI concentrations above the proposed MEC was approximately 80% in those receiving lopinavir/ritonavir and 100% in those using nelfinavir, saquinavir, amprenavir, atazanavir or indinavir (Table 2).
Out of the 14 patients achieving NNRTI or PI plasma concentrations below the MEC, nonadherence was found to be a possible explanation for five cases (36%). The reason that the remainder of this group had subtherapeutic drug concentrations is unknown.
Overall, 17 patients developed virological failure during the 48-week period which followed blood sampling. Drug concentrations were above the proposed MEC in 16 patients and only one subject had subtherapeutic drug concentrations (P = 0.69). No patient receiving nevirapine or atazanavir therapy developed significant drug-related toxicity during the follow-up. On the other hand, nine out of the 42 patients who were receiving efavirenz experienced central nervous system disturbances. However, concentrations of this drug in plasma were considered optimal in most of the patients and only two (22%) had concentrations >4.0 mg l−1. Similarly, the proportion of subjects who developed central nervous system symptoms while receiving efavirenz was similar in patients with drug concentrations above and below 4.0 mg l−1 (P = 0.451).
Discussion
Our results show that plasma concentrations of NNRTI and PI may vary widely among HIV-infected adults in a routine outpatient setting. In addition, drug concentrations were outside the recommended therapeutic window in almost one-third of our patients.
Overall, our results are concordant with previously reported data. In a study performed by de Maat et al. of 97 subjects, drug concentrations were subtherapeutic in one-quarter of the 1145 samples analysed [20]. The lower prevalence of subtherapeutic drug concentrations observed in our study is probably because the drug analyses in the study by de Maat et al. were all requested for TDM purposes and samples were not collected in a systematic way. In addition, many patients in that study had repeated drug determinations over time. As a consequence, the inclusion of patients whose drug concentrations were suspected to be outside the therapeutic range might have been favoured, leading to an overestimation of the true prevalence of subtherapeutic drug concentrations. The present study included all subjects on NNRTI or PI therapy attending our clinic over a representative period of time and only one sample was collected from each patient. Thus, our results may represent more accurately the actual prevalence of drug concentrations outside the therapeutic window among HIV-infected adults in routine clinical practice.
Variability in drug concentrations may have important consequences for antiretroviral therapy. Subtherapeutic drug concentrations may prevent sustained viral suppression in HIV-infected patients and may predispose them to the development of viral resistance, limiting future re-utilization of antiretroviral agents [27]. On the other hand, inappropriately high drug concentrations are associated with adverse events, which may have a negative effect on quality of life and treatment adherence [18, 25, 26]. In our study, interindividual variability in drug concentrations in subjects receiving the same dose of each NNRTI or PI was about 50%. Fourteen out of the 117 patients studied showed drug concentrations below the proposed MEC and one-third of the patients receiving NNRTI therapy had potentially toxic drug concentrations. In addition, inappropriate adherence explained less than half of the subtherapeutic drug concentrations. This finding suggests that individual differences in drug absorption, distribution, metabolism or elimination may be responsible for variability in drug concentrations and that, although other factors such as intraindividual variability should also be considered [5], monitoring drug concentrations of antiretroviral agents might be of help in clinical practice.
We found no relationship between drug exposure and the development of virological failure or drug-related toxicity in this study. Thus, the proportion of patients who developed virological failure was similar between patients with drug concentrations below and above the proposed MEC. Our results are in disagreement with previous observations [11–19] and may suggest that the role of TDM is limited. However, several points should be considered when interpreting these findings. First, the main objective of this study was to assess interindividual variability in drug concentrations in clinical practice and only few patients developed treatment failure or drug-related toxicity during follow-up. As a consequence, the study may not have been powerful enough to relate clinical outcome to drug exposure. In addition, we measured drug concentrations at only a single time point, which may not have been representative of values throughout follow-up. Finally, the clinical usefulness of the limits of the therapeutic window proposed for some antiretroviral agents continues being debated. In this regard, although Marzolini et al. identified efavirenz concentrations >4.0 mg l−1 as being associated with central nervous system disorders [15], Kappelhoff et al. found no relationship between efavirenz exposure and the development of neuropsychiatryic disturbances [28]. In addition, the target MEC may vary widely between individuals, or even within the same subject with time, due to the accumulation of drug resistance mutations in the viral genome and to the gradual decrease in drug susceptibility.
In conclusion, wide interindividual variability in plasma concentrations of PI and NNRTI among HIV-infected adults in routine clinical practice was observed, with drug concentrations being outside the proposed therapeutic window in a significant proportion of patients. These results, in combination with the lower intraindividual variability in PI and NNRTI concentrations previously reported [15, 29], support a potential role for TDM of antiretroviral therapy. Prospective, randomized, appropriately powered trials assessing the clinical usefulness of this strategy are needed.
J.M. is supported by FIS trough grant 030135 from the Fundació per a la Recerca Biomédica Germans Trias i Pujol in collaboration with the Spanish Health Department. M.V. is supported by FIS trough grant CP04/00121 from the Spanish Health Department in collaboration with Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona.
References
- 1.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fhurer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;238:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr, Feinberg JE, Balfour HH, Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimetre or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Hecht FM, Swanson M, Elbeik T, Loftus R, Cohen PT, Grant RM. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13:F35–F43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 4.Barlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antirretroviral-naive HIV-1 infected adults. AIDS. 2001;15:1369–77. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 5.Back D, Gatti G, Fletcher C, Garraffo R, Haubrich R, Hoetelmans R, Kurowski M, Luber A, Merry C, Perno CF. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS. 2002;16(Suppl. 1):S5–S37. doi: 10.1097/00002030-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Murphy R. Antirretroviral therapy in patients with suboptimal virologic suppression. AIDS Rev. 1999;1:205–12. [Google Scholar]
- 7.Drusano GL, Biello JA, Stein DS, Nessly M, Meibohm A, Emini EA, Deustch P, Condra J, Chodakewitz J, Holder DJ. Factors influencing the emergence of resistance to indinavir: role of virologic, immunologic, and pharmacologic variables. J Infect Dis. 1998;78:360–7. doi: 10.1086/515631. [DOI] [PubMed] [Google Scholar]
- 8.Reijers MH, Weigel HM, Hart AA, Ten Kate RW, Mulder JW, Reiss P, Schuitemaker H, Hoetelmans RM, Weverling GJ, Lange JM. Toxicity and drug exposure in a quadruple drug regimen in HIV-1 infected patients participating in the ADAM study. AIDS. 2000;14:59–67. doi: 10.1097/00002030-200001070-00007. [DOI] [PubMed] [Google Scholar]
- 9.Burger DM, Hoetelmans RM, Hugen PW, Mulder JW, Meenhorst PL, Koopmans PP, Brinkman K, Keuter M, Dolmans W, Hekster YA. Low plasma concentrations of indinavir are related to virological treatment failure in HIV-1 infected patients on indinavir-containing triple therapy. Antivir Ther. 1998;3:215–20. [PubMed] [Google Scholar]
- 10.Dieleman JP, Gyssens IC, van der Ende ME, de Marie S, Burger DM. Urological complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS. 1999;13:473–8. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 11.Gieschke R, Fotteler B, Buss N, Steiner JL. Relationship between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin Pharmacokinet. 1999;37:75–86. doi: 10.2165/00003088-199937010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Hugen PW, Aarnoutse RE, Hoetelmans R, Jambroes M, Nieuwkerk PT, Schreij G, Schneider MM, van der Ende ME, Lange JM ATHENA Study Group. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther Drug Monit. 2003;25:73–80. doi: 10.1097/00007691-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Sadler BM, Guillotin C, Lou Y, Stein DS. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob Agents Chemother. 2001;45:30–7. doi: 10.1128/AAC.45.1.30-37.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masquelier B, Breilh D, Neau D, Lawson-Ayayi S, Lavignolle V, Ragnaud JM, Dupon M, Morlat P, Dabis F, Fleury H. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46:2926–32. doi: 10.1128/AAC.46.9.2926-2932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1 infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 16.Veldkamp AL, Weverling GJ, Lange JM, Montaner JS, Reiss P, Cooper DA, Vella S, Hall D, Beijnen JH, Hoetelmans RM. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1 infected individuals. AIDS. 2001;15:1089–95. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Havlir D, Cheeseman SH, McLaughlin M, Murphy R, Erice A, Spector SA, Greenough TC, Sullivan JL, Hall D, Myers M. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–45. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez de Requena D, Bonora S, Canta F, Marrone R, D’Avolio A, Sciandra M, Milia M, Di Garbo A, Sinicco A, Di Perri G. Atazanavir Ctrough is Associated with Efficacy and Safety: Definition of Therapeutic Range. 12th Conference on Retroviruses and Opportunistic Infections; Boston. [Abstract 645]. [Google Scholar]
- 19.Torti C, Quiros-Roldan E, Tirelli V, Regazzi-Bonora M, Moretti F, Pierotti P, Orani A, Maggi P, Cargnel A, Patroni A, De Luca A, Carosi G. Lopinavir plasma levels in salvage regimens by a population of highly active antiretroviral therapy-treated HIV-1-positive patients. AIDS Patient Care Stds. 2004;18:629–34. doi: 10.1089/apc.2004.18.629. [DOI] [PubMed] [Google Scholar]
- 20.de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp ECM, Mairuhu ATA, Beijnen JH. Subtherapeutic antiretroviral concentrations in routine clinical outpatient HIV care. Ther Drug Monit. 2003;25:367–73. doi: 10.1097/00007691-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Langmann P, Zilly M, Weissbrich B, Schlor C, Vath T, Richter E, Klinker H. Therapeutic drug monitoring of saquinavir in patients during protease inhibitor therapy with saquinavir alone or in combination with ritonavir or nelfinavir. Eur J Med Res. 2000;5:59–62. [PubMed] [Google Scholar]
- 22.Burger D, Hughen P, Reiss P, Gyssens I, Schneider M, Kroon F, Schreij G, Brinkman K, Richter C, Prins J, Aaranoutse R, Lange J ATHENA Study Group. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naïve HIV-infected individuals. AIDS. 2003;17:1157–65. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- 23.Sarasa-Nacenta M, López-Púa Y, López-Cortés LF, Mallolas J, Gatell JM, Carne X. Determination of efavirenz in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromat B Biomed Sci Appl. 2001;763:53–9. doi: 10.1016/s0378-4347(01)00357-7. [DOI] [PubMed] [Google Scholar]
- 24.Droste JAH, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J Acquir Immune Defic Syndr. 2003;32:287–91. doi: 10.1097/00126334-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 25.Back DJ, Blashke TF, Boucher C, Burguer D, Fletcher C, Flexner C, Gerber J, Shapiro J. Optimising TDM in HIV Clinical Care: a Practical Guide to Performing Therapeutic Drug Monitoring (TDM) for Antiretroviral Agents [online] Available from URL http://www.hivpharmacology.com Last accessed 10 January 2005.
- 26.de Maat MM, ter Heine R, Mulder JW, Meenhorst PL, Mairuhu ATA, van Gorp ECM, Huitema AD, Beijnen JH. Incidence and risk factors for nevirapine-associated rash. Eur J Clin Pharmacol. 2003;59:457–62. doi: 10.1007/s00228-003-0613-3. [DOI] [PubMed] [Google Scholar]
- 27.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS, Montaner JS. Predictors of HIV drug-resistance mutations in a large antiretroviral-naïve cohort initiating antiretroviral therapy. J Infect Dis. 2005;191:339–47. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 28.Kapelhoff BS, van Leth F, Robinson PA, MacGragor TR, Baraldi E, Montella F, Uip DE, Thompson MA, Russell DB, Lange JM, Beijnen JH, Huitema AD. Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther. 2005;10:489–98. [PubMed] [Google Scholar]
- 29.Marzolini C, Buclin T, Decosterd LA, Biollaz J, Telenti A. Nelfinavir plasma levels under twice-daily and three-times-daily regimens: high interpatient variability and low intrapatient variability. Ther Drug Monit. 2001;23:394–8. doi: 10.1097/00007691-200108000-00012. [DOI] [PubMed] [Google Scholar]